Abstract
Alternatively activated macrophages play an important role in host defense in the context of a T helper type 2 (Th2) microenvironment such as parasitic infection. However, the role of these macrophages during secondary challenge with Th1 pathogens is poorly defined. In this study, thioglycollate-elicited mouse peritoneal macrophages were treated with interleukin-4 (IL-4) or IL-13 in vitro and challenged with Neisseria meningitidis. After 8 to 12 hours of IL-4 pretreatment, the nonopsonic phagocytic uptake of N meningitidis was markedly reduced, depending on the common IL-4Rα chain, but independent of Scavenger receptor A and macrophage receptor with collagenous structure (MARCO), 2 known receptors for N meningitidis. Inhibition of phagocytosis extended to several other microbial particles, zymosan, and other bacteria. Concomitantly, IL-4 potentiated the secretion of proinflammatory cytokines, after additional bacterial stimulation, which depended on the MyD88 signaling pathway. Similar results were obtained after intraperitoneal stimulation of IL-4 and N meningitidis in vivo. Further in vitro studies showed a striking correlation with inhibition of Akt phosphorylation and stimulation of the mitogen-activated protein kinase pathway; inhibition of phagocytosis was associated with inhibition of phagosome formation. These findings are relevant to host defense in mixed infections within a Th2 microenvironment and shed light on immunologic functions associated with alternative priming and full activation of macrophages.
Introduction
Macrophages (MΦs) play an important role in the innate and acquired host response to intracellular and extracellular pathogens. They contribute to the recognition, uptake, and killing of microorganisms and multicellular parasites, antigen presentation to T and B lymphocytes, and inflammation during both acute and chronic infections.1 The phenotype of MΦs is markedly heterogeneous,2 with distinct signatures of gene expression and effector functions associated with Toll-like receptor (TLR; innate),3 interferon-γ (IFN-γ; classical activation)4 and interleukin-4 (IL-4)/IL-13 (alternative activation)5 pathways. Although the role of MΦs in T helper type 1 (Th1)–dependent antimicrobial responses is well defined, their functions in Th2-dependent or mixed responses remain poorly understood. IL-4 and IL-13 have overlapping but distinct effects on MΦs, dependent on a common IL-4Rα,6 with profound changes in the expression of a range of cellular proteins and functions broadly implicated in the regulation of inflammation and repair.5 Most studies hitherto have focused on IL-4 as a sole differentiating cytokine, without further TLR, Th1, or Th2 stimuli, which may be required to induce full expression of MΦ effector mechanisms. It is known that IL-4 pretreatment of MΦs can potentiate lipopolysaccharide (LPS)–induced cytokine and chemokine production.7–10 IL-4, by itself, has profound effects on fluid phase and mannose receptor (MR)–dependent and independent endocytosis, as well as modifying other elements of the endocytic pathway.11–13 However, the effects of IL-4 on phagocytosis of opsonized and unopsonized bacteria, yeasts, or other particles are not clear,14–16 nor has the effect of phagocytic stimuli on intracellular signaling and secretion by IL-4–treated MΦs been defined.
We have studied the effect of IL-4 pretreatment on a well-characterized phagocytic model, nonopsonic recognition of Neisseria meningitidis by the class A Scavenger receptors, SRA I/II and macrophage receptor with collagenous structure (MARCO). N meningitidis, a Gram-negative diplococcus, is an important cause of bacterial meningitis and septic shock in humans.17 We observed a striking reduction in the uptake of N meningitidis after IL-4 pretreatment of thioglycollate-elicited mouse peritoneal MΦs (ThioMΦs), which extended to a range of particles. At the same time, IL-4 induced a remarkable shift to enhanced secretion of proinflammatory cytokines, after secondary microbial challenge. These alterations in cell function occurred in parallel with a switch in phosphorylation of key signal transducers. Our studies show that IL-4 can prime MΦs to undergo additional, microbial-induced changes in cellular properties, relevant to host defense and pathogenesis of infectious and immune diseases.
Methods
Animals
The mice used in this study were older than 8 weeks on a C57/BL6J background. We used the following knockout (KO) mouse strains: SRA (SRA−/−),18 MARCO (MARCO−/−),19 SRA/MARCO double knockout (SRA−/−/MARCO−/−),20 IL-4Rα (IL-4Rα−/−),21 and MyD88 (MyD88−/−).22 All animals were housed under specific pathogen-free conditions and handled in accordance with guidelines issued by the United Kingdom Home Office.
Reagents
Mouse recombinant IL-4 and mouse recombinant IL-13 were obtained from R&D Systems. PD98059 (MEK inhibitor), SB202190 (p38 inhibitor), and wortmannin (phosphatidylinositol 3-kinase [PI3K] inhibitor) were purchased from Sigma-Aldrich. Fluorescein isothiocyanate–labeled zymosan and Rhodamine Green X (RdGnX) were obtained from Invitrogen. Bacteriologic plastic plates were obtained from Greiner. All the electron microscopy supplies are from Agar Scientific.
Bacterial culture and labeling
N meningitidis serogroup B (strain MC58),23 a kind gift of Dr Richard Moxon (Weatherall Institute of Molecular Medicine, University of Oxford), was cultivated as described.23 For fluorescent labeling, N meningitidis was resuspended in 70% ethanol overnight at 4°C and labeled with RdGnX (RdGnX-N meningitidis).24 Ethanol inactivation of the intact organism preserves ligand activity for both SR-A and TLR-4 and allows ligation of both receptors.25
Particle uptake assay
For in vitro uptake by MΦs, peritoneal cells were isolated by lavage with ice-cold PBS from mice that had been treated intraperitoneally 4 days previously with 1 mL 4% thioglycollate broth (Sigma-Aldrich). MΦs were plated in 6-well bacteriologic plastic vessels at a density of 106 cells per well in OPTIMEM medium, with or without IL-4 or IL-13, for 48 hours. Cells were washed with PBS and challenged with RdGnX-N meningitides (100 bacteria/MΦ) for 2 hours at 37°C. After incubation, unbound particles were removed by washing 3 times with cold PBS. MΦs were harvested with cold PBS containing 4 mg/mL lidocaine-HCl and 10mM EDTA (PBS/EDTA/lidocaine), before fixation with 2% paraformaldehyde (PFA). Fluorescence was determined on a FACScan instrument (Becton Dickinson), and the results were analyzed with FlowJo (TreeStar Inc) software. The mean fluorescence of unchallenged control cells was subtracted from the mean fluorescence of each test sample, and the average was determined. Results are representative of at least 3 independent experiments. Statistical significance was determined using the Student t test.
The effect of IL-4 on N meningitidis binding was determined as described in the paragraph above, but each step was performed at 4°C.
Lysate preparation, SDS–polyacrylamide gel electrophoresis, and Western blotting
To prepare whole-cell lysates, ThioMΦs (106 cells/well) were cultivated on bacteriologic plastic. The cells were washed with PBS and lysed with ice-cold RIPA buffer (25mM Tris-HCl pH 7.6, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 1mM PMSF and protease inhibitor cocktail (Roche Applied Science). Twenty micrograms of protein were separated on a 6% SDS–polyacrylamide gel and transferred to nitrocellulose (Amersham). The membrane was blocked in 5% milk in PBS/0.1% Tween 20 and probed overnight with primary antibodies. After washing with PBS/Tween, blots were probed with HRP-conjugated secondary antibody (The Jackson Laboratory) for 1 hour at room temperature. Staining was shown by chemiluminescence (ECL protein detection system; Amersham), and bands were visualized on Hyperfilm-ECL (Amersham). The Abs used were 2F8 (rat IgG2b anti–mouse SRA mAb)26; ED31 (rat IgG2b anti–mouse MARCO mAb),27 and 5D3 (rat IgG2a anti–mouse MR mAb).28 All other antibodies were purchased from Cell Signaling.
Immunofluorescent detection of SRA, MARCO, and Dectin 1 expression by FACS
ThioMΦs (106 cells/well) were cultivated on bacteriologic plastic and stimulated for different periods with IL-4. Cells were washed 3 times with PBS, detached with PBS/EDTA/lidocaine then incubated in blocking buffer (PBS, 0.5% BSA, 2mM NaN3, 2mM EDTA, 5% goat serum, 5% rabbit serum) for 30 minutes at 4°C. Cells were incubated with primary Abs for 1 hour at 4°C in blocking buffer and washed 3 times with fluorescence-activated cell sorting (FACS) wash buffer (PBS 0.5% BSA, 2mM NaN3, 2mM EDTA). Cells were probed with an Alexa 488–secondary antibody (Molecular Probes) for 1 hour at 4°C in blocking buffer and washed 3 times with FACS wash buffer. Cells were fixed with 2% PFA, and fluorescence was analyzed on a FACScan (Becton Dickinson), using FlowJo (TreeStar Inc) software. The primary Abs used in this study were 2F8, ED31, and 2A11 (rat IgG2b anti–mouse Dectin 1 mAb).29
Determination of cytokine release
ThioMΦs (106 cells/ well) were plated on bacteriologic plastic, stimulated for 48 hours with IL-4, and challenged with N meningitidis (100 bacteria /MΦ) for 24 hours. Supernatants were harvested, centrifuged at 8000g, and stored at −80°C. Levels of tumor necrosis factor (TNF-α; eBioscience) and IL-6 (BD Biosciences) were determined by enzyme-linked immunoabsorbent assay (ELISA), according to the manufacturer's instructions. The level of IL-12p70 was determined using the mouse FlowCytomix system (Bender MedSystems GmbH).
Determination of in vivo uptake of N meningitidis after IL-4 treatment
C57/BL6J mice (older than 8 weeks) were injected intraperitoneally with 100 ng IL-4, 12 hours and 2 hours (injection of PBS for the control) before challenge with 108 ethanol-killed RdGnX-N meningitidis for 2 hours. The infiltrate was collected by lavage with PBS, and cells were fixed with 2% paraformaldehyde. Fluorescence was analyzed by flow cytometry, using FlowJo (TreeStar Inc) software. The scatter profile and labeling with F4-80 were used to gate MΦs in the peritoneal washout.
Determination of in vivo cytokine production after IL-4 treatment
C57/BL6J mice (older than 8 weeks) were injected intraperitoneally with 100 ng IL-4, 12 hours and 2 hours (injection of PBS for the control) before challenge with 2 × 106 N meningitidis for 2 hours. The infiltrate was collected by lavage with PBS and spun down. TNF-α and IL-6 were assayed by ELISA in the supernatants.
Electron microscopy
ThioMΦs were treated for 48 hours with IL-4 and challenged with zymosan (20 particles/MΦ) for 30 minutes at 37°C. For transmission electron microscopy (TEM), ThioMΦs were plated on bacteriologic plastic and fixed with the use of a mixture of 2.5% glutaraldehyde, 2% paraformaldehyde, and 0.1% picric acid in 100mM cacodylate buffer (pH 7.0) containing 2mM EGTA and 1mM MgCl2. The samples were post-fixed in 1% osmium in 100mM cacodylate buffer (pH 7.0) for 1 hour at 4°C, washed with distilled water, and stained en bloc with 2% aqueous uranyl acetate for 2 hours at 4°C, in the dark. The samples were dehydrated with ethanol, and the cells were released from plastic using propylene oxide. The cells were pelleted and washed several times with propylene oxide (to remove the dissolved plastic still present in solution) and embedded in resin. Ultrathin (∼ 70-nm thick) sections were cut, stained with uranyl acetate and lead citrate, and examined in a FEI Tecnai 12 electron microscope.
For scanning electron microscopy, ThioMΦs were plated on coverslips and fixed as described in the paragraph above. Samples were rinsed several times with distilled water, dehydrated through a series of ethanol washes, and critical point-dried. After sputter-coating with gold, samples were examined in a JOEL JSM 5510 scanning electron microscope. Images were viewed with an FEI Tecnai 12 microscope (FEI UK Ltd) operating at 80 kV with a 20-μm objective aperture using a Gatan US1000 1 2k-2k CCD camera and Gatan DigitalMicrograph software Version 3.11.1. Images were analyzed with Adobe Photoshop software.
Results
Alternative activation of MΦs decreases N meningitidis uptake
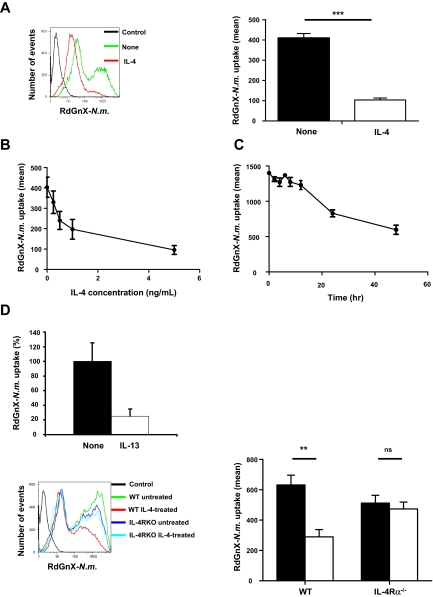
Alternatively activated MΦs are present during Th2-type responses, particularly in allergic, cellular, and humoral reactions to extracellular pathogens. However, the phagocytic capacity of alternatively activated MΦs for microorganisms is poorly defined. To examine this function, ThioMΦs were stimulated for 48 hours with recombinant IL-4 and challenged with RdGnX-N meningitidis (100 bacteria/MΦ) for 2 hours in the absence of serum. FACS analysis showed heterogeneity in the uptake of N meningitidis by wild-type MΦs (WT MΦs). IL-4 treatment decreased N meningitidis uptake by ThioMΦs and more particularly induced the loss of the population with the higher capacity for uptake (P < .001; Figure 1A). Moreover, IL-4 induced a dose-dependent decrease of N meningitidis uptake (Figure 1B). A time course study showed that inhibition of uptake by IL-4 became evident after 12 hours of treatment (Figure 1C) and was sustained for the duration of the experiment (48 hours), without loss of MΦ viability. Similar results were obtained with Biogel polyacrylamide bead–elicited MΦs (data not shown). We determined that IL-4 also affected uptake of other bacteria such as Escherichia coli as well as of other particles such as zymosan and latex beads (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Viability of the cells, verified by Trypan blue exclusion and phase contrast microscopy, was not modified by IL-4 treatment. To confirm the specific effect of alternative activation of MΦs, we demonstrated that IL-13 also inhibited N meningitidis uptake by ThioMΦs (Figure 1D). Moreover, we treated ThioMΦs lacking the common IL-4/IL-13 receptor α chain, shared by IL-4 and IL-13 (IL-4Rα−/− ThioMΦs) with IL-4, followed by challenge with N meningitidis. IL-4–treated WT ThioMΦs had an approximately 50% to 80% dose-dependent reduction in ingestion of N meningitidis, which was absent in IL-4Rα−/− ThioMΦs (Figure 1D).
Figure 1.
Alternative activation of MΦs decreases N meningitidis uptake. (A) Flow cytometric analysis of ThioMΦ ingestion of Rhodamine Green labeled Neisseria (RdGnX-N.m.). ThioMΦs were stimulated with IL-4 for 48 hours and challenged with ethanol-fixed RdGnX-N.m. (100 bacteria/MΦ) for 2 hours at 37°C. The mean fluorescence for each treatment was determined by flow cytometry. The histogram shows the effect of IL-4 treatment on uptake of N meningitidis. (B) IL-4 induces a dose-dependent inhibition of N meningitidis uptake. ThioMΦs were stimulated with different concentrations of IL-4 for 48 hours and challenged as described. The mean fluorescence for each population was determined by flow cytometry. The average mean fluorescence for each condition is shown. Error bars indicate SDs. (C) IL-4–inhibited RdGnX-N.m. uptake by MΦs in a time-dependent manner. ThioMΦs were stimulated for different periods with IL-4 and challenged as above. Unchallenged cells served as a negative control. The mean fluorescence for each population was determined by flow cytometry. The average mean fluorescence for each condition is shown. Error bars indicate SDs. (D) The inhibition of N meningitidis uptake is specific for alternative activation of MΦs. (Left) ThioMΦs were stimulated with IL-13 (10 ng/mL) for 48 hours and challenged with ethanol-fixed RdGnX-N.m. The mean fluorescence for each treatment was determined by flow cytometry. The histogram shows the effect of IL-13 treatment on uptake of N meningitidis. (Right) IL-4 inhibited N meningitidis uptake by ThioMΦs via IL-4Rα. WT or IL-4Rα−/− ThioMΦs were incubated for 48 hours with IL-4 (5 ng/mL) and challenged with RdGnX-N.m. as described. After fixation with 2% paraformaldehyde, the mean fluorescence for each population was determined by flow cytometry. The figure showed the average mean fluorescence intensity of 3 independent experiments. Error bars indicate SDs.
Inhibition of N meningitidis phagocytosis by IL-4 treatment of MΦs is independent of SRA and MARCO expression
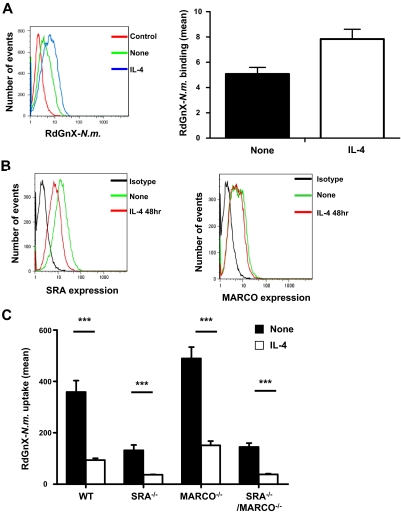
Phagocytosis is a complex mechanism that begins with the recognition and binding of pathogens by pattern-recognition receptors, followed by rearrangement of the actin cytoskeleton, leading to internalization. To elucidate the effect of IL-4 on N meningitidis binding, ThioMΦs were stimulated for 48 hours with IL-4 and challenged for 2 hours with RdGnX-N meningitidis at 4°C to inhibit ingestion. The association of RdGnX-N meningitidis was dependent on temperature, with more bacteria associated with ThioMΦs at 37°C than at 4°C (MFI 500 vs 4); however, our results indicated that IL-4 did not reduce N meningitidis binding at the cell surface (Figure 2A).
Figure 2.
IL-4 priming of MΦs does not modify SRA and MARCO expression or decrease N meningitidis binding at MΦ surface. (A) Effect of IL-4 on N meningitidis binding at the MΦ surface. ThioMΦs were treated for 48 hours with IL-4 and incubated for 2 hours at 4°C with RdGnX-N.m. (Nm; 100 bacteria/MΦ). The average mean fluorescence intensity of 2 independent experiments is presented as a bar diagram. Error bars indicate SDs. (B) Effect of IL-4 on surface expression of SRA and MARCO. ThioMΦs were treated for 48 hours with IL-4, and SRA and MARCO cell surface expressions were determined by flow cytometry using αMARCO mAb (ED31) or αSRA mAb (2F8). One experiment representative of 2 is shown. (C) Effect of alternative activation on RdGnX-N.m. uptake by WT, SRA−/−, MARCO−/−, and SRA−/−/MARCO−/− MΦs. ThioMΦs were treated for 48 hours with IL-4 and incubated for 2 hours at 37°C with RdGnX-N.m. (100 bacteria/MΦ). The average mean fluorescence intensity for 4 independent experiments is presented as a bar diagram. Error bars indicate SDs.
To confirm these data, we studied the effect of IL-4 treatment on expression of SRA and MARCO, well-characterized nonopsonic pattern recognition receptors for N meningitidis.24,25 ThioMΦs were stimulated for 48 hours with IL-4 and analyzed for SRA and MARCO surface expression. IL-4 treatment decreased the surface expression of SRA receptor to a limited extent, whereas the level of MARCO surface expression was not altered (Figure 2B). The observed decrease of SRA and MARCO expressions was out of proportion to, and did not correlate with, the drastic inhibition of N meningitidis uptake.
To extend this analysis, SRA KO (SRA−/−), MARCO KO (MARCO−/−), and SRA/MARCO double KO (SRA−/−/MARCO−/−) ThioMΦs were stimulated with IL-4 and challenged with RdGnX-N meningitidis. We confirmed that untreated SRA-deficient MΦs had a substantial reduction in N meningitidis ingestion25 (Figure 2C). By contrast, loss of MARCO expression was associated with a modest increase of N meningitidis uptake in untreated MΦs, whereas IL-4 treatment of MARCO−/− MΦs inhibited N meningitidis ingestion similarly to that observed with WT MΦs (P < .001). The SRA−/−/MARCO−/− ThioMΦs showed a similar response profile to that of the SRA−/− MΦs (Figure 2C). Our data confirmed that, although SRA is a major pattern recognition receptor for N meningitidis in ThioMΦs, IL-4 inhibition of N meningitidis uptake was independent of the expression of either scavenger receptor. Moreover, surface expression of Dectin-1 and MR, phagocytic receptors thought to play a role in zymosan recognition,30 was significantly higher on IL-4–stimulated ThioMΦs (supplemental Figure 1C; data not shown), confirming previous results.31 Despite increased receptor expression, IL-4 treatment decreased zymosan uptake (supplemental Figure 1C). These data strongly suggest that alternative activation of MΦs inhibited a phagocytic mechanism independent of pattern recognition receptor expression.
Inhibition of N meningitidis phagocytosis by IL-4 treatment of MΦs is associated with increased proinflammatory cytokine secretion
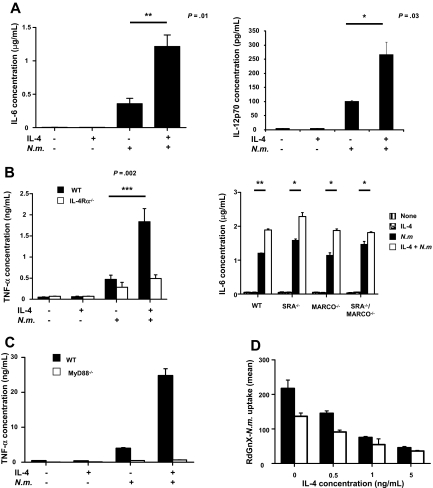
Protection against pathogens is mediated mainly by phagocytosis, inducing an immune response characterized by production of inflammatory cytokines.25 ThioMΦs were treated for 48 hours with IL-4 and challenged for 24 hours with N meningitidis. Results showed that IL-4 by itself did not modify cytokine production, whereas N meningitidis, in the absence of IL-4, increased TNF-α, IL-6, and IL-12p70 production, as expected.25 Strikingly, IL-4 and N meningitidis–cotreated MΦs secreted substantially higher levels of proinflammatory cytokines (Figure 3A-B), not observed after alternative activation of IL-4Rα−/− ThioMΦs (Figure 3B), confirming that increased proinflammatory secretion depended on the IL-4 receptor pathway. The anti-inflammatory cytokine IL-10 was not detected in our system (data not shown).
Figure 3.
Alternative activation potentiates N meningitidis–induced proinflammatory cytokine secretion via MyD88, independent of SRA and MARCO. (A) WT ThioMΦs were cultivated 48 hours in the presence or absence of IL-4 and incubated for 24 hours with or without N meningitidis (Nm; 100 bacteria/cell). The culture supernatant was analyzed for production of TNF-α and IL-6 by ELISA and by FlowCytomix for IL-12 p70 production. Data represent the mean ± SEM of replicates from 1 experiment, representative of 3 experiments. (B) Increased proinflammatory secretion depended on the IL-4 receptor pathway but was independent of pathogen recognition receptor expression. (Left) WT (■) and IL-4Rα−/− (□) ThioMΦs were cultivated 48 hours in the presence or absence of IL-4 and incubated for 24 hours with or without N meningitidis (100 bacteria/cell). The culture supernatant was analyzed for production of TNF-α and IL-6 by ELISA. Data represent the mean ± SEM of replicates from 1 experiment, representative of 3 experiments. (Right) WT, SRA−/−, MARCO−/−, and SRA−/−/MARCO−/− ThioMΦs were cultivated for 48 hours in the presence or absence of IL-4 and challenged with or without N meningitidis (100 bacteria/cells) for 24 hours. The culture supernatant was harvested and analyzed for IL-6 secretion by ELISA. (C) MyD88−/− ThioMΦs were cultivated for 48 hours in the presence or absence of IL-4 and challenged with or without N meningitidis for 24 hours. Cell supernatants were assayed for TNF-α by ELISA. (D) Flow cytometry of ingestion of RdGnX-N meningitidis by WT and MyD88−/− ThioMΦs. ThioMΦs were treated for 48 hours with different concentrations of IL-4 and incubated for 2 hours at 37°C with RdGnX-N meningitidis (100 bacteria/MΦ). The average mean fluorescence intensity of 3 independent experiments is shown as a bar diagram. Error bars indicate SDs.
Comparison of cytokine production by WT and SRA−/−, MARCO−/−, and SRA−/−/MARCO−/− ThioMΦs showed the same profile, independent of phagocytosis (Figure 3B). These data suggested that ingestion of N meningitidis via SRA is not necessary for MΦ activation and that increased cytokine production after alternative activation does not depend on SRA and MARCO receptors. LPS is a potent stimulus for MΦ gene expression, especially of TNF-α, by engaging a TLR4 membrane signaling complex. Peiser et al25 demonstrated previously that MΦs require TLR signaling for MΦ activation after N meningitidis exposure; we, therefore, determined the role of the MyD88 pathway in the enhanced secretion of proinflammatory cytokines by IL-4–treated MΦs. MyD88−/− MΦs had a greatly reduced response to N meningitidis, and the enhanced production of cytokines induced by IL-4 after N meningitidis challenge was abrogated (Figure 3C). However, the absence of MyD88 had no comparable effect on the uptake of RdGnX-N meningitidis by ThioMΦs (Figure 3D). These results confirmed that IL-4 pretreatment reduced phagocytosis but increased cytokine secretion, but they established that these responses differed in their requirement for MyD88.
IL-4 treatment in vivo impairs phagocytosis but also increases proinflammatory cytokine secretion
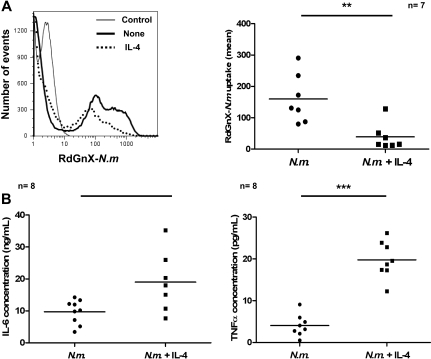
The results described in the paragraph above have shown that alternative activation inhibited phagocytosis of RdGnX-N meningitidis while potentiating particle-induced proinflammatory cytokine production in vitro. To confirm the biologic relevance of these findings, we assayed the effect of IL-4 on phagocytosis of N meningitidis in vivo, in the absence of thioglycollate stimulation. We injected RdGnX-N meningitidis into the peritoneum of C57/BL6J mice preinjected or not with IL-4 and analyzed N meningitidis uptake by peritoneal MΦs after 2 hours, by FACS. Preinjection of IL-4 decreased N meningitidis uptake significantly (P = .004; Figure 4A). Moreover, the decrease of uptake was associated with increased proinflammatory cytokine production in the peritoneal cavity over a period of 2 hours (IL-6, P = .01; TNF-α, P = .002; Figure 4B). These results have shown that the modulation of MΦ phenotype induced by IL-4 in vitro could also be shown in vivo.
Figure 4.
IL-4 impairs uptake of RdGnX-N meningitidis and increases antibacterial proinflammatory response in vivo. (A left) Flow cytometry profile of uptake of RdGnX-N.m. (Nm) by peritoneal cells. RdGnX-N meningitidis cells (108) were injected in the peritoneum of C57/BL6J mice. Animals were preinjected with IL-4, and the inflammatory infiltrate was collected by lavage. Mice preinjected with PBS were used as control. (Right) Scatter plot showing effect of IL-4 on RdGnX-N meningitidis uptake by MΦs. (B) Cytokine ELISA for TNF-α and IL-6 production in peritoneal lavage fluid. Ethanol-killed N meningitidis cells (2 × 106) were injected into the peritoneum of C57/BL6J mice, preinjected with IL-4. The inflammatory infiltrate was collected by lavage, and the supernatant was assayed for TNF-α and IL-6. Mice preinjected with PBS were used as controls. Each symbol represents 1 animal.
IL-4 pretreatment inhibits Akt but stimulates p42/p44 and p38 phosphorylation
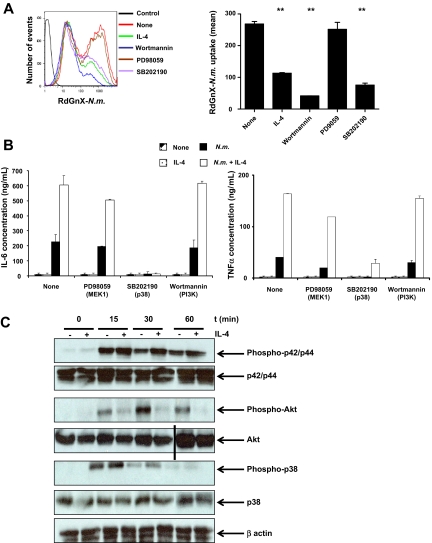
In MΦs, Fc-receptor–mediated phagocytosis is regulated by phospholipid-modifying enzymes such as PI3K,32 and cytokine secretion is associated with mitogen-activated protein kinase (MAPK) activation. To characterize the signaling pathways required during phagocytosis of N meningitidis and cytokine secretion, ThioMΦs were stimulated with inhibitors of p38 (SB202190), MEK1 (p42/p44 activator kinase; PD98059), or PI3K (wortmannin) for 1 hour, before and during 2-hour challenge with RdGnX-N meningitidis. Effects of the different inhibitors on N meningitidis uptake were determined by FACS. Inhibition of PI3K completely abolished N meningitidis uptake (P = .001), and inhibition of p38 decreased N meningitidis uptake to the same extent as IL-4 (P = .003). By contrast, inhibition of the p42/p44 pathway had no effect on N meningitidis uptake (Figure 5A). Phagocytosis of N meningitidis, therefore, required the activation of PI3K and the p38 kinase pathways. Furthermore, we demonstrated that TNF-α and IL-6 secretion depended on p38 phosphorylation, but it was independent of the PI3K and p42/p44 pathways (Figure 5B). Because IL-4 inhibited phagocytosis of N meningitidis by MΦs, we determined the effect of IL-4 on kinase activities. ThioMΦs were treated for 48 hours with IL-4 and challenged for different periods with N meningitidis, and the total levels of proteins and the phosphorylated forms of p38, Akt (kinase activated by PI3K), and p42/p44 were determined by Western blotting. Our results showed that IL-4 did not modify phosphorylation of the different kinases in the absence of N meningitidis. We demonstrated that N meningitidis by itself induced maximal activation of Akt after 30 minutes of stimulation, whereas Akt phosphorylation was markedly reduced in the presence of IL-4. Maximal phosphorylation of p42/p44 and p38 was observed after 15 minutes of N meningitidis stimulation. Cotreatment with IL-4 potentiated the activation of p42/p44 and p38, which was sustained over time (Figure 5C). We concluded that IL-4 treatment inhibited the PI3K pathway, required for N meningitidis phagocytosis, and that the PI3K pathway could be one of the targets of IL-4 that lead to inhibition of phagocytosis. Conversely, enhanced p38 phosphorylation was consistent with the increased proinflammatory cytokine secretion observed after IL-4 priming.
Figure 5.
IL-4 pretreatment and N meningitidis challenge inhibit Akt phosphorylation, but stimulate p42/p44 and p38 pathways. (A) Phagocytosis of N meningitidis by ThioMΦs is dependent on the p38 and PI3K pathway. ThioMΦs were treated with a kinase inhibitor (PD98059, 50μM; wortmannin, 100nM; SB202190, 50μM) for 1 hour before and during challenge (2 hours at 37°C) with RdGnX-N.m. (Nm; 100 bacteria/cell), and phagocytosis was determined by flow cytometry. (Right) FACS profile of RdGnX-N.m. uptake after treatments. The mean fluorescence of 1 experiment representative of 3 is shown as a bar diagram (left). (B) Proinflammatory cytokine secretion is dependent on the p38 pathway. ThioMΦs were treated with a kinase inhibitor (PD98059, 50μM; wortmannin, 100nM; or SB202190, 50μM) for 1 hour before and during challenge (24 hours at 37°C) with N meningitidis (100 bacteria/cell), and cytokine production was determined by ELISA. Results from 1 experiment of 2 are presented. (C) ThioMΦs were stimulated for 48 hours in the presence or absence of IL-4 and challenged with N meningitidis (100 bacteria/cell) for different periods. Expression of total protein levels and of the phosphorylated forms of p38, p42/p44, and Akt was determined by Western blotting. The results of 1 experiment, representative of 2, are presented. A vertical line has been inserted to indicate a repositioned gel lane.
IL-4 pretreatment inhibits phagosome formation
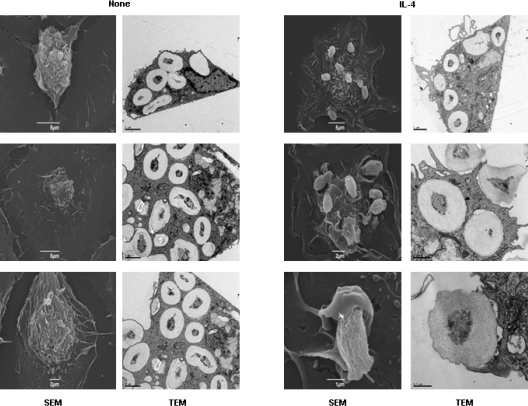
In MΦs, inhibition of PI3K arrests phagocytic cup closure.32 To determine whether the inhibition of PI3K by IL-4 modulates phagosome formation, ThioMΦs were stimulated for 48 hours with IL-4 and challenged with zymosan, a more suitable particle for morphologic studies. Scanning electron microscopy showed that, by 30 minutes, no zymosan particle was detected on the surface of untreated ThioMΦs, and TEM showed that zymosan particles were all intracellular. By contrast, in IL-4–treated cells, many zymosan particles were observed on the cell surface in phagocytic cups, in which distal margins remained open (Figure 6).
Figure 6.
Microscopy analysis of zymosan phagocytosis by untreated and IL-4–treated ThioMΦs. ThioMΦs were plated on bacteriologic plastic for TEM or on coverslips for scanning electron microscopy in the same well. Cells were treated for 48 hours with IL-4 and challenged with zymosan (20 particles/MΦ) for 30 minutes at 37°C. Scanning electron microscopy showed that zymosan particles were no longer seen on the cell surface of untreated ThioMΦs, because all had been internalized by the cells (TEM data). In IL-4–treated ThioMΦs, many incomplete phagocytic cups were observed on the surface of MΦs, showing the arrest of the phagocytic cup closure (white arrows) induced by IL-4 treatment. Moreover, TEM showed that few zymosan particles were internalized in IL-4–treated ThioMΦs compared with untreated MΦs.
Discussion
Using a 2-stage model of IL-4 pretreatment followed by N meningitidis challenge, our studies have shown a striking switch of MΦ phenotype from efficient phagocytosis to reduced uptake (Figure 1), coupled to potentiation of proinflammatory cytokine release in response to microbial stimulation (Figure 3), and associated with altered phosphorylation of signaling pathways implicated in phagocytosis and secretion (Figure 5; schematic representation in Figure 7). The alteration of phenotype depended on the IL-4Rα chain shared with IL-13, and a more than 12-hour delay after IL-4 treatment, consistent with IL-4–induced changes in MΦ gene expression (Figure 1). Down-regulation of uptake by IL-4 was also observed with other bacteria such as E coli and with other phagocytic particles, including zymosan (supplemental Figure 1), but was not observed in assays of MR-dependent and independent endocytosis of soluble ligands (data not shown).
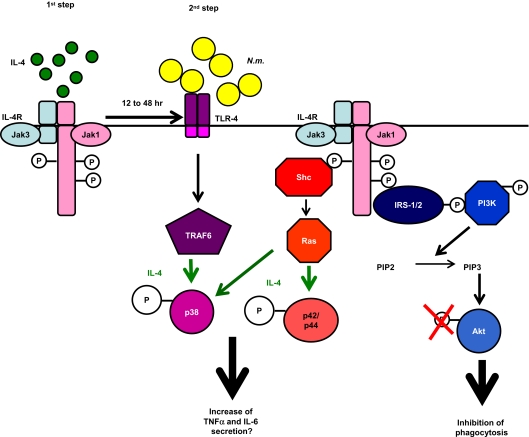
Figure 7.
Schematic summary of effect of IL-4 priming on phagocytic and secretory capacities of MΦs. IL-4 treatment (first step) inhibited the PI3K pathway, required for N meningitidis (Nm) phagocytosis and increased proinflammatory cytokine secretion after N meningitidis challenge (second step), consistent with enhanced p38 phosphorylation.
We demonstrated that IL-4 priming of MΦs increased proinflammatory cytokine secretion after N meningitidis stimulation. Our findings confirm and extend earlier studies on LPS7–10,33,34 and staphylococcal enhancement of proinflammatory cytokine release by IL-4–pretreated MΦs, with altered gene expression and signal transduction.7 Interestingly, Mylonas et al35 showed that nematode-elicited peritoneal MΦs, which display IL-4–dependent features, secrete more proinflammatory cytokines in response to LPS/IFN-γ challenge. The investigators also demonstrated that IL-4 priming of uninfected ThioMΦs did not modify cytokine secretion after LPS/IFN-γ stimulation in vitro. Our protocol used intact N meningitidis rather than LPS alone. Other studies have also shown that IL-4 treatment can increase inflammation and exacerbate the development of Th1-mediated diseases, such as colitis36 or experimental autoimmune uveoretinitis.37
At the cellular level, our studies provide evidence that alternative activation of MΦs extensively remodels membrane traffic involved in phagocytosis, signaling, and secretion, together with changes in gene expression.38 The effect of IL-4 on phagocytosis has been controversial. Several previous reports have shown that IL-4 did not modify the uptake of particles such as latex beads or bacteria,14,15 whereas others have shown increased phagocytosis of apoptotic neutrophils39,40 or protozoan parasites.41 Our results showing the inhibitory effect of IL-4 on phagocytosis are supported by those of Moreno et al,16 who demonstrated that the IL-4/STAT6 pathway decreased the phagocytosis of small particles by MΦs, and by Leidi et al,42 who demonstrated that IL-4 reduced phagocytosis of rituximab-opsonized B cells. Use of particulate pathogen-derived stimuli (zymosan) or intact N meningitidis made it possible to characterize the effect of IL-4 pretreatment on phagocytosis and to explore its mechanisms. IL-4 induced changes in scavenger receptor and Dectin-1 expression,31,43 major receptors for N meningitidis and zymosan, respectively, as well as MR, which did not correlate with reduced phagocytosis (Figure 2; supplemental Figure 1). Although ingestion of N meningitidis and zymosan was markedly reduced by IL-4, enhanced cytokine secretion may result, in part, from persistence of a TLR-dependent stimulus at the cell surface. It will be important to establish whether nonphagocytic or non-TLR challenges, eg, by immune complexes acting through FcR, alter signaling and secretion after IL-4 treatment.
We demonstrated that IL-4 altered phosphorylation signaling pathways implicated in phagocytosis and secretion. Decreased phosphorylation of Akt, a downstream target of PI3K, was induced by IL-4 pretreatment, and pharmacologic inhibitors of these signaling molecules mimicked its effects (Figure 5). Conversely, the MAPK pathway could be implicated in the potentiation of proinflammatory cytokine secretion (TNF-α, IL-6). The role of p38 phosphorylation in cytokine production has been controversial.44 Whereas, Rawadi et al45 showed that the production of proinflammatory cytokines in response to LPS depends on p38 activity, the decrease of MAPK activation in MKK3−/− MΦs did not modify LPS-stimulated TNF-α production. IL-12p70 release was also enhanced by IL-4 followed by N meningitidis, as reported in earlier studies7–10,34; we did not detect changes in IL-10 or nitric oxide secretion under the present conditions (A.V., unpublished data, June 2008).
PI3K is known to be required for phagosome closure32 and using zymosan, more suitable for microscopic analysis than N meningitidis, we observed that IL-4 treatment, which inhibited PI3K activity, reduced phagocytic cup closure, consistent with a role in inhibition of uptake (Figure 6).
The 2-stage murine model used here is not restricted to ThioMΦs, because our unpublished studies showed identical effects with Biogel-elicited MΦs. We validated our observations in vivo by treating mice intraperitoneally with IL-4 followed by N meningitidis challenge, in the absence of thioglycollate stimulation (Figure 4). Although the regime adopted (IL-4 pretreatment, 2-hour phagocytosis assay, 2-hour cytokine release) could alter the cell population within the peritoneal cavity (data not shown), FACS and cytokine analysis of ex vivo F4/80+ MΦs showed similar effects on phagocytosis and cytokine secretion as in vitro studies with costimulated ThioMΦs.
It will be important to extend our phagocytic studies to human MΦs; earlier related studies on HIV-1–infected subjects46 indicate that similar effects on cytokine production could be induced by IL-4 and LPS challenge. Further studies are also required to establish the relevance of the present experimental model to coinfections in vivo, in particular its effect on microbial killing by MΦs. In vitro studies by Stenger et al34 and Sadick et al47 indicated that IL-4 pretreatment of Leishmania-infected mouse MΦs in vitro reduced parasite survival. IL-4 priming followed by microbial or TLR challenge, eg, could have broad significance in immunity, with deleterious as well as beneficial effects. Babu et al48 demonstrated that filariasis, associated with a strong Th2 response, significantly decreased the Mycobacterium tuberculosis–immune response in patients with latent tuberculosis.48 Moreover, alveolar MΦs in Th2-biased asthmatic subjects display reduced phagocytic activity.49 We emphasize that the microbial challenge, although not restricted to N meningitidis, a potent MyD88-dependent stimulus, implicates TLR pathways in full activation of selected MΦ effector functions after IL-4 priming, thus representing a response likely to be significant in a mixed Th1/Th2 cytokine and TLR ligand–rich microenvironment.
Our data show that highly differentiated MΦs can radically alter their phenotype and intracellular signaling in response to a further challenge. These findings are consistent with recent evidence that, despite long-term exposure to Th2 cytokines in vivo, MΦs could respond to Th1 stimuli and display a classically activated phenotype.35 Moreover, Hagemann et al50 demonstrated that tumor-promoting alternatively activated MΦs can be reprogrammed to promote tumor killing. Our data confirm that highly polarized MΦs maintain remarkable plasticity in their microenvironment.
Taken together, these studies indicate that IL-4 and IL-13 can prime MΦs for subsequent challenge by an alternative activation process parallel to, but distinct from, classic priming by IFN-γ and LPS.
Supplementary Material
Acknowledgments
We thank Mike Shaw and Nick White at the Bio-imaging Facility of the Dunn School of Pathology for help and electron microscopy and the animal facility staff for care of the animals. We thank Katherine Makepeace and Dr Richard Moxon for kindly providing Neisseria meningitidis. We thank Philip Ahern and Dr Kevin Maloy for providing the mouse FlowCytomix system kit to assess IL-12p70 concentration. We thank Dr Martin Stacey for critical review of the manuscript and helpful discussions.
This work was supported by grants from the Medical Research Council UK, by the Fondation pour la Recherche Médicale (A.V.), by the E. . Abraham Trust (S.M.), and by grants from the University of Franche-Comté (G.H.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.V., S.M., and S.G. conceived and designed the experiments; A.V. and S.M. performed the experiments; A.V., S.M., G.H., and S.G. analyzed the data; and A.V. and S.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for S.G. is National Cancer Institute, PO Box B, Frederick, MD 21702.
Correspondence: Siamon Gordon, Sir William Dunn School of Pathology, South Park Roads, OX1 3RE Oxford, United Kingdom; e-mail: siamon.gordon@path.ox.ac.uk.
References
- 1.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 3.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 4.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 6.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J Exp Med. 1995;181(2):537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambayashi T, Jacob CO, Strassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell Immunol. 1996;171(1):153–158. doi: 10.1006/cimm.1996.0186. [DOI] [PubMed] [Google Scholar]
- 9.Roy S, Charboneau R, Melnyk D, Barke RA. Interleukin-4 regulates macrophage interleukin-12 protein synthesis through a c-fos mediated mechanism. Surgery. 2000;128(2):219–224. doi: 10.1067/msy.2000.108063. [DOI] [PubMed] [Google Scholar]
- 10.Major J, Fletcher JE, Hamilton TA. IL-4 pretreatment selectively enhances cytokine and chemokine production in lipopolysaccharide-stimulated mouse peritoneal macrophages. J Immunol. 2002;168(5):2456–2463. doi: 10.4049/jimmunol.168.5.2456. [DOI] [PubMed] [Google Scholar]
- 11.Montaner LJ, da Silva RP, Sun J, et al. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-gamma or IL-10. J Immunol. 1999;162(8):4606–4613. [PubMed] [Google Scholar]
- 12.Cipriano IM, Mariano M, Freymuller E, Carneiro CR. Murine macrophages cultured with IL-4 acquire a phenotype similar to that of epithelioid cells from granulomatous inflammation. Inflammation. 2003;27(4):201–211. doi: 10.1023/a:1025084413767. [DOI] [PubMed] [Google Scholar]
- 13.Wainszelbaum MJ, Proctor BM, Pontow SE, Stahl PD, Barbieri MA. IL4/PGE2 induction of an enlarged early endosomal compartment in mouse macrophages is Rab5-dependent. Exp Cell Res. 2006;312(12):2238–2251. doi: 10.1016/j.yexcr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Raveh D, Kruskal BA, Farland J, Ezekowitz RA. Th1 and Th2 cytokines cooperate to stimulate mannose-receptor-mediated phagocytosis. J Leukoc Biol. 1998;64(1):108–113. [PubMed] [Google Scholar]
- 15.Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scand J Immunol. 2005;61(1):10–17. doi: 10.1111/j.0300-9475.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- 16.Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol. 2007;82(6):1542–1553. doi: 10.1189/jlb.0107058. [DOI] [PubMed] [Google Scholar]
- 17.van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13(1):144–166. doi: 10.1128/cmr.13.1.144-166.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386(6622):292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 19.Arredouani M, Yang Z, Ning Y, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200(2):267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Pikkarainen T, Elomaa O, et al. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J Immunol. 2005;175(12):8173–8180. doi: 10.4049/jimmunol.175.12.8173. [DOI] [PubMed] [Google Scholar]
- 21.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162(12):7302–7308. [PubMed] [Google Scholar]
- 22.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 23.Virji M, Kayhty H, Ferguson DJ, Alexandrescu C, Heckels JE, Moxon ER. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5(8):1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Chen Y, Sankala M, et al. MARCO, an innate activation marker of macrophages, is a class A scavenger receptor for Neisseria meningitidis. Eur J Immunol. 2006;36(4):940–949. doi: 10.1002/eji.200535389. [DOI] [PubMed] [Google Scholar]
- 25.Peiser L, De Winther MP, Makepeace K, et al. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect Immun. 2002;70(10):5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364(6435):343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 27.van der Laan LJ, Kangas M, Dopp EA, et al. Macrophage scavenger receptor MARCO: in vitro and in vivo regulation and involvement in the anti-bacterial host defense. Immunol Lett. 1997;57(1–3):203–208. doi: 10.1016/s0165-2478(97)00077-1. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Pomares L, Reid DM, Brown GD, et al. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J Leukoc Biol. 2003;73(5):604–613. doi: 10.1189/jlb.0902450. [DOI] [PubMed] [Google Scholar]
- 29.Brown GD, Taylor PR, Reid DM, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196(3):407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor PR, Tsoni SV, Willment JA, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8(1):31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willment JA, Lin HH, Reid DM, et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171(9):4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 32.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135(5):1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaliñski P, Smits HH, Schuitemaker JH, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165(4):1877–1881. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 34.Stenger S, Solbach W, Rollinghoff M, Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis, II: endogenous tumor necrosis factor-alpha production by macrophages is induced by the synergistic action of interferon (IFN)-gamma and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-gamma and IL 4. Eur J Immunol. 1991;21(7):1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- 35.Mylonas KJ, Nair MG, Prieto-Lafuente l, Paape D, Allen JE. Alternative activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J Immunol. 2009;182:3084–3094. doi: 10.4049/jimmunol.0803463. [DOI] [PubMed] [Google Scholar]
- 36.Fort M, Lesley R, Davidson N, et al. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001;166(4):2793–2800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- 37.Ramanathan S, de Kozak Y, Saoudi A, et al. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol. 1996;157(5):2209–2215. [PubMed] [Google Scholar]
- 38.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113(9):2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loke P, Gallagher I, Nair MG, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 41.Wirth JJ, Kierszenbaum F, Zlotnik A. Effects of IL-4 on macrophage functions: increased uptake and killing of a protozoan parasite (Trypanosoma cruzi). Immunology. 1989;66(2):296–301. [PMC free article] [PubMed] [Google Scholar]
- 42.Leidi M, Gotti E, Bologna L, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182(7):4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 43.de Villiers WJ, Fraser IP, Gordon S. Cytokine and growth factor regulation of macrophage scavenger receptor expression and function. Immunol Lett. 1994;43(1–2):73–79. doi: 10.1016/0165-2478(94)00148-0. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2(9):717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 45.Rawadi G, Ramez V, Lemercier B, Roman-Roman S. Activation of mitogen-activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J Immunol. 1998;160(3):1330–1339. [PubMed] [Google Scholar]
- 46.Marshall JD, Robertson SE, Trinchieri G, Chehimi J. Priming with IL-4 and IL-13 during HIV-1 infection restores in vitro IL-12 production by mononuclear cells of HIV-infected patients. J Immunol. 1997;159(11):5705–5714. [PubMed] [Google Scholar]
- 47.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babu S, Bhat SQ, Kumar NP, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis. 2009;200(2):288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121(6):1372–1378. 1378, e1371–1373. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagemann T, Lawrence T, McNeish I, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.