Plant cortical microtubules self-organize into parallel yet dispersed arrays transverse to the elongation axis of elongating plant cells. Computational modeling yields several novel predictions and shows that plus-end entrainment can give rise to order.
Abstract
Microtubules confined to the two-dimensional cortex of elongating plant cells must form a parallel yet dispersed array transverse to the elongation axis for proper cell wall expansion. Some of these microtubules exhibit free minus-ends, leading to migration at the cortex by hybrid treadmilling. Collisions between microtubules can result in plus-end entrainment (“zippering”) or rapid depolymerization. Here, we present a computational model of cortical microtubule organization. We find that plus-end entrainment leads to self-organization of microtubules into parallel arrays, whereas catastrophe-inducing collisions do not. Catastrophe-inducing boundaries (e.g., upper and lower cross-walls) can tune the orientation of an ordered array to a direction transverse to elongation. We also find that changes in dynamic instability parameters, such as in mor1-1 mutants, can impede self-organization, in agreement with experimental data. Increased entrainment, as seen in clasp-1 mutants, conserves self-organization, but delays its onset and fails to demonstrate increased ordering. We find that branched nucleation at acute angles off existing microtubules results in distinctive sparse arrays and infer either that microtubule-independent or coparallel nucleation must dominate. Our simulations lead to several testable predictions, including the effects of reduced microtubule severing in katanin mutants.
INTRODUCTION
Microtubules (MTs) are ubiquitous biopolymers that endow animal cells with structural rigidity, intracellular transport, and the ability to proliferate. Without the constraints of an MT-organizing center, the MTs at the cortex of plant cells are able to self-organize into robust yet dynamic arrays. After cell division and disassembly of the phragmoplast array, MTs form de novo on the inside of the plasma membrane, where a strong association with the cell cortex forces their dynamics to play out on an effectively two-dimensional geometry (Wasteneys, 2002). Through polymer dynamics, interactions with the membrane and microtubule-associated proteins (MAPs), and MT–MT interaction, the thousands of MTs at the cortex (Dixit and Cyr, 2004) form a strikingly parallel array transverse to, but dispersed along, the cell's axis of elongation. This highly ordered transverse array can reform within 120 min of the initiation of MT assembly after drug-induced depolymerization (Wasteneys and Williamson, 1989a), and it is required to generate anisotropic mechanical properties of the cell wall, although the relationship between the cortical MT array and the oriented deposition of cellulose microfibrils is not yet clear (Himmelspach et al., 2003; Wasteneys, 2004; Wasteneys and Fujita, 2006). For recent reviews, see Wasteneys and Ambrose (2009), Ehrhardt and Shaw (2006), Hashimoto and Kato (2006), and Wasteneys and Fujita (2006).
The study of MT organization in the absence of an MT-organizing center is important for understanding plant cell growth, but it also has implications for understanding acentrosomal MT organization in general. Systems for which organization relies on MT dynamics in concert with activity of MT cross-linking and bundling proteins have occurred in numerous contexts. These systems include mitotic spindle organization (Burbank et al., 2007; Groen et al., 2009), aster formation (Surrey et al., 2001; Pinot et al., 2009), and organelle positioning (Malikov et al., 2005).
MTs are stiff, polar polymers composed of tubulin. One end of the polymer, known as the plus-end, randomly switches between states of rapid growth and rapid shrinkage (Mitchison and Kirschner, 1984) as well as intermittent pauses (Shaw et al., 2003) in a phenomenon known as dynamic instability. Transitions from growth to shrinkage and shrinkage back to growth are known as catastrophe and rescue, respectively. The minus-end might remain static or can slowly depolymerize (Shaw et al., 2003). Although photobleaching studies show that individual tubulin subunits remain mostly fixed relative to the cell cortex (Shaw et al., 2003), this hybrid-treadmilling mechanism (dynamic instability at the plus-end and on-average shrinkage of the minus-end) allows individual MTs to migrate as the plus-end polymerizes (on average) and the minus-end depolymerizes.
The collision of two cortical MTs may result in several possible outcomes. If the angle of collision is steep, the incident MT may switch to the shrinking state, or it may continue to grow unperturbed, crossing over the barrier MT. If the angle of collision is shallow, the incident MT may become entrained with the barrier MT, after which the plus-end grows parallel to the barrier MT, resulting in a sharp bend in the MT at the site of collision. This phenomenon is commonly referred to as “zippering” (Dixit and Cyr, 2004), but recently it has been suggested that this term should be reserved for situations in which two preformed MTs are progressively coaligned (Wasteneys and Ambrose, 2009). Here, we refer to this phenomenon as plus-end entrainment or, simply, entrainment. After an MT is entrained by another, the MTs form a bundle most likely mediated by members of the MAP65 class of MAPs, which cross-link adjacent MTs together with a spacing of 20–30 nm (Chan et al., 1999). Once bundled, MTs remain dynamic (Shaw et al., 2003), although possibly with different polymerization properties (Van Damme et al., 2004). Other collision outcomes are possible: the incident MT may buckle before the barrier (Wightman and Turner, 2007); it may cross over the barrier and continue in a perturbed direction (Hashimoto and Kato, 2006); or, it may become severed at the cross-over point with the new MT formed from the leading end undergoing a gradual reorientation via treadmillling (Wightman and Turner, 2007).
In the absence of an organizing center, nucleation of new MTs occurs throughout the cortex. As in other eukaryotes, nucleation is mediated by the γ-tubulin ring complex (γ-TuRC) (Murata et al., 2005). There is evidence that nucleation can occur in the absence of existing MTs (Wasteneys and Williamson, 1989b; Wasteneys et al., 1993; Chan et al., 2003) and also in an MT-dependent manner where γ-TuRC is distributed along extant MTs. New MTs have been reported to branch off extant MTs at a specific angle of 40° from (Wasteneys and Williamson, 1989b; Murata et al., 2005) or parallel with (Wasteneys and Ambrose, 2009) the extant MTs. One hypothesis is that the minus-end of the new MT remains statically associated with the γ-TuRC for a short time until it is severed by the MT-severing protein katanin (Wasteneys, 2002; Murata et al., 2005).
MOR1 is an MT-associated protein with the ability to alter several dynamic instability parameters, including increasing both shrinking and growing velocities. It is a homologue of XMAP215, which has MT polymerase activity in vitro (Brouhard et al., 2008). Altering these parameters has a dramatic effect on the cortical MT array. The temperature-sensitive mutant mor1-1 has an organized array at permissive temperature 21°C, whereas the dynamic instability parameters are modified significantly at 31°C and the MTs become short and disorganized (Whittington et al., 2001; Kawamura and Wasteneys, 2008).
Strong association of cortical MTs to the cortex is essential for a properly organized array (Dhonukshe et al., 2003; Ambrose and Wasteneys, 2008). Anchoring is believed to involve phospholipase D (Dhonukshe et al., 2003) and the CLASP protein (Ambrose and Wasteneys, 2008). Inhibiting or perturbing either of these perturbs the MT array organization. In particular, in the clasp-1 mutant in which CLASP transcripts are not present, anchor density is decreased and the distance between anchors is increased (Ambrose and Wasteneys, 2008). The free, unanchored length at the plus-end of a growing MT seems to entrain more readily in these mutants, as the free end can explore more space and be entrained with less curvature. Furthermore, the clasp-1 mutant's array is more highly ordered (that is, with fewer deviations from the dominant orientation) than the wild-type array.
It has been widely hypothesized (Dixit and Cyr, 2004; Ehrhardt and Shaw, 2006; Wasteneys and Ambrose, 2009) that the MT–MT interactions outlined above can lead to the formation of an ordered array, where the majority of MTs point in the transverse direction relative to the major growth axis. However, this hypothesis remains to be tested theoretically. What MT–MT interactions are sufficient to lead to an organized array, and how long would this self-organization take to emerge? What are the relative importance of various aspects, such as the density and length of MTs? Computer simulations can address these questions in a way that experiments cannot. In a recent study simulating a similar system, by Baulin et al. (2007), a dominant direction indeed emerged. In their caricature of the plant system, each MT is constantly growing at one end and shrinking at the other end (at a slower rate) unless it encounters a barrier MT, in which case it pauses until the barrier MT has treadmilled out of its way. In a more closely related study by Dixit and Cyr (2004), simulations were carried out including collision-induced catastrophe (CIC) as well as plus-end entrainment (which they refer to as zippering). However, given the computational difficulty of the problem, the authors were only able to consider at most 20 MTs for 10 min, and a statistically meaningful interpretation is difficult to extract from their results.
Here, we present a computational study of cortical MTs in plants. We simulate several thousands of MTs over time scales of minutes to hundreds of minutes, including the effects of CIC, plus-end entrainment and MT-dependent nucleation. We explicitly model the mor1-1 and the clasp-1 mutants of Arabidopsis thaliana and find agreement with experiments for mor1-1, but not clasp-1. Our results illustrate assumptions under which an ordered array will emerge, and assumptions under which it does not.
MATERIALS AND METHODS
Ambrose and Wasteneys (2008) and Shaw et al. (2003) report cortical MTs switching spontaneously between growth (g), pause (p), and shrinkage (s). This three-state dynamic instability model thus involves eight parameters: six transition rates between the states fij, where i,j = g,p,s, and growth and shrinkage velocities vg and vs. As a simplification of this three-state model, a two-state dynamical instability model involving four parameters has been studied previously (Rubin, 1988; Dogterom and Leibler, 1993) and used extensively in cell biology (Grill and Hyman, 2005; Wollman et al., 2005; Gardner et al., 2008). The mean length (Dogterom and Leibler, 1993) and mean lifetime (Rubin, 1988) of an MT in the two-state model depend on a threshold quantity fgs vs − fsg vg. If the quantity is positive, the MTs tend to shrink more than they grow, and the MTs will have a finite mean length and mean lifetime. Otherwise, on average, they tend to grow forever. For the three-state case, we compute the equivalent equations in the Supplemental Material. There is an equivalent threshold quantity that determines if the MTs tend to remain finite or grow indefinitely. Note that these simplified models only consider dynamic instability: they are only valid in the absence of interactions between the MTs and any growth boundaries, and in the abundance of free tubulin. Thus, the mean length and mean lifetime should be thought of as characteristic scales that are perturbed by MT–MT interactions and the action of MAPs. Tables 1 and 2 summarize parameters from the literature (Shaw et al., 2003; Dixit and Cyr, 2004; Kawamura and Wasteneys, 2008) that we use in this article.
Table 1.
Dynamic instability parameters from three-state models using data from Kawamura and Wasteneys (2008) for wild type (WT) and the mor1-1 mutant and Shaw et al. (2003), and two-state models using data from Dixit and Cyr (2004)
| WTa 21°C | WTa 31°C | mor1-1 21°C | mor1-1 31°C | Shaw et al. (2003) | Dixit and Cyr (2004) | |
|---|---|---|---|---|---|---|
| Kawamura and Wasteneys | ||||||
| fgp | 0.20 | 0.380 | 0.200 | 0.960 | 0.47 | |
| fgs | 0.17 | 1.590 | 0.380 | 0.820 | 0.97 | 1.61 |
| fpg | 2.01 | 1.400 | 1.560 | 0.700 | 0.51 | |
| fps | 1.02 | 0.700 | 0.560 | 0.620 | 0.24 | |
| fsg | 1.00 | 1.990 | 1.180 | 0.610 | 0.87 | 3.26 |
| fsp | 0.31 | 0.440 | 0.590 | 1.210 | 0.23 | |
| vpg | 3.50 | 6.500 | 2.500 | 2.000 | 3.690 | 5.6 |
| vps | 9.00 | 12.000 | 6.200 | 3.800 | 5.800 | 10.09 |
| vms | 0.530 | |||||
| Minus-end stationary | ||||||
| l̄ (μm) | −15.12 | 13.55 | −11.47 | 3.27 | 7.89 | 8.58 |
| τ (min) | −6.46 | 3.83 | −7.38 | 4.47 | 5.26 | 5.51 |
| Minus-end vms = 0.53 | ||||||
| l̄ (μm) | −21.46 | 9.49 | −49.67 | 1.74 | 5.20 | 8.58 |
| τ (min) | −10.18 | 2.79 | −37.03 | 2.81 | 3.75 | 5.51 |
The mean length l̄ and mean lifetime τ in the absence of interactions or cell boundaries are computed using Eqs. 3–10 in Supplemental Material. The minus-end shrinking velocity is taken from the average shrinking velocity, including pauses, in Shaw et al. (2003). Because l̄ and τ consider only the effects of dynamic instability, they should be thought of as characteristic scales that are perturbed by MT–MT interactions and the action of MAPs.
a Wild type.
Table 2.
Parameters used in the model in addition to the dynamic instability parameters in Table 1
| Parameter | Meaning | Value | Reference |
|---|---|---|---|
| θZ | Critical entrainment angle | 40°, 60° | Ambrose and Wasteneys (2008),Dixit and Cyr (2004) |
| θnuc | Branched nucleation angle | 40° | Murata et al. (2005) |
| k0 | Background nucleation rate | 10 μm−2 min−1 | Estimated |
| k1 | MT-dependent nucleation rate | 103 min−1 | Estimated |
| pcat | Probability of catastrophe upon steep collision | 0.09–0.6 | Wightman and Turner (2007),Dixit and Cyr (2004) |
| pzip | Probability of entrainment upon shallow collision | ≈1 | Dixit and Cyr (2004) |
| δ | Spacing between bundled MTs | 25 nm | Chan et al. (1999) |
We assume the minus-end is either always static, or continuously shrinking with constant rate vms. Shaw et al. (2003) report MT minus-ends spending 25.3% of the time shrinking at, on average, 2.78 μm/min, and 8.4% of the time growing at 1.96 μm/min, and the remaining time (66.3%) paused. Thus, for instances in which we assume minus-ends shrink, we use an appropriately weighted average of these data: vms = 0.253 (2.78 μm/min) + 0.084 (−1.96 μm/min) + 0.663 (0) = 0.53 μm/min.
When two MTs collide, the outcome depends on the angle between the incident and barrier MTs (Wasteneys and Ambrose, 2009), which we call the collision angle X. We define the critical entrainment angle θZ as follows. If θX > θZ, the collision is steep and catastrophe occurs with probability pcat, otherwise the incident MT crosses over the barrier MT (with probability 1 − pcat). In A. thaliana, 9% of steep-angle collisions result in catastrophe in petiole epidermal cells and 25% in leaf pavement cells (Wightman and Turner, 2007), whereas in tobacco BY2 cells, catastrophe results 60% of the time (Dixit and Cyr, 2004). In these studies, the angles 45° and 40°, respectively, were found to delineate the transition between entrainment and catastrophe.
If θX < θZ, the collision is shallow and plus-end entrainment occurs with probability pzip. After an entrainment event, the extant segment of the incident MT remains in its precollision configuration, but the plus-end continues to grow parallel to the barrier MT. Thus, the MT is now composed of two line segments with a kink. In this article, we assume this phenomenological description of entrainment, neglecting fine-grain biophysical properties of the kink. The segment from the kink to the plus-end of the entrained MT is kept a distance of δ = 25 nm from the barrier MT in agreement with electron microscopy of cross-linking due to MAP65 (Chan et al., 1999).
We consider two modes of MT nucleation. The first is independent of the extant MT array. MTs of zero initial length and uniform random orientation are inserted randomly into the cortex, at rate k0 in micrometers−2 minute−1. The second is MT-dependent nucleation, where new MTs are nucleated off extant MTs. The rate of MT-dependent nucleation will depend upon both the length of existing polymer and the number of available γ-TuRC in the cytoplasm (Murata et al., 2005). However, we assume the γ-TuRC is rate limiting and thus MT-dependent nucleation occurs at a constant rate k1 in minutes−1. Once the new MT is nucleated, its plus-end immediately begins dynamic instability, and if vms >0, the minus-end immediately begins shrinking. In reality, there is likely to be a delay before katanin severs the minus-end (Sedbrook and Kaloriti, 2008), but because an actual lag time is unknown, we assume this is negligible. Also, we find that a completely static minus-end only delays the onset of self-organization, suggesting the katanin delay would not change our results significantly.
There are physical details not explicitly included in the model, such as the mechanical properties of the MTs, the cortex and the anchors connecting them. We assume that anchors are sufficiently strong and densely distributed along MTs so that 1) the radius of curvature of the cortex is not mechanically significant and 2) the kink in an entrained MT remains in place even if the barrier MT is removed through depolymerization.
We simulate on a 10- by 10-μm domain, which is a typical size of a plant cell face in early interphase, with periodic boundary conditions in both directions, except where we explicitly explore the consequences of boundaries. All simulations are run for 1000 min. We use a Gillespie algorithm to simulate switching between MT states (competing Poisson processes with rates fij) and constant growth and shrinkage velocities, resulting in a variable timestep on the order of Δt = 10−3 min. To detect and resolve collisions, we use a fixed timestep of Δt = 0.05 min. All simulations were replicated ten times with different, random initial conditions. We use dynamic instability parameters from Table 1, and, unless otherwise noted, other parameters from Table 2.
There are several ways to measure how well-oriented an array is. A common qualitative approach is to plot a histogram of MT angles defined so that the dominant direction is either 0 or 90° (Himmelspach et al., 2003; Ambrose and Wasteneys, 2008). If the angle distribution is unimodal, its SD serves as a quantitative measure of orientation. As an extension of this, the distribution can be weighted by length, i.e., the angles of longer MTs count for more in the histogram.
More generally, Baulin et al. (2007) define an order parameter 0 < Sl < 1 based on an angular cost function, weighted by li2, where li is the length of each MT. Here, we define a slightly modified version of their order parameter, S = Σili (cos2(θi − Ω) − sin2(θi − Ω))/Σili, where θi is the angle of each straight MT segment and Ω is the dominant angle. Heuristically, S represents the relative difference between the projected polymer length in the dominant direction and the projected polymer length in its perpendicular direction.
RESULTS
Collision-induced Catastrophe Does Not Lead to Ordering for Physiological Kinetic Parameters
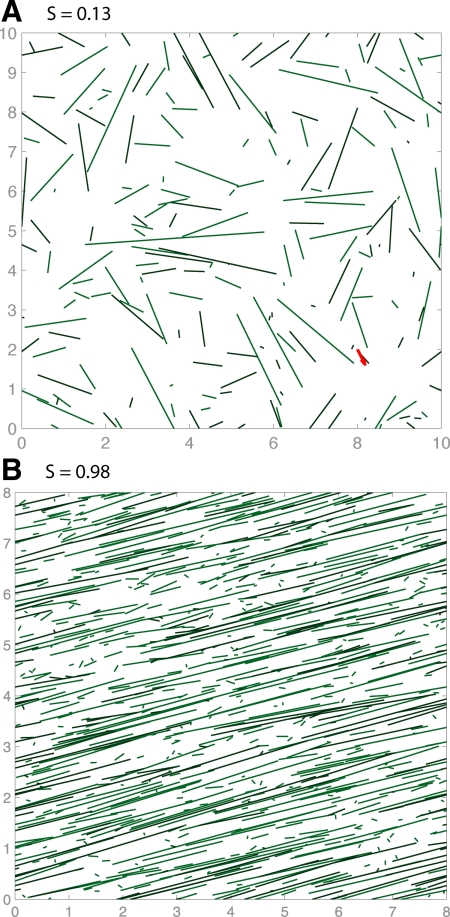
With parameters as given in Tables 1 and 2, CIC did not lead to any ordering. Instead, they lead to sparse arrays shown in Figure 1A. For high values of pcat = 0.9 (shown in the figure), the MTs were too short in length and lifetime for orientation to emerge, whereas for low values of pcat = 0.1, they simply did not interact enough. Intermediate values of pcat also failed to organize. However, Baulin et al. (2007) report that pause-inducing collisions alone are enough to give rise to an oriented array, a result we confirm with our simulations (see Figure 1B and Supplemental Movies S1 and S2). In the Supplemental Material, we show that the pause-inducing collision model is a limiting case of the catastrophe-inducing collision model. After conducting a random sweep of 103 kinetic parameter sets, we conclude that CIC only leads to self-organization in the limit where the shrinkage rate and catastrophe rate are approximately 0 (vps fgs ≈ 0) and the rescue rate is much larger than the catastrophe rate (fsg ≫ fgs), consistent with Baulin et al. (2007).
Figure 1.
Simulation snapshots at t = 60 min with collision-induced catastrophe only, using parameters from wild type at 31°C (Kawamura and Wasteneys 2008) (A) and collision-induced pauses, using the single-state model of Baulin et al. (2007) (B).
Plus-End Entrainment, with or without CIC, Results in an Ordered Array
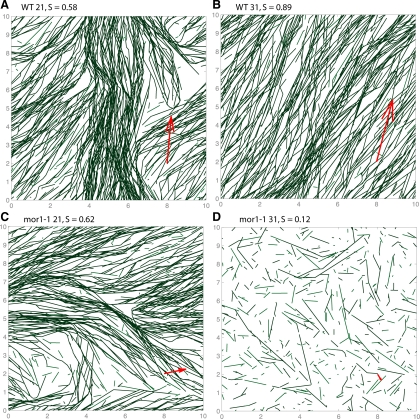
Simulations that include entrainment gave rise to significant order parameters within the first 60 min. In Figure 2, we display snapshots from the simulations for the four kinetic parameter sets taken from Kawamura and Wasteneys (2008) for plus-end dynamics and the average vms from Shaw et al. (2003). In Figure 2, A–C, a single dominant direction is evident with patches of deviation present. The dominant direction (red arrows) is uniformly random (data not shown) across simulations and persists for at least 103 min.
Figure 2.
Collision-induced catastrophe at steep angles (>40°) and entrainment at shallow angles (<40°) for four sets of kinetic parameters from Kawamura and Wasteneys (2008) and continuously depolymerizing minus-end (Shaw et al., 2003). The top (A and B) and bottom (C and D) rows are wild-type and mor1-1 kinetic parameters, respectively, and the left (A and C) and right (B and D) are at 21 and 31°C, respectively. New MTs are inserted randomly at a rate of k0 = 10 μm−2 min−1. The boundaries are periodic in both directions. After 60 min, order emerges in local domains in all cases except mor1-1 at 31°C. The direction of the red arrow indicates the dominant direction of the MT array, whereas their lengths are proportional to the order parameter.
The time course of some of these simulations are shown in Supplemental Movies S3–S7. Initially, several locally ordered domains emerge, grow and shrink (but never rotate, as reported in Chan et al. (2007)). By 103 min, typically a single orientation dominates. However, sometimes the cortex is divided into two domains with distinct dominant orientations. These directions were never observed to differ by more than the critical entrainment angle θZ. It remains possible that one of these domains becomes globally dominant on time scales much larger than 103 min.
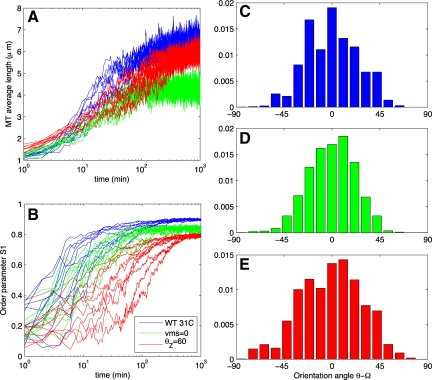
In Figure 3, A and B, we show time series from 10 runs with wild-type 31°C parameters (blue curves). The mean lengths increase slowly. After ∼103 min, in each simulation, the mean MT length converges to a value below l̄, the predicted mean length in the absence of interaction (Table 1 and Eq. 8 in Supplemental Material). For comparison, single cortical MTs have been reported to be 2–4 μm when measured by transmission electron microscopy (Hardham, 1978). The number of MTs converges quickly to roughly 103 (data not shown). Note that the steady-state number of MTs depended on k0, and we chose k0 = 10 μm −2 min−1 to give ∼103 MTs.
Figure 3.
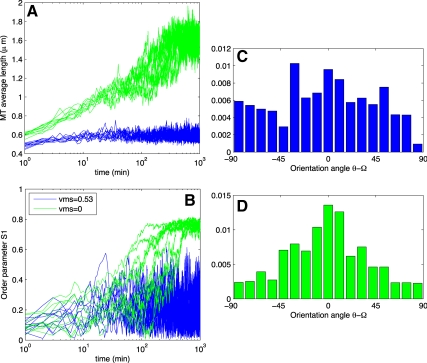
Data from wild-type (WT) simulation runs. (A) Average length of an MT. (B) Order parameter S, given by the equation in Materials and Methods [a modified version of the Baulin et al. (2007) order parameter]. All simulations use kinetics from wild type at 31°C in Table 1. (C–E) Histograms from selected runs depicted in Figure 2. Blue curves in A and B and histogram in C have a depolymerizing minus end and a critical entrainment angle of θZ = 40°. Green curves in A and B and histogram in D have a static minus-end (vps = 0) with θZ = 40°, and red curves in A and B and histogram in E have depolymerizing minus end with θZ = 60°. Time series from 10 independent simulations are shown in each case.
Using S as our measure of order, we conclude that plus-end entrainment does give rise to order. This order emerges with a time scale <102 min (Figure 3B), in agreement with the observed time-to-order in vivo (Wasteneys and Williamson, 1989a). The full orientational distribution is shown in Figure 3C. Notably, without CIC in the simulations (i.e., pcat = 0) ordering still emerged, see Figure 5A. Ordering also occurred using parameters from Shaw et al. (2003) and for the two-state model using parameters from Dixit and Cyr (2004) (data not shown).
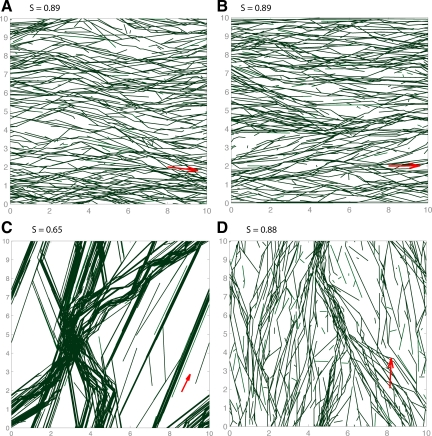
Figure 5.
Simulation snapshots at t = 60 min using kinetic parameters from wild type at 31°C. (A) Entrainment at shallow angles (<40°) and no collision-induced catastrophe (pcat = 0). (B) A biased, transverse dominant angle that arises if two edges (here, the top and bottom) induce catastrophe. This provides a possible mechanism for selecting a direction transverse to the cell elongation axis. (C) Sparse array that results if all nucleation is branched MT-dependent nucleation. (D) Moderately sparse array arising from a combination of MT-dependent and MT-independent nucleation.
With Kinetic Parameters Taken from the mor1-1 Mutant at 31°C, Ordered Arrays Do Not Form
In simulations of the mor1-1 mutant at 31°C, the MTs were short and therefore much lower in total polymer density. From Figure 4A, we see their average length is 0.5 μm, roughly one sixth of their mean free length in the absence of interactions. Reducing the nucleation rate increased their mean length slightly but still did not allow for ordering.
Figure 4.
Data from simulations of the mor1-1 mutant. (A) Average length of an MT. (B) Order parameter S, given by the equation in Materials and Methods [a modified version of the Baulin et al. (2007) order parameter]. All simulations use kinetics from mor1-1 at 31°C in Table 1. Blue curves in A and B and histogram in C have a depolymerizing minus end, whereas green curves and histogram in D have a static minus-end (vps = 0).
Static Minus-Ends Delay, but Do Not Prevent, Array Organization
Although three-state dynamic instability of MT plus-ends has been reported extensively (Shaw et al., 2003; Dixit and Cyr, 2004; Kawamura and Wasteneys, 2008), the hybrid treadmilling has been reported less often (Shaw et al., 2003). To explore the consequences of a freely depolymerizing minus-end, we ran simulations with static minus-ends (vms = 0).
A typical array arising from vms = 0 is qualitatively similar to the arrays in Figure 2B, the equivalent runs with vms = 0.53 μm/min. The order parameter S after 102 min is, on average, also comparable (0.9 and 0.8 for vms = 0.53 μm/min and vms = 0, respectively). However, static minus-ends seemed to delay the onset of self-organization. In Figure 3B, we show the order parameter's time evolution for both vms = 0.53 μm/min (blue curves) and vms = 0 (green curves). Although the simulation with a shrinking minus-end has reached its well-ordered steady-state (within 10% of its steady-state order parameter S) within 20 min, it takes the simulation with static minus-ends roughly 80 min, which is 4 times longer.
Static Minus-Ends Allow “mor1-1” to Self-Organize
The dynamics of the minus-ends affect the average length of MTs (Table 1) in that if minus-ends are static, the MTs grow slightly longer. In simulations with kinetic parameters from the mor1-1 mutant at 31°C, we find that static minus-ends induce a change sufficient to allow for ordering. Time series data for this kinetic parameter set are shown in Figure 4, A and B. The order parameter in Figure 4B shows that the mutant with a depolymerizing minus-end does not organize (blue curve). With static minus ends, organization is rescued (green curve). In this case, however, self-organization still takes longer than in simulations of wild-type plants with nonstatic minus ends.
Increasing the Critical Entrainment Angle Does Not Enhance, but Rather Delays, Array Organization
MTs in the clasp-1 mutant described by Ambrose and Wasteneys (2008) entrain over a wider range of incident angles, with a mean entrainment angle roughly 11° larger than in wild type and demonstrate “hyperparallel” arrays, indicated by a smaller SD of MT orientations. To test whether a higher critical entrainment angle can explain the hyperparallel arrays, we ran simulations in which we increased the critical entrainment angle from θZ = 40° to θZ = 60°, which is equivalent to increasing the mean entrainment angle by 10°.
Qualitatively, the resulting arrays seem indistinguishable from the corresponding array in Figure 1 (see Supplemental Movie S8). However, examining the time course of the order parameter S reveals that the increased θZ delays and slightly reduces array ordering, similar to the case of static minus-ends. The time series of ordering is shown in red in Figure 3B, with a typical angle distribution in Figure 3E. This suggests that the clasp-1 hyperparallel MT phenotype is dependent on another mechanism.
MT-dependent Branched Nucleation Leads to Unrealistic Array Structures
Up to this point, all MT nucleation has been assumed to occur uniformly in space and at random angles, referred to here as background nucleation. To explore the reports of MT-dependent branched nucleation, we ran simulations without background nucleation (k0 = 0) and nonzero MT-dependent nucleation rate k1 = 103 min−1.
These simulations always resulted in arrays with a distinct structure reminiscent of shattered glass. As with background nucleation, one or a few dominant angles emerged locally at early times, the domains grew or shrank, and often one direction dominated globally. However, the arrays were always sparse with large gaps free of persisting MTs. A typical snapshot is shown in Figure 5C. Time courses of typical simulations are shown in Supplemental Movies S9 and S10.
Although MT-dependent branch nucleation alone leads to an unrealistic array organization, this does not necessarily mean it is not important. We ran simulations with a combination of MT-dependent and MT-independent nucleation. As MT-independent nucleation is reduced, the arrays seemed more and more sparse. A simulation with a combination of nucleation types, k1 = 500 min−1 and k0 = 5 min−1 μm−2, is shown in Figure 5D.
Catastrophe-inducing Boundaries Are Enough to Bias the Dominant Orientation
Up to now, all simulations described were carried out on a square cortex with periodicity in both directions. That is, an MT that disappears off any edge appears from the opposite edge. When a dominant angle emerges in our simulations, it is uniformly random. In diffusely elongating plant cells, the dominant angle is transverse to the direction of elongation, indicating that there must be a symmetry-breaking mechanism that signals a preferred orientation to the MTs. One candidate for this mechanism is interaction with the apical and basal poles of the plant cell (Ehrhardt and Shaw, 2006). Real cells have distinct faces. Side walls may allow MTs oriented transverse to the elongation axis to continue growing indefinitely, unperturbed by boundaries between faces. In the longitudinal direction, MTs can treadmill onto the cross walls but rarely do (Collings and Wasteneys, 2005). It has been suggested that the boundaries of the poles inhibit MT growth, either sterically or through MAP activity (Ehrhardt and Shaw, 2006). We represent this interaction by imposing catastrophe on any MT that collides with the top or bottom of the cylinder.
We find that this catastrophe-inducing boundary effect is enough to cause selection of the transverse angle as the dominant orientation. Snapshots from these simulations are shown in Figure 5B. At t = 60 min, not all nontransversely oriented patches disappear, yet the dominant transverse angle always persists.
In fact, even in the complete absence of MT–MT interactions, catastrophe-inducing boundaries at the cross-walls will lead to a certain amount of ordering. MTs transverse to the elongation axis can treadmill indefinitely, whereas those parallel to the elongation axis will quickly encounter a boundary. The ordering of MTs is therefore strongest near the cross walls and decays toward the mid-cell over a distance of roughly the mean length in the absence of interaction (see Supplemental Material for details). MT–MT interaction allows this boundary-induced orientation to propagate further into the mid-cell cortex. We also found that the introduction of catastrophe-inducing boundaries does not induce ordering in the CIC-only model described above.
DISCUSSION
Recent genetic and pharmacological experiments on cortical MTs in plants have given rise to a model for the self-organization of these MTs into a parallel array. Here, we have presented the results of large-scale simulations of a quantitative implementation of this model. We find that self-organization into a parallel array can arise from a combination of MT dynamic instability, plus-end entrainment, and, in certain cases, CIC. The arrays that arise in our simulations seem qualitatively similar to the arrays in plant cells, including the local domains of orientation that are similar to the patchwork patterns in maturing Chara cells (Wasteneys et al., 1993) and the outer epidermis of Arabidopsis hypocotyl cells (Chan et al., 2007). In addition to recapitulating wild-type behavior, our model also agrees with mutant studies (Kawamura and Wasteneys, 2008).
It has been proposed that CIC can explain ordering. Our results show that CIC is neither necessary nor sufficient for ordering when physiologically reasonable dynamic instability parameters are used. Previous modeling efforts have focused on the role of CIC in the emergence of order. Dixit and Cyr (2004) showed that CIC in combination with plus-end entrainment leads to ordering. In light of our results, we suggest ordering in their model arises due to entrainment rather than CIC. The model of Baulin et al. (2007) corresponds to a limit in which the growth rate dominates the shrinkage rate, and the rescue frequency dominates the catastrophe frequency. This model fails to self-organize when extended to a regime that matches reported kinetic parameters (Shaw et al., 2003; Dixit and Cyr, 2004; Kawamura and Wasteneys, 2008).
Plus-end entrainment with branched MT-dependent nucleation gives rise to an oriented array that seems sparse because areas of low MT content have no candidate nucleation sites, whereas areas of high MT content have many. From this, we conclude that exclusive MT-dependent branched nucleation leads to unrealistic array structures. Free nucleation seems to be necessary to explain the dispersed arrays seen in vivo. This seems to contradict the hypothesis that branched nucleation helps to disperse the array throughout the cortex (Wasteneys and Ambrose, 2009), but is consistent with the observation that during recovery from drug-induced disassembly, the initial transverse order of freely nucleated MTs is progressively lost when most subsequent MTs are produced by branch-form nucleation (Wasteneys and Williamson, 1989a). Although MT-dependent branch-form nucleation alone leads to unrealistic arrays, this does not suggest that it does not occur. As proposed by Wasteneys and Ambrose (2009), it may be specifically promoted under conditions where it is beneficial to change the predominant orientation of MTs. Recent improvements in live cell imaging has enabled the detection of microtubule-dependent nucleation that is parallel to the parent MT (see figure 1C in Ambrose and Wasteneys 2008) and this alternative form of MT-dependent nucleation might prove to be much more common than previously thought (Wasteneys and Ambrose 2009).
We find that increasing the critical entrainment angle does not enhance the array order. In fact, order is reduced slightly and delayed. This conflicts with recent experiments in which Ambrose and Wasteneys (2008) observed “hyperparallel” arrays in the clasp-1 mutant. This suggests that the CLASP protein affects array organization through more than simply modulating the critical entrainment angle.
Two of the phenomena we neglect in our simulations are increased MT stability through bundling, and severing after crossover. MTs within bundles remain dynamic, however, with slightly modified kinetic parameters (Van Damme et al., 2004). It is unknown whether this effect arises simply through the reduced collision frequency or whether it is important for MT array organization. MT severing at sites of existing crossovers, possibly mediated by katanin, also has been reported (Wightman and Turner, 2007); however, this occurs rarely as severing of elongated MTs is rare (Shaw et al., 2003).
Three novel predictions arise from this work. First, if the transverse dominant direction is selected by catastrophe-inducing boundaries at the top and bottom edges of the cell, then the time to order will increase as the cell length increases, as it takes longer for the signal to propagate inward.
The other two predictions demonstrate the paradoxical influence of static minus ends. First, if minus-ends do not become mobile in wild type (e.g., in a katanin knockout), we predict ordering to take fourfold longer. Second, we predict that a similar perturbation of the mor1-1 31°C mutant rescues ordering.
This last set of predictions illustrates one of the values of computational modeling. The subtle influence of static as opposed to mobile minus-ends, which in one case promotes and in the other inhibits organization, is essentially impossible to tease out without recourse to computational techniques.
Supplementary Material
ACKNOWLEDGMENTS
Computation was carried out on the WestGrid network. We thank Christian Ambrose (University of British Columbia) for useful discussion. This work was supported financially by Natural Sciences and Engineering Research Council of Canada and Canadian Institutes of Health Research.
Abbreviations used:
- CIC
collision-induced catastrophe
- MAP
microtubule-associated protein
- MT
microtubule
- γ-TuRC
γ-tubulin ring complex.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0579) on November 12, 2009.
REFERENCES
- Ambrose J. C., Wasteneys G. O. CLASP modulates microtubule-cortex interaction during self-organization of acentrosomal microtubules. Mol. Biol. Cell. 2008;19:4730–4737. doi: 10.1091/mbc.E08-06-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulin V. A., Marques C. M., Thalmann F. Collision induced spatial organization of microtubules. Biophys. Chem. 2007;128:231–244. doi: 10.1016/j.bpc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Brouhard G. J., Stear J. H., Noetzel T. L., Al-Bassam J., Kinoshita K., Harrison S. C., Howard J., Hyman A. A. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank K. S., Mitchison T. J., Fisher D. S. Slide-and-cluster models for spindle assembly. Curr. Biol. 2007;17:1373–1383. doi: 10.1016/j.cub.2007.07.058. [DOI] [PubMed] [Google Scholar]
- Chan J., Calder G., Fox S., Lloyd C. Cortical microtubule arrays undergo rotary movements in Arabidopsis hypocotyl epidermal cells. Nat. Cell Biol. 2007;9:171–175. doi: 10.1038/ncb1533. [DOI] [PubMed] [Google Scholar]
- Chan J., Calder G. M., Doonan J. H., Lloyd C. W. EB1 reveals mobile microtubule nucleation sites in Arabidopsis. Nat. Cell Biol. 2003;5:967–971. doi: 10.1038/ncb1057. [DOI] [PubMed] [Google Scholar]
- Chan J., Jensen C. G., Jensen L.C.W., Bush M., Lloyd C. W. The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc. Natl. Acad. Sci. USA. 1999;96:14931–14936. doi: 10.1073/pnas.96.26.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings D., Wasteneys G. Actin microfilament and microtubule distribution patterns in the expanding root of Arabidopsis thaliana. Can. J. Bot. 2005;83:579–590. [Google Scholar]
- Dhonukshe P., Laxalt A. M., Goedhart J., Gadella T.W.J., Munnik T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell. 2003;15:2666. doi: 10.1105/tpc.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Cyr R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell. 2004;16:3274–3284. doi: 10.1105/tpc.104.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogterom M., Leibler S. Physical aspects of the growth and regulation of microtubule structures. Phys. Rev. Lett. 1993;70:1347–1350. doi: 10.1103/PhysRevLett.70.1347. [DOI] [PubMed] [Google Scholar]
- Ehrhardt D. W., Shaw S. L. Microtubule dynamics and organization in the plant cortical array. Annu. Rev. Plant Biol. 2006;57:859–875. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- Gardner M. K., Hunt A. J., Goodson H. V., Odde D. J. Microtubule assembly dynamics: new insights at the nanoscale. Curr. Opin. Cell Biol. 2008;20:64–70. doi: 10.1016/j.ceb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill S. W., Hyman A. A. Spindle positioning by cortical pulling forces. Dev. Cell. 2005;8:461–465. doi: 10.1016/j.devcel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Groen A. C., Maresca T. J., Gatlin J. C., Salmon E. D., Mitchison T. J. Functional overlap of microtubule assembly factors in chromatin-promoted spindle assembly. Mol. Biol. Cell. 2009;20:2766. doi: 10.1091/mbc.E09-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham A. Structure of cortical microtubule arrays in plant cells. J. Cell Biol. 1978;77:14–34. doi: 10.1083/jcb.77.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Kato T. Cortical control of plant microtubules. Curr. Opin. Plant Biol. 2006;9:5–11. doi: 10.1016/j.pbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Himmelspach R., Williamson R. E., Wasteneys G. O. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J. 2003;36:565. doi: 10.1046/j.1365-313x.2003.01906.x. [DOI] [PubMed] [Google Scholar]
- Kawamura E., Wasteneys G. O. MOR1, the Arabidopsis thaliana homologue of Xenopus MAP215, promotes rapid growth and shrinkage, and suppresses the pausing of microtubules in vivo. J. Cell Sci. 2008;121:4114. doi: 10.1242/jcs.039065. [DOI] [PubMed] [Google Scholar]
- Malikov V., Cytrynbaum E. N., Kashina A., Mogilner A., Rodionov V. Centering of a radial microtubule array by translocation along microtubules spontaneously nucleated in the cytoplasm. Nat. Cell Biol. 2005;7:1213–1218. doi: 10.1038/ncb1332. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability in microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murata T., Sonobe S., Baskin T. I., Hyodo S., Hasezawa S., Nagata T., Horio T., Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of big gamma-tubulin in higher plants. Nat. Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- Pinot M., Chesnel F., Kubiak J. Z., Arnal I., Nedelec F. J., Gueroui Z. Effects of confinement on the self-organization of microtubules and motors. Curr. Biol. 2009;19:954–960. doi: 10.1016/j.cub.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Rubin R. J. Mean lifetime of microtubules attached to nucleating sites. Proc. Natl. Acad. Sci. USA. 1988;85:446–448. doi: 10.1073/pnas.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook J. C., Kaloriti D. Microtubules, MAPs and plant directional cell expansion. Trends Plant Sci. 2008;13:303–310. doi: 10.1016/j.tplants.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Shaw S. L., Kamyar R., Ehrhardt D. W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science. 2003;300:1715–1718. doi: 10.1126/science.1083529. [DOI] [PubMed] [Google Scholar]
- Surrey T., Nedelec F., Leibler S., Karsenti E. Physical properties determining self-organization of motors and microtubules. Science. 2001;292:1167–1171. doi: 10.1126/science.1059758. [DOI] [PubMed] [Google Scholar]
- Van Damme D., Van Poucke K., Boutant E., Ritzenthaler C., Inze D., Geelen D. In vivo dynamics and differential microtubule-binding activities of MAP65 proteins 1. Plant Physiol. 2004;136:3956–3967. doi: 10.1104/pp.104.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteneys G., Gunning B., Hepler P. Microinjection of fluorescent brain tubulin reveals dynamic properties of cortical microtubules in living plant-cells. Cell Motil. Cytoskeleton. 1993;24:205–213. [Google Scholar]
- Wasteneys G. O. Microtubule organization in the green kingdom: chaos or self-order? J. Cell Sci. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]
- Wasteneys G. O. Progress in understanding the role of microtubules in plant cells. Curr. Opin. Plant Biol. 2004;7:651–660. doi: 10.1016/j.pbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wasteneys G. O., Ambrose J. C. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Wasteneys G. O., Fujita M. Establishing and maintaining axial growth: wall mechanical properties and the cytoskeleton. J. Plant Res. 2006;119:5–10. doi: 10.1007/s10265-005-0233-3. [DOI] [PubMed] [Google Scholar]
- Wasteneys G. O., Williamson R. E. Reassembly of microtubules in Nitella tasmanica: quantitative analysis of assembly and orientation. Eur. J. Cell Biol. 1989a;50:76–83. [Google Scholar]
- Wasteneys G. O., Williamson R. E. Reassembly of microtubules in Nitella tasmanica: assembly of cortical microtubules in branching clusters and its relevance to steady-state microtubule assembly. J. Cell Sci. 1989b;93:705–714. [Google Scholar]
- Whittington A., Vugrek O., Wei K., Hasenbein N., Sugimoto K., Rashbrooke M., Wasteneys G. MOR1 is essential for organizing cortical microtubules in plants. Nature. 2001;411:610–613. doi: 10.1038/35079128. [DOI] [PubMed] [Google Scholar]
- Wightman R., Turner S. R. Severing at sites of microtubule crossover contributes to microtubule alignment in cortical arrays. Plant J. 2007;52:742. doi: 10.1111/j.1365-313X.2007.03271.x. [DOI] [PubMed] [Google Scholar]
- Wollman R., Cytrynbaum E., Jones J., Meyer T., Scholey J., Mogilner A. Efficient chromosome capture requires a bias in the ‘search-and-capture‘ process during mitotic-spindle assembly. Curr. Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.