Abstract
A conformational transition of normal cellular prion protein (PrPC) to its pathogenic form (PrPSc) is believed to be a central event in the transmission of the devastating neurological diseases known as spongiform encephalopathies. The common methionine/valine polymorphism at residue 129 in the PrP influences disease susceptibility and phenotype. We report here seven crystal structures of human PrP variants: three of wild-type (WT) PrP containing V129, and four of the familial variants D178N and F198S, containing either M129 or V129. Comparison of these structures with each other and with previously published WT PrP structures containing M129 revealed that only WT PrPs were found to crystallize as domain-swapped dimers or closed monomers; the four mutant PrPs crystallized as non-swapped dimers. Three of the four mutant PrPs aligned to form intermolecular β-sheets. Several regions of structural variability were identified, and analysis of their conformations provides an explanation for the structural features, which can influence the formation and conformation of intermolecular β-sheets involving the M/V129 polymorphic residue.
Keywords: crystal structure, prion proteins, spongiform encephalopathies
Introduction
Prion diseases, or transmissible spongiform encephalopathies (TSE), are an unusual group of invariably fatal mammalian neurodegenerative diseases (reviewed by Prusiner, 1998; Collinge, 2001; Aguzzi et al, 2008). These rare disorders can be acquired by infection, inheritance via mutations in the gene encoding for the prion protein (PrP), or occur spontaneously. Typical features of the TSEs are long incubation periods and characteristic brain pathology including spongiform degeneration, astrogliosis, and accumulation of misfolded protein deposits. These diseases are intimately associated with conformational conversion of the normal cellular PrP (PrPC) to a pathogenic form (PrPSc). According to the ‘protein-only' model, PrPSc itself represents the infectious prion agent: it is believed to self-propagate by the mechanism involving binding to PrPC and templating the conversion of the latter protein to the PrPSc state.
The human prion diseases include Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker disease (GSS), fatal familial insomnia (FFI), and kuru. The common methionine/valine polymorphism at residue 129 influences disease susceptibility and phenotype. It is striking that the new variant CJD, believed to be transmitted by dietary exposure to bovine spongiform encephalopathy (BSE)-contaminated beef, has to date afflicted only individuals who are homozygous for M129 (Brandel et al, 2009). Also, sporadic CJD cases display a variety of clinicopathological symptoms, which depend on the M/V129 genotype, and a majority of patients are homozygous at position 129 (Alpérovitch et al, 1999). Finally, M/V129 heterozygosity appears to be protective against kuru in the Fore tribe (Cervenáková et al, 1998). Thus, the M/V129 polymorphism has an influence in sporadic and transmitted prion diseases. The inherited diseases include GSS, FFI, and some forms of CJD, and are associated with mutations in the PRNP gene. More than 20 disease predisposing point mutations have been reported, with the M/V129 polymorphism also affecting familial prion disease phenotype. For example, the D178N mutation co-segregates with V129 in FFI, but with M129 in CJD (Goldfarb et al, 1992). Other inherited pathogenic mutations also typically co-segregate with only either M129 or V129 (reviewed by Aguzzi et al, 2008).
The family of mammalian prion diseases includes scrapie in sheep, BSE in cattle, and chronic wasting disease in elk and deer. Disease transmission between species is usually much less efficient than within the same species, leading to the concept of ‘species barrier'. While these barriers are closely related to differences in PrP primary sequence between the donor and recipient species, another factor contributing to TSE transmissibility is the existence of multiple prion strains even within the same animal species. These distinct strains of the prion agent (leading to distinct disease phenotypes) appear to be associated with different conformational states of the PrPSc aggregate, although high-resolution insight into these conformational differences is still missing (reviewed by Collinge and Clarke, 2007).
The PrPC to PrPSc transformation involves a conversion from a soluble and predominantly α-helical protein to an aggregated form, which is substantially enriched in β-sheet. Bacterially expressed recombinant PrPs, which can be easily purified, have been more amenable to high-resolution structural studies than the brain-derived proteins; PrPSc is particularly problematic due to its aggregated nature. Although recombinant PrPs lack the C-terminal glycophosphatidyinositol (GPI) anchor and N-linked glycosylation at two sites, both of these post-translational modifications are not essential for infectivity (Taraboulos et al, 1990; Chesebro et al, 2005; Tuzi et al, 2008). NMR and circular dichroism studies of bovine PrPC purified from healthy brains showed that the thermal stability and three-dimensional structure of the purified glycoprotein and non-glycosylated recombinant protein are essentially identical (Hornemann et al, 2004). NMR structures of various mammalian PrPs revealed a conserved monomeric protein fold with a highly flexible N-terminus (residues 23–124) and a globular C-terminal domain (125–231), which contains a small two-stranded, anti-parallel β-sheet and three long α-helices (Wüthrich and Riek, 2001). This predominantly α-helical fold is reiterated in the crystal structures of a domain-swapped human PrP (hPrP) (Knaus et al, 2001) and of monomeric hPrP and ovine PrP (ovPrP) (Eghiaian et al, 2004; Haire et al 2004; Antonyuk et al, 2009). The published structures all contain M129. To investigate the structural consequences of the M/V129 polymorphic residue and understand how it may play a determinant role in prion disease susceptibility, we have solved the crystal structures of recombinant wild-type (WT) hPrP containing V129. We have also determined the crystal structures of the pathogenic mutants D178N and F198S, with both M129 and V129, to further probe the conformational effects of the polymorphic residue and also to investigate the structural consequences of the disease predisposing mutations themselves.
Results
WT-V129 in three different crystal forms reveals both dimeric and monomeric structures
Three different WT-V129 crystal forms were obtained: WT-V129_1, which has space group C2221, is isomorphous with WT-M129, and was solved at 2.26-Å resolution; WT-V129_2 and WT-V129_3 both have space group P212121, but different unit cell dimensions, and were refined at 3.1 and 1.8-Å resolution, respectively (Table I).
Table 1.
Data collection and refinement statistics
| Crystal | WT-V129_1 | WT-V129_2 | WT-V129_3 | D178N/M129 | D178N/V129 | F198S/M129 | F198S/V129 |
|---|---|---|---|---|---|---|---|
| Data collection | |||||||
| Source | APS 19BM | APS 19ID | APS 19ID | NSLS X25 | APS 19BM | NSLS X25 | APS 19ID |
| Wavelength (Å) | 0.9787 | 1.0332 | 0.9791 | 1.1000 | 0.9198 | 1.1000 | 0.9786 |
| Space group | C2221 | P212121 | P212121 | P43212 | I212121 | P43212 | P43212 |
| Unit cell (Å) | a=85.3 | a=28.7 | a=32.5 | a=b=57.5 | a=39.2 | a=b=57.0 | a=b=57.6 |
| b=86.4 | b=72.0 | b=49.1 | b=57.7 | ||||
| c=40.7 | c=125.9 | c=56.9 | c=168.0 | c=93.3 | c=167.4 | c=163.3 | |
| Resolution (Å) | 30.0-2.26 (2.38–2.26) | 50.0-3.1 (3.21–3.1) | 30.0-1.8 (1.86–1.8) | 50.0-1.8 (1.86–1.8) | 50.0-2.0 (2.07–2.0) | 50.0-2.0 (2.07–2.0) | 50.0-1.85 (1.92–1.85) |
| Rsym (%) | 8.5 (38.4) | 6.6 (27.7) | 4.2 (19.8) | 5.7 (21.6) | 6.7 (47.1) | 4.0 (40.5) | 5.2 (65.6) |
| I/σI | 15.0 (2.3) | 16.4 (4.4) | 30.8 (6.8) | 36.9 (4.8) | 23.2 (3.3) | 27.8 (2.5) | 41.0 (3.5) |
| Completeness (%) | 91.8 (59.3) | 91.9 (73.0) | 99.4 (99.8) | 95.8 (80.3) | 99.5 (99.9) | 94.2 (65.1) | 98.4 (96.6) |
| Redundancy | 5.7 (3.5) | 4.3 (4.0) | 4.5 (4.5) | 10.1 (3.0) | 5.0 (4.7) | 8.1 (8.1) | 14.6 (14.6) |
| No. reflections | 6679 | 4728 | 8871 | 25 609 | 7405 | 19 275 | 24 056 |
| Refinement | |||||||
| Resolution (Å) | 27.3-2.26 | 27.9-3.1 | 16.1-1.8 | 40.1-1.8 | 27.4-2.0 | 41.9-2.0 | 28.8-1.85 |
| R-factor/Rfree (%) | 20.3/27.8 | 23.0/29.9 | 18.5/24.1 | 20.6/23.2 | 20.5/26.6 | 21.6/24.9 | 22.6/26.5 |
| Number of atoms | |||||||
| Protein/ions/waters | 878/2/52 | 1701/0/0 | 897/0/105 | 1728/2/217 | 799/3/56 | 1645/2/172 | 1654/2/138 |
| Average B-factors (Å2) | |||||||
| All protein | 46.6 | 76.2 | 18.8 | 25.6 | 32.9 | 38.0 | 34.3 |
| 164–170 loop (A/B) | 56.1 | 78.7/88.0 | 17.0 | 30.1/36.4 | 46.6 | 46.4/50.8 | 40.3/29.9 |
| Ions/waters | 63.3/57.0 | —/— | —/33.0 | 14.8/34.8 | 21.5/39.3 | 28.6/47.1 | 33.5/45.2 |
| r.m.s. deviations | |||||||
| Bond length (Å) | 0.013 | 0.008 | 0.012 | 0.011 | 0.012 | 0.011 | 0.013 |
| Bond angle (degrees) | 1.43 | 1.08 | 1.35 | 1.24 | 1.46 | 1.24 | 1.41 |
| Ramachandran plot (%) | |||||||
| Most favoured region | 98.9 | 91.3 | 96.8 | 95.1 | 91.8 | 95.9 | 94.7 |
| Additionally allowed regions | 1.1 | 8.2 | 3.2 | 4.9 | 4.7 | 4.1 | 5.3 |
| Disallowed regions | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PDB accession code | 3HAF | 3HJ5 | 3HAK | 3HEQ | 3HJX | 3HES | 3HER |
Values in parentheses are for the highest-resolution shell.
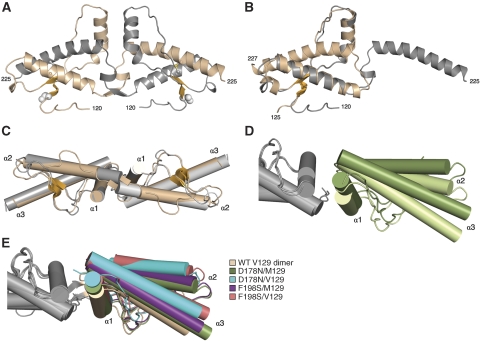
Both WT-V129_1 and WT-V129_2 reveal disulfide-linked, 3D-domain-swapped dimers nearly identical to the WT-M129 structure (Figure 1A and Supplementary Figure S1A, additional details are provided in the Supplementary data; Knaus et al, 2001). V129 is well-defined in density located in the first short β-strand and, consistent with NMR studies (Hosszu et al, 2004), the M129-to-V129 substitution does not seem to cause any local conformational changes. In both crystal forms, the V129 side chain is on the protein surface, exposed to solvent, and is not involved in any intermolecular interactions. The elucidation of domain-swapped PrP dimers in two different crystal forms, in which the two halves are related by different types of symmetry (crystallographic in WT-V129_1 and non-crystallographic in WT-V129_2), confirms that dimerization is a characteristic behaviour of recombinant PrP rather than merely a crystallization artefact. This is also supported by the observation of a small amount of disulfide-linked dimer in solutions of WT and variant PrP proteins (Supplementary Figure S1C). WT-V129_3 contains a single PrP polypeptide in the asymmetric unit (Figure 1B); the electron density defines a closed hinge-loop conformation corresponding to a compactly folded, non-swapped monomer (Supplementary Figure S1B). V129 is a solvent-exposed surface residue, near a crystal packing interface with H155 as its closest neighbour.
Figure 1.
Crystal structures of WT-V129 and mutant hPrPs. (A) Ribbon diagram of the WT-V129_1 3D-domain-swapped dimer. The two polypeptide chains are in beige and grey, with the V129 side chains shown as space-filling spheres and the intermolecular disulfide bonds as ball-and-stick structures. The two-stranded, antiparallel β-sheet is coloured in orange. (B) Ribbon diagram of the WT-V129_3 monomer (beige). Superimposed is one chain of the WT-V129_1 domain-swapped dimer (grey), to illustrate the differences in hinge region conformation. (C) Non-swapped hPrP dimers cannot adopt the 3D-domain-swapped dimer organization. Superimposed on the WT-V129_1 domain-swapped dimer (beige) are two copies of the non-swapped WT-V129_3 monomers (grey); the helices-2 and hinge loops sterically clash at the dimer interface. Helices are drawn as cylinders. (D) Asymmetry in the D178N/M129 dimer. Superimposition of two copies of the dimer shows that the two halves (green) are rotated ∼10 degrees with respect to each other. (E) Variations in the bending angles of the hPrP dimer. When one set of monomers is superimposed, the second set of monomers is oriented differently in mutant and WT dimers. D178N/M129 (green) and F198S/M129 (purple) dimers have the same bending angle, D178N/V129 (cyan) and F198S/V129 (pink) superimpose well, and the WT-V129_1 dimer (beige) is similar to WT-V129_2 (not shown) and different from both pairs of mutant dimers.
The ordered structures for the three crystal forms (encompassing residues A120–Y225 in WT-V129_1 and L125–Q227 in WT-V129_2 and WT-V129_3) appear to be N-terminally truncated as was seen for WT-M129, crystals of which were shown to contain N-terminally proteolysed protein (Knaus et al, 2001). That the N-terminus is susceptible to proteolysis is not surprising since NMR studies have shown that this region is flexible to about residue 125 in full-length (23–230) and truncated (90–230) recombinant PrP. This flexible N-terminus appears to have little effect on the structure of the C-terminal domain (Zahn et al, 2000). Furthermore, glycosylated PrP purified from bovine brains (Hornemann et al, 2004) shows a similar ordered structure limited to the C-terminal region.
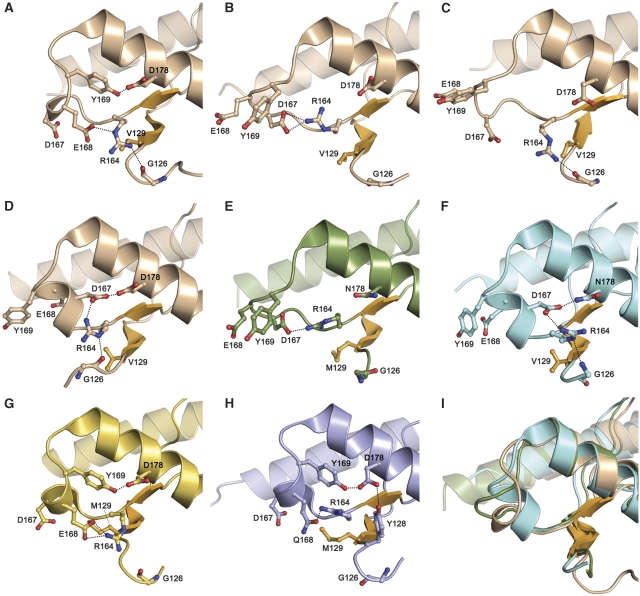
By superimposing two WT-V129_3 monomers onto the two halves of the WT-V129_1 dimer, the resulting steric clashes of the hinge loops and helices-2 show that a dimer of non-swapped monomers would require substantial conformational adjustments (Figure 1C), and confirm that the WT-V129 dimers are domain-swapped. In addition to the hinge loop, which changes structure upon helix-3 swapping, the (R164–S170) loop adjacent to the small β-sheet with M/V129 is conformationally variable. This loop is nearly identical in both WT-V129_1 and WT-M129 dimers, with characteristic side-chain hydrogen bonding interactions between Y169 and D178, and between R164 and E168 (Figure 2A). In one chain of the WT-V129_2 dimer, R164 forms a salt bridge with E167, while in the other there is no intra-loop interaction (Figure 2B and C). The WT-V129_3 monomer loop includes a helical turn such that D167 swings in to interact with D178 (Figure 2D). In all WT-V129 and WT-M129 crystal structures, R164 hydrogen bonds to the G126 main chain.
Figure 2.
Variable conformation of the (R164–S170) loop in PrP crystal structures. Three different conformations of the (R164–S170) loop, adjacent to the β-strand containing M/V129, are observed. Side chains of selected loop residues are shown as ball-and-stick structures; hydrogen bonds are represented by dashed lines. (A) In the WT-V129_1 dimer loop, Y169 forms a hydrogen bond with D178, while R164 interacts with E168 and the main chain of G126. (B, C) The two loops in the WT-V129_2 dimer have similar main-chain conformations, but different hydrogen-bonding interactions. (D) The WT-V129_3 monomer loop has a helical turn and a network of hydrogen bonds formed by D178, D167, R164, and the main chain of G126. (E) The D178N/M129 loop resembles that in the WT-V129_2 chain shown in panel B. (F) The D178N/V129 loop is similar to that observed in WT–V129_3 shown in panel D. (G) In the monomeric hPrP–Fab structure, the loop resembles that in WT-V129_1 except there is no interaction between R164 and G126. (H) In WT ovPrP, the loop is similar to that in WT-V129_1 except there is no interaction between R164 and G126, and there is an additional hydrogen bond between Y128 and D178 that is observed only in the WT and mutant ovPrP crystal structures. (I) Superimposition of the loops from D178N/M129 (green), D178N/V129 (cyan), and V129_1 (beige), highlighting the three distinct conformations.
CJD D178N/M129 and FFI D178N/V129 crystal structures exhibit different conformations and a non-conserved intermolecular β-sheet
The D178N mutation is associated with two pathologically distinct inherited prion diseases, CJD and FFI, when the mutation colocalizes with M129 or V129, respectively (Goldfarb et al, 1992). Both versions of the mutant have been crystallized. The CJD D178N/M129 crystal has space group P43212 and was solved at 1.8-Å resolution, while the FFI D178N/V129 crystal has space group I212121 and yielded a 2.0-Å resolution structure (Table I).
In D178N/M129, there is no continuous density for ∼35 N-terminal residues, several hinge-loop residues, and for a few C-terminal residues. Thus, the refined model contains residues G126–T192 and K194–R228 for chain-A and G126–T192 and E196–R228 for chain-B. The two chains form a dimer, which is very similar to those observed in the WT crystals, except that the D178N/M129 dimer appears to be composed of two non-swapped, closed monomers. The hinge-loop density gaps correspond to only 1–3 residues and indicate that the D178N/M129 dimer is most likely composed of non-swapped monomers. In contrast, the D178N/V129 crystal contains only a single chain in the asymmetric unit, but it combines with a symmetry-related molecule to form a dimer that is similar to that seen for D178N/M129. The refined D178N/V129 protein model consists of residues G126–T192 and E196–Q223; its small hinge-loop density gap also suggests a dimer of two non-swapped monomers. Due to scarcity of crystals, it was not possible to determine the disulfide-linked dimer content, or the extent of N-terminal proteolysis, of either of these two mutant crystals.
There is no substantial difference in the overall protein fold in D178N/M129 or D178N/V129 as compared with the WT dimers, other than the hinge loops, which are in a partially disordered closed conformation in the mutants and an ordered open conformation for the WT dimers (Figure 3A and C). However, the local environment surrounding the altered D178N residue shows differences between the M129 and V129 isoforms. In the WT crystal structures, D178 is a mostly solvent-exposed residue, and in several crystalline forms the D178 side-chain hydrogen bonds to either Y169 or D167 in the variable (R164–S170) loop. In the two D178N structures, the N178 residues are in nearly identical conformations as D178 in the WT structures; the local structural variability is in the adjacent (R164–S170) loop. In D178N/M129, N178 does not interact with the (R164–S170) loop (Figure 2E), while in D178N/V129, the N178 side chain interacts with D167, which in turn forms a salt bridge with R164 (Figure 2F), corresponding to two very different loop conformations (3.1 Å r.m.s.d. for Cα superimpositions). As is also the case for the dimeric WT crystal structures, the average loop B-factors for the M129 and V129 isoforms of D178N are higher than the corresponding overall average B for the two proteins (Table I). In accordance with correspondingly higher loop B-factors, the electron density in this region for D178N/V129 is less clear than for the different loop conformation in D178N/M129 (Supplementary Figure S2A) or the similar conformation in WT-V129_3.
Figure 3.
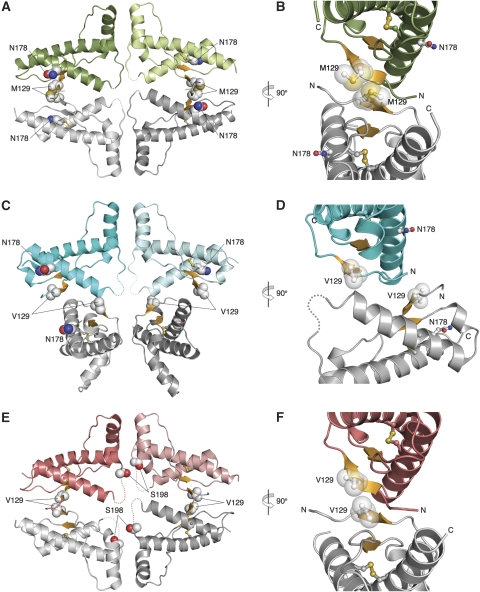
Crystal structures of hPrP mutants. (A) Packing between two D178N/M129 dimers. An intermolecular antiparallel, four-stranded β-sheet (orange) containing M129 (space-filling spheres) is formed at the dimer:dimer interface; the variant N178 side chains are also shown. (B) A close-up view of the D178N/M129 intermolecular β-sheet containing M129. (C) D178N/V129 dimers do not pack to form intermolecular β-sheets; instead these dimers are rotated ∼90 degrees with respect to each other. (D) A close-up view of the D178N/V129 β-sheets. The V129 side chains do not approach each other and the β-sheets do not interact. (E) F198S/V129 dimers form an intermolecular β-sheet. While the F198S/M129 dimer:dimer interaction (Supplementary Figure S1C) is nearly identical to that for D178N/M129, the F198S/V129 is slightly twisted in comparison. (F) A close-up view of the F198S/V129 intermolecular β-sheet. The disordered/flexible hinge loop residues are represented by dashed lines.
An even more striking difference between the D178N and WT structures, and between the two D178N structures, is in the environment about the M/V129 polymorphic residue and the intermolecular packing of dimers. In crystals containing WT-M129 and WT-V129 dimers, the M129 and V129 side chains are solvent-exposed and not involved in intermolecular contacts. In D178N/M129, two dimers related by crystallographic symmetry come together such that the two small intramolecular β-sheets combine to form continuous intermolecular, four-stranded, antiparallel β-sheets at the dimer:dimer interface (Figure 3A and B). The N-terminal β-strands in the middle of the sheet contain M129 side chains that pack against each other to form part of the ‘sheet interface.' In contrast, the presence of V129 in D178N/V129 yields a crystal with very different packing between dimers, which are rotated by ∼90 degrees with respect to each other as compared with their approach in the M129 isoform (Figure 3C). Due to this rotation, the short β-strands in the neighbouring molecules do not combine to form a continuous intermolecular β-sheet, and the V129 side chains do not interact with each other but instead are exposed to solvent (Figure 3D).
F198S/M129 and GSS F198S/V129 structures have similar conformations and intermolecular β-sheets
F198S is a mutation, which colocalizes with the V129 polymorphism in GSS (Dlouhy et al, 1992; Hsiao et al, 1992); colocalization with M129 has not yet been reported. To provide a full structural investigation of the pathogenic F198S substitution in the context of both M129 and V129, both proteins were crystallized. Both crystals have space group P43212 and similar cell dimensions. The F198S/M129 structure was solved at 2.0-Å resolution, while the GSS F198S/V129 structure was refined to 1.85-Å resolution (Table I).
The refined F198S/M129 model includes residues L125–T193 and S198–Y226 for chain-A, and G126–V189 and N197–Y226 for chain-B. In F198S/V129, there is continuous density for residues L125–T192 and S198–Y226 in chain-A, and G126–T190 and S198–Q227 in chain-B. The two F198S crystals are isomorphous not only with each other, but also with D178N/M129. It is not surprising then that both F198S crystals contain dimers similar to those seen in the D178N structures (Figure 3E and Supplementary Figure S2C). As in the D178N structures, there is discontinuous hinge-loop density. F198S/M129 crystallization was reproducible enough to provide adequate samples for Western blot analysis, which shows a mixture of proteins of different sizes, but with the major component being intact monomeric PrP(90–231), while WT crystals contain only the N-terminally proteolysed protein and are enriched in disulfide-linked dimers (Supplementary Figure S1C; details in Supplementary data). This correlates well with the observation of continuous density for open hinge loops in the swapped WT dimers, and broken density for flexible closed hinge loops in the mutant dimers.
In the WT-V129_1 swapped dimer and WT-V129_3 closed monomer, the F198 side chain is buried in a hydrophobic environment (Supplementary Figure S1A and B). In both D178N mutant structures, the F198 side chain remains solvent-inaccessible even with increased flexibility of the adjacent hinge loop. In contrast, in both F198S mutant structures the S198 side chain is largely solvent-exposed (Supplementary Figure S2B). The S198 hinge-loop density gaps correspond to 4–7 residues as compared with 1–3 residues in the D178N dimers. Thus a structural effect of the F198S substitution appears to be an increase in hinge-loop flexibility as a result of replacing a large buried hydrophobic residue with a small hydrophilic amino acid that prefers to be exposed to solvent.
The increase in hinge-loop flexibility in the two F198S isoforms does not interfere with the mutants' ability to crystallize as non-covalent dimers. The protein fold and crystal packing are very similar in both the F198S and D178N/M129 structures (Figure 3A and E; Supplementary Figure S2C, Cα r.m.s.d.s of 0.6–1.1 Å), including the (R164–S170) loop (Cα r.m.s.d.s 0.14–0.4 Å). In both F198S/M129 and F198S/V129, R164 forms a salt bridge with D167 and D178 is left to interact with the solvent. These loop interactions and conformations are very similar to those in WT-V129_2 and D178N/M129 (Figure 2C and E). Also in keeping with the other dimeric PrP structures, the average loop B-factor is higher than the overall protein average B for the F198S/M129 structure; F198S/V129 is the exception since its loop and overall average B-factors are nearly identical (Table I).
Unlike the two D178N structures, which showed that the dimers in the M129 and V129 isoforms participate in dramatically different intermolecular interactions, the two F198S structures reveal a relatively subtle structural effect of the M/V129 substitution. As for D178N/M129, F198S/M129 dimers come together with M129 side chains packing against each other at an intermolecular β-sheet interface. A similar β-sheet is formed between F198S/V129 dimers, except that this interface is bent by ∼10 degrees as compared with that in the M129 isoform. The result is that one face of the β-sheet becomes more exposed such that the V129 side chains, which are short and branched as compared with that of M129, do not pack tightly against each other (Figure 3F).
Discussion
NMR studies of recombinant PrPs have thoroughly characterized a conserved monomeric fold presumed to represent PrPC (Wüthrich and Riek, 2001). The crystal structure of hPrP WT-M129 revealed an unexpected 3D-domain-swapped dimer and expanded the understanding of the PrP conformational repertoire (Knaus et al, 2001). Single-amino-acid substitutions such as the M/V129 isoforms, and pathogenic mutations identified in inherited prion diseases, influence disease susceptibility and phenotype. Structural comparison of WT and variant PrPs is an important step in understanding how variant PrPs may contribute to prion disease mechanisms. Detailed structural information on hPrP disease variants has been limited to the NMR structure of the familial CJD-related Q200K mutant, which was nearly identical to that of WT hPrP except for minor differences in flexible regions such as both termini and two loops (Zhang et al, 2000). NMR structures of non-pathogenic hPrP variants also do not reveal any significant structural differences (Calzolai et al, 2000). The ensemble of seven hPrP crystal structures described here, for WT-V129 and pathogenic D178N and F198S mutants, substantially increases the characterization of PrP conformational behaviour, and highlights structural details, which may be relevant to prion disease mechanisms.
PrP dimers exhibit differences in 3D-domain swapping and dimer organization
Dimers are observed in the crystals of both WT and mutant PrPs, but vary in several different ways (details in the Supplementary data). The first is that both WT-M129 and WT-V129 can crystallize as swapped dimers, while D178N and F198S mutants crystallize as non-swapped dimers. Secondly, PrP dimers vary in the orientation of the two monomers with respect to each other. The two mutant dimers containing M129 are asymmetric and bent, while the two mutant dimers containing V129 are symmetric (Figure 1D). The WT dimers are symmetric irrespective of whether they contain M129 or V129. When one monomer of each dimer is superimposed and the orientations of the second monomers are compared, the WT, M129-containing mutants and V129-containing mutants are found to exhibit three different dimer conformations (Figure 1E). This segregation of mutant dimer organization according to the M/V129 polymorphism is intriguing since the polymorphic M/V129 residues are predominantly solvent-exposed in the context of the dimers, and they are distant from the dimer interface.
The presence of 3D-domain swapping can explain the significant difference in organization between the WT and mutant dimers. When two copies of the WT-V129_3 monomer are superimposed onto the two halves of the WT-V129_1 swapped dimer, the C-termini of the two helices-2 and the adjacent closed hinge loops introduce severe steric clashes (Figure 1C). Thus, for two monomers to form a non-swapped dimer, shifts of the helices-2 and the hinge loops are necessary. Substantial shifts of helix-2 in either one or both monomers produce asymmetric M129-containing or symmetric V129-containing mutant dimers, respectively (Supplementary Figure S3).
Intermolecular β-sheets containing the polymorphic residue 129 are observed only in non-swapped structures
In WT PrP structures, the M129/V129 residues do not directly participate in intermolecular contacts. In contrast, in three of the four mutant structures, the M129/V129 side chains pack against each other at an intermolecular β-sheet interface. Intermolecular β-sheets have been observed in two other mammalian PrP crystal structures (hPrP residue numbering for all species is for simplicity). The structure of WT ovPrP contains symmetry-related monomers that form intermolecular β-sheets with M129 at the interface (Haire et al, 2004). Superimposition of the structure of WT ovPrP with the two hPrP mutants reveals substantial differences in the sheet interface angle, by up to ∼40 degrees (Figure 4A). In contrast, several ovPrP scrapie-susceptibility variants crystallized as Fab complexes did not exhibit intermolecular β-sheets, apparently due to potential steric clashes involving the Fab, which is only ∼10 Å from M129 (Eghiaian et al, 2004). The crystal structure of monomeric WT hPrP bound to the ICSM 18-Fab also has an intermolecular β-sheet with M129 (Antonyuk et al, 2009). The hPrP M129/Fab sheet interface differs substantially from those of ovPrP and the hPrP mutants, by up to ∼70 degrees (Figure 4A). In the ovPrP and hPrP mutant sheets, the M/V129 residues interact with each other more intimately (Cβ–Cβ distances of 5.3–5.8 Å) than in WT hPrP–Fab (Cβ–Cβ distance of 6.8 Å). Thus while intermolecular β-sheets containing M/V129 are observed in five different mammalian PrP structures, there are differences in the interactions involving M/V129 and a wide variation in the conformation of the sheet and the angle between the monomers forming the interface.
Figure 4.
Variability in PrP intermolecular β-sheet interactions. (A) Superimposition of hPrP F198S/M129 (purple) and F198S/V129 (pink) with the ovPrP (indigo) and hPrP-Fab (yellow) structures. With one monomer from each structure superimposed (grey), the orientation of the second monomer in each interface (coloured) can vary up to ∼70 degrees. Helices are drawn as cylinders and numbered; the interface β-sheets are in orange and the M/V129 Cα atoms are shown as spheres. (B) Superimposition of hPrP D178N/M129 (green) and D178N/V129 (cyan). With monomers from one dimer superimposed (grey), the monomers from the second dimer (coloured) are rotated ∼90 degrees with respect to each other and an intermolecular β-sheet is present in only D178N/M129. (C) Scheme showing the relationship of the conformationally variable regions, which may influence intermolecular β-sheet formation. This is a composite figure showing conformations and interactions from both WT and mutant hPrP crystal structures. In the non-swapped dimer (green, blue for helices-2), (1) the hinge loops (dashed blue lines) and helices-2 (blue) must shift from their corresponding positions in the swapped dimer (pale blue) to relieve steric clashes. The helix-2 orientation influences (2) the conformation of the (R164–S170) loop (from pale red to red) and in turn, (3) the adjacent β-sheet containing M/V129 (orange), and (4) its ability to form an intermolecular β-sheet of a particular conformation with another PrP molecule (grey).
The M/V129 polymorphic residue influences intermolecular β-sheet formation and conformation
There is no significant difference in local protein fold between M129- and V129-containing structures. This is consistent with observations that the polymorphism has no effect on the stability, folding, or dynamics of PrPC (Hosszu et al, 2004). The most striking difference is in the formation and conformation of the intermolecular β-sheets, which are observed in a subset of the structures, and is most clear when comparing the mutant hPrP structures. The two M129-containing mutants, D178N/M129 and F198S/M129, form identical intermolecular β-sheets with the M129 side chains interacting at the interface (Figure 3A and B; Supplementary Figures S2C, S5A and C). When V129 is present, the sheet interface in F198S/V129 is bent ∼10 degrees to prevent the branched V129 side chains from sterically clashing with each other (Figures 3E, F and 4A; Supplementary Figure S5D), and in D178N/V129 the sheet interface is absent entirely (Figures 3C, D and 4B; Supplementary Figure S5B). One possible speculation from the comparison of these four hPrP mutant structures is that M129 is more compatible with the formation of an intermolecular β-sheet than V129. In this context, it is interesting to note that both the ovPrP and the hPrP M129/Fab complex structures, which exhibit β-sheet interfaces, also contain M129.
In contrast, no sheet interfaces are observed for the hPrP WT-M129 or WT-V129 dimers, or the WT-V129 monomer (Supplementary Figure S4). The presence of V129 may explain the absence of sheet interface in the three WT-V129 crystals. A comparison of the hPrP crystal structures containing a sheet interface with that of the hPrP WT-M129 dimer was performed to understand why the interface is not observed for swapped WT dimers. When WT-M129 is superimposed on D178N/M129, the different conformation of the WT-M129 N-terminal residues, V121–G126, would introduce steric clashes that prevent intermolecular sheet formation. The WT-M129 N-terminal structure appears to be stabilized by an (R164–S170) loop conformation whose R164 side chain is shifted outward towards G126 (Figure 2A), instead of participating in intra-loop interactions as seen in the mutant (Figure 2E). Comparison of WT-M129 with M129/Fab shows a less direct impediment: with a modelled M129/Fab-like interface, the C-termini of the two WT-M129 halves would sterically clash. It is interesting to note that these N-terminal and C-terminal residues, which would interfere with the formation of ‘mutant-like' and ‘Fab-complex-like' sheet interfaces in the WT-M129 dimer, flank the opposite sides of the (R164–S170) loop.
The (R164–S170) loop may act as a conformational bridge via R164 interactions
The ensemble of PrP crystal structures reveals three distinct regions of conformational variability. The first is the dimer interface, where the hinge-loop conformation and flexibility, and the helix-2 C-terminus orientation are very different between swapped WT dimers and non-swapped mutant dimers. The second region is the small β-sheet containing the M/V129 polymorphic residue, which can form intermolecular sheet interfaces of variable conformation. There appears to be a relationship between these two regions since formation of the sheet interface between hPrP dimers correlates with the non-swapped dimer conformation. However, there is limited/no direct interaction between the β-strand containing M/V129 and either helix-2 or the hinge loop. Instead, a third, structurally variable region, the (R164–S170) loop located between the first strand and the N-terminus of helix-2, can potentially allow transmission of conformational information between the dimer interface and the β-sheet (Figure 4C).
Specific interactions involving the different (R164–S170) loop conformations may influence the M/V129-containing β-strand and its role in intermolecular β-sheet formation (Figure 2A–I). When the loop conformation is such that the R164 side-chain hydrogen bonds with G126, the M/V129-containing β-strand is tethered to the (R164–S170) loop and does not form an intermolecular β-sheet. This is observed in the WT-M129, the three WT-V129, and the D178N/V129 crystal structures, which all lack the β-sheet interface and which have R164 side-chain nitrogen–G126 oxygen (nitrogen for D178N/V129) distances ranging from 2.7 to 3.2 Å (Figure 2A–C and F). Conversely, for those hPrP structures in which the R164 side chain is too far from G126 for hydrogen bonding, the M/V129-containing β-strand is not directly tethered to the (R164–S170) loop and is able to form the sheet interface. This is the case for the three hPrP mutants and the M129/Fab complex, which all exhibit the intermolecular β-sheet and in which R164–G126 oxygen distances range from 4.2 to 12.8 Å (Figure 2E and G).
The correlation between the R164–G126 interaction and absence of intermolecular β-sheet is observed among all nine hPrP crystal structures. In the WT ovPrP crystal structure, which exhibits the sheet interface, R164 does not hydrogen bond to G126 (a distance of 6.4 Å to the G126 carbonyl; Figure 2H). Thus, the connection between the (R164–S170) loop conformation and the sheet interface holds for both hPrP and ovPrP structures. The Fab in the three ovPrP variant antibody complex structures sterically prevent intermolecular β-sheet formation, explaining the lack of R164–G126 hydrogen bonds (distances of 4.3–4.7 Å to the G126 carbonyl) in these structures, which also lack the sheet interface.
Conformations of the (R164–S170) loop and β-sheet interface containing M/V129 may be species-specific markers
The different (R164–S170) loop conformations seen in the WT and mutant hPrP structures can be sorted into three distinct groups: ‘loop-A' in the WT-M129 and WT-V129_1 swapped dimers (Figure 2A), two variations of ‘loop-B' in the WT-V129_2 swapped dimer (Figure 2B and C), and ‘loop-C' in the WT-V129_3 monomer (Figure 2D). These WT loop conformations are reiterated in the four mutant hPrP structures. The three mutants, which exhibit a β-sheet interface, all have (R164–S170) loop conformations similar to ‘loop-B' (Figure 2E), while the one mutant, which does not form a sheet interface, adopts the ‘loop-C' conformation instead (Figure 2F). The flexibility of the (R164–S170) loop is well-documented for many mammalian PrPs. NMR studies show no observable backbone resonances for hPrP, bovine PrP, or mouse PrP (mPrP) for residues 166–172 (Riek et al, 1998; López Garcia et al, 2000; Zahn et al, 2000). However, this loop region becomes partially stabilized in Syrian hamster PrP (shPrP) (Liu et al, 1999) and is very well defined in elk PrP (ePrP) (Gossert et al, 2005).
The (R164–S170) loop is one of two regions in PrP with most significant inter-species sequence variation; mutations of residues in/near this loop inhibited PrPSc formation in a cell culture model, and residues within this region have been identified as part of the epitope for binding of a hypothetical ‘protein X' (Telling et al, 1995; Kaneko et al, 1997). In the designed hPrP S170N mutant (in which the hPrP serine was replaced with the shPrP asparagine) or mPrP S170N/N174T mutant (in which mPrP residues 170 and 174 were replaced with the corresponding residues in ePrP), NMR data show a switch from a flexible to a partially stabilized (shPrP-like), and a highly ordered (ePrP-like) loop structure, respectively (Calzolai et al, 2000; Gossert et al, 2005). Thus, this loop may represent a conformational marker for species-specific susceptibility to prion diseases, as well as their transmissibility barriers. Consistent with this notion, a recent study demonstrated that transgenic mice expressing the mPrP variant with loop-ordering mutations S170N/N174T spontaneously developed a fully penetrant TSE disease (Sigurdson et al, 2009).
The crystal structures reveal one (R164–S170) loop interaction in ovPrP, which is not observed in hPrP: in all four ovPrP crystal structures, D178 forms an additional hydrogen bond with Y128, adjacent to M129 (Figure 2H). The Y128–D178 interaction is observed in both free ovPrP and ovPrP–Fab complex structures, whether or not the intermolecular β-sheet is present. Similarly, although Y128 is conserved, the Y128–D178 interaction is absent in all nine hPrP crystal structures irrespective of the presence or absence of the sheet interface or bound Fab. The Y128–D178 hydrogen bond represents a striking species-dependent conformation near the polymorphic M/V129, and highlights residues N-terminal to M/V129 as another potential conformational marker of species-specific disease strains.
Comparison of the hPrP D178N(M/V129) and F198S(M/V129) crystal structures with those of hPrP-M129/Fab (Antonyuk et al, 2009) and WT ovPrP (Haire et al, 2004) reveal very different sheet interface conformations, which are not only mutant- and polymorphism-dependent, but also species-dependent (Figure 4A). The conformation of the (R164–S170) loop and its interactions with residues in or adjacent to the M/V129-containing β-strand appear to influence the formation and conformation of the intermolecular β-sheets. This link between the (R164–S170) loop and sheet interface conformations suggests that both may play a role in determining the species-dependent conformational strain of mammalian prions.
D178N and F198S pathogenic mutant substitutions influence two highly conformationally variable regions
Mutants associated with familial prion diseases are expected to be more susceptible to structural transformation to PrPSc. Studies of recombinant hPrP and mPrP variants show that pathogenic mutants do not necessarily exhibit decreased thermodynamic stability relative to the WT proteins (Swietnicki et al, 1998; Liemann and Glockshuber, 1999). The ensemble of crystal structures reported here allows the first detailed comparison of WT with pathogenic mutants and reveals that the D178N and F198S substitutions can affect the conformations of two regions, which are highly variable conformationally.
The GSS F198S substitution results in the replacement of a large hydrophobic phenylalanine side chain with a small hydrophilic serine. The structural effect appears to be increased flexibility of the hinge loop at the dimer interface. It is possible that this may influence the propensity of the protein to undergo 3D-domain swapping, a behaviour that is exhibited by other amyloidogenic proteins (reviewed by Bennett et al, 2006). The increased hinge-loop flexibility may also affect the mobility of helix-2, which in turn can influence the conformation of the (R164–S170) loop and the adjacent M/V129-containing β-strand (Figure 4C).
The structural consequences of the FFI and CJD D178N substitution are more subtle. The D178N mutation alters a helix-2 surface residue, which interacts directly with the (R164–S170) loop in two of the three hPrP conformations as well as in ovPrP. The ovPrP crystal structures show an additional D178–Y128 hydrogen bond, which is not present in any hPrP crystal structure. Since the D178N and loop conformations in the two mutant structures are similar to their counterparts in two WT hPrP structures, a possible effect of the D178N replacement is that it may select for a single local conformation out of the several WT possibilities. Removal of the negative charge of D178 would be expected to alter the strength of electrostatic interactions, and play a role in influencing and/or selecting conformations of the (R164–S170) loop and the adjacent β-strand containing M/V129.
A complicated interplay among loop conformation, 3D-domain swapping, helix-2 orientation, and β-sheet interface may influence prion conversion and strain
The observation of an intermolecular β-sheet containing M129 was initially made in the crystal structure of monomeric ovPrP, and this region was proposed as a possible initiation point for β-sheet-mediated polymerization (Haire et al, 2004). Since this interface is small and does not result in a significant increase in β-sheet structure, it is expected that substantial refolding of other regions of the protein is still required in PrPSc conversion. In vitro studies of recombinant hPrP variants show that D178N/M129 undergoes the α-helix-to-β-sheet transition and forms amyloid fibrils more quickly than D178N/V129 or either WT polymorphic forms, and that the secondary structures of amyloids are slightly different for the two variant proteins (Apetri et al, 2005). The correlation of these observations with the presence of the sheet interface in the D178N/M129 crystal structure and absence of a corresponding sheet in D178N/V129, WT-M129, and WT-V129 structures supports the possible role of the sheet interface in initiating extended β-sheet formation. The in vitro biophysical studies also show that, at least under some experimental conditions, both polymorphic WT hPrP forms have similar propensity to form amyloid fibrils (Apetri et al, 2005; Tahiri-Alaoui and James, 2005), consistent with our observation of similar 3D-domain-swapped dimers and absence of sheet interfaces in all the free WT-M129 and WT-V129 hPrP crystal structures.
The crystal structures reported here represent a small sample of the conformational states that hPrP may adopt as a consequence of different pathogenic mutations or during the course of pathogenesis. These seven structures identify several conformationally variable features, which appear to be correlated and allow us to speculate on a sequence of related structural shifts, which may play a role in the pathogenic mechanism(s) (Figure 4C). 3D-domain swapping appears to be coupled with conformational changes in the hinge loop and helix-2 orientation, which in turn can influence the structure and interactions of the (R164–S170) loop and subsequently the adjacent β-strand containing M/V129. In addition, the pathogenic D178N and F198S substitutions can influence the hinge-loop and (R164–S170) loop conformations, respectively. Overall, the nature of the specific conformations in each of these variable regions can combine to determine whether the M/V129-containing β-strand forms an intermolecular sheet interface. Finally, the presence of an M129 or V129 polymorphic residue influences the conformation of the sheet interface. While the mechanisms of major conformational changes underlying PrP conversion to the infectious aggregated form are still largely unknown, identification of these polymorphism-dependent differences in intermolecular contact sites may provide a basis for understanding the initial events in this complex reaction.
Materials and methods
Purification and crystallization
WT and variant hPrPs containing residues 90–231 were expressed in Escherichia coli as inclusion bodies, refolded and purified as previously described (Zahn et al, 1997; Morillas et al, 1999). Additional details for protein preparation, crystallization, and cryoprotection are provided in the Supplementary data. Briefly, all crystals were grown by sitting-drop vapour diffusion methods. WT-V129_1 crystals were obtained with 0.1 M Tris (pH 10.0), 3.4 M NaCl, and 5 mM CdCl2 at 20°C. WT-V129_2 crystals were obtained at 4°C with 0.1 M succinic acid (pH 8.0) and 15% PEG 6K. WT-V129_3 crystals were grown at 4°C from 0.1 M Na/K phosphate (pH 5.2) and 20% PEG 4K. D178N/M129 and F198S/M129 were both crystallized at 4°C using 0.1 M Tris (pH 8.0), 0.2 M Mg acetate, 5% PEG 4K, and 5 mM CdCl2 for D178N/M129, and 0.1 M Tris (pH 8.5), 1.0–1.2 M NaCl, 10–12% PEG 8K, and 5 mM CdCl2 for F198S/M129. D178N/V129 and F198S/V129 crystals were both obtained at 20°C. D178N/V129 crystals were grown using 0.1 M Tris (pH 7.0–8.0), 1.9–2.1 M NaCl, and 5 mM CdCl2, while F198S/V129 crystals were grown using 0.1 M Tris (pH 8.5), 0.2 M Mg acetate, 8–12% PEG 6 or 8K, and 5–10 mM CdCl2. WT and mutant proteins crystallized under different conditions; attempts made to crystallize each protein under the conditions for the others were unsuccessful.
Data collection and structure determination
Crystals of D178N/M129 and F198S/M129 were grown first and their structures were solved by SIRAS to avoid model bias from molecular replacement calculations (Supplementary Table SI). Heavy-atom derivatization was performed by soaking the crystals in artificial mother liquor containing 5 mM K2PtCl4 for 2 days. Crystals of the other hPrPs were much more difficult to obtain and their structures were solved by molecular replacement. Native and derivative data were measured using either synchrotron sources or an in-house Rigaku R-AXIS IV imaging plate detector and CuKα radiation from a Rigaku H3R rotating anode X-ray generator equipped with Yale focusing mirrors (Table I and Supplementary Table SI). All data were processed using HKL (Otwinowski and Minor, 1997). Phasing and density modification calculations were performed with SOLVE and RESOLVE (Terwilliger and Berendzen, 1999; Terwilliger 2000), while molecular replacement solutions were obtained with EPMR (Kissinger et al, 1999) or MOLREP (Vagin and Teplyakov, 1997), using the hPrP WT-M129 crystal structure (Knaus et al, 2001) with the variant residue truncated to alanine, and the hinge loop (V189–P198) and disordered N-terminal region (G90-G124) removed. Cycles of interactive model building using Coot (Emsley and Cowtan, 2004) and restrained refinement using Refmac (Murshudov et al, 1997) were performed. Final refined models were validated using Procheck (Laskowski et al, 1993) and MolProbity (Davis et al, 2007). All molecular figures were prepared using PyMol (DeLano Scientific, San Carlos, CA, USA).
Western blot analysis of crystals
In cases where there were enough PrP crystals available, Western blots were performed to detect the presence of monomer and/or disulfide-linked 3D-domain-swapped dimer, and N-terminally cleaved or intact protein. WT-M129 protein stock solution and crystals, and F198S/M129 crystals were resolved by 15% SDS–PAGE and analysed by immunoblotting with the monoclonal antibody 7A12 (Li et al, 2000). WT-M129 crystals were obtained as described previously (Knaus et al, 2001). Five fresh crystals each of WT-M129 and F198S/M129 were boiled in non-reducing loading buffer for 3 min. Each dissolved crystal sample was then divided into two aliquots, one of which was boiled for an additional 3 min with reducing loading buffer containing 6% 2-mercaptoethanol. Crystals of WT-V129 and of the other F198S and D178N mutants were not abundant enough to be analysed by Western blots at a similar rate of success.
Accession numbers
Coordinates and structure factors for all structures have been deposited at the Protein Data Bank. The accession codes are given in Table I.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank Dr Man-Sun Sy for kindly providing monoclonal antibody 7A12 and Dr David Vanik for providing protein for initial experiments. Data were measured at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source, and at beamline X25 of the National Synchrotron Light Source. Argonne is operated by UChicago Argonne, LLC, for the US Department of Energy, Office of Biological and Environmental Research. Financial support for NSLS comes principally from the Offices of Biological and Environmental Research, and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources of the NIH. This work was supported by a Brain Disorders Award from the McKnight Endowment Fund for Neurosciences, and NIH grant DK075897 to VCY, who is an Established Investigator of the American Heart Association.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aguzzi A, Baumann F, Bremer J (2008) The prion's elusive reason for being. Annu Rev Neurosci 31: 439–477 [DOI] [PubMed] [Google Scholar]
- Alpérovitch A, Zerr I, Pocchiari M, Mitrova E, de Pedro Cuesta J, Hegyi I, Collins S, Kretzschmar H, van Dujin C, Will RG (1999) Codon 129 prion protein genotype and sporadic Creutzfeldt–Jakob disease. Lancet 353: 1673–1674 [DOI] [PubMed] [Google Scholar]
- Antonyuk SV, Trevitt CR, Strange RW, Jackson GS, Sangar D, Batchelor M, Cooper S, Fraser C, Jones S, Georgiou T, Khalili-Shirazi A, Clarke AR, Hasnain SS, Collinge J (2009) Crystal structure of human prion protein bound to a therapeutic antibody. Proc Natl Acad Sci USA 106: 2554–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetri AC, Vanik DL, Surewicz WK (2005) Polymorphism at residue 129 modulates the conformational conversion of the D178N variant of human prion protein 90–231. Biochemistry 44: 15880–15888 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Sawaya MR, Eisenberg D (2006) Deposition diseases and 3D domain swapping. Structure 14: 811–824 [DOI] [PubMed] [Google Scholar]
- Brandel JP, Heath CA, Head MW, Levavasseur E, Knight R, Laplanche JL, Langeveld JP, Ironside JW, Hauw JJ, Mackenzie J, Alpérovitch A, Will RG, Haïk S (2009) Variant Creutzfeldt–Jakob disease in France and the United Kingdom: evidence for the same agent strain. Ann Neurol 65: 249–256 [DOI] [PubMed] [Google Scholar]
- Calzolai L, Lysek DA, Güntert P, von Schroetter C, Riek R, Zahn R, Wüthrich K (2000) NMR structures of three single-residue variants of the human prion protein. Proc Natl Acad Sci USA 97: 8340–8345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenáková L, Goldfarb LG, Garruto R, Lee HS, Gajdusek DC, Brown P (1998) Phenotype-genotype studies in kuru: implications for new variant Creutzfeldt–Jakob disease. Proc Natl Acad Sci USA 95: 13239–13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308: 1435–1439 [DOI] [PubMed] [Google Scholar]
- Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24: 519–550 [DOI] [PubMed] [Google Scholar]
- Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318: 930–936 [DOI] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB III, Snoeyink J, Richardson JS, Richardson DC (2007) MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res 35: W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy SR, Hsiao K, Farlow MR, Foroud T, Conneally PM, Johnson P, Prusiner SB, Hodes ME, Ghetti B (1992) Linkage of the Indiana kindred of Gerstmann–Sträussler–Scheinker disease to the prion protein gene. Nat Genet 1: 64–67 [DOI] [PubMed] [Google Scholar]
- Eghiaian F, Grosclaude J, Lesceu S, Debey P, Doublet B, Tréquer E, Rezaei H, Knossow M (2004) Insight into the PrPC → PrPSc conversion from the structures of antibody-bound ovine prion scrapie-susceptibility variants. Proc Natl Acad Sci USA 101: 10254–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Petersen RB, Tabaton M, Brown P, LeBlanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, Haltia M, Wills PR, Hauw JJ, McKeever PE, Monari L, Schrank B, Swergold GD, Autilio-Gambettie L, Gajdusek DC, Lugaresi E et al. (1992) Fatal familial insomnia and familial Creutzfeldt–Jakob disease: disease phenotype determined by a DNA polymorphism. Science 258: 806–808 [DOI] [PubMed] [Google Scholar]
- Gossert AD, Bonjour S, Lysek DA, Fiorito F, Wüthrich K (2005) Prion protein NMR structures of elk and mouse/elk hybrids. Proc Natl Acad Sci USA 102: 646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire LF, Whyte SM, Vasisht N, Gill AC, Verma C, Dodson EJ, Dodson GG, Bayley PM (2004) The crystal structure of the globular domain of sheep prion protein. J Mol Biol 336: 1175–1183 [DOI] [PubMed] [Google Scholar]
- Hornemann W, Schorn C, Wüthrich K (2004) NMR structure of the bovine prion protein isolated from healthy calf brains. EMBO Rep 5: 1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosszu LL, Jackson GS, Trevitt CR, Jones S, Batchelor M, Bhelt D, Prodromidou K, Clarke AR, Waltho JP, Collinge J (2004) The residue 129 polymorphism in human prion protein does not confer susceptibility to Creutzfeldt–Jakob disease by altering the structure or global stability of PrPC. J Biol Chem 279: 28515–28521 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Dlouhy SR, Farlow MR, Cass C, Costa MD, Conneally PM, Hodes ME, Ghetti B, Prusiner SB (1992) Mutant prion proteins in Gerstmann–Sträussler–Scheinker disease with neurofibrillary tangles. Nat Genet 1: 68–71 [DOI] [PubMed] [Google Scholar]
- Kaneko K, Zulianello L, Scott M, Cooper CM, Wallace AC, James TL, Cohen FE, Prusiner SB (1997) Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc Natl Acad Sci USA 94: 10069–10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger CR, Gehlhaar DK, Fogel DB (1999) Rapid automated molecular replacement by evolutionary search. Acta Crystallogr D 55: 484–491 [DOI] [PubMed] [Google Scholar]
- Knaus KJ, Morillas M, Swietnicki W, Malone M, Surewicz WK, Yee VC (2001) Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat Struct Biol 8: 770–774 [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26: 283–291 [Google Scholar]
- Li R, Liu T, Wong BS, Pan T, Morillas M, Swietnicki W, O'Rourke K, Gambetti P, Surewicz WK, Sy MS (2000) Identification of an epitope in the C terminus of normal prion protein whose expression is modulated by binding events in the N terminus. J Mol Biol 301: 567–573 [DOI] [PubMed] [Google Scholar]
- Liemann S, Glockshuber R (1999) Influence of amino acid substitutions related to inherited human prion diseases on the thermodynamic stability of the cellular prion protein. Biochemistry 38: 3258–3267 [DOI] [PubMed] [Google Scholar]
- Liu H, Farr-Jones S, Ulyanov NB, Llinas M, Marqusee S, Groth D, Cohen FE, Prusiner SB, James TL (1999) Solution structure of Syrian hamster prion protein rPrP(90–231). Biochemistry 38: 5362–5377 [DOI] [PubMed] [Google Scholar]
- López Garcia F, Zahn R, Riek R, Wüthrich K (2000) NMR structure of the bovine prion protein. Proc Natl Acad Sci USA 97: 8334–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillas M, Swietnicki W, Gambetti P, Surewicz WK (1999) Membrane environment alters the conformational structure of the recombinant human prion protein. J Biol Chem 274: 36859–36865 [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D 53: 240–255 [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymol 276: 307–325 [DOI] [PubMed] [Google Scholar]
- Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95: 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek R, Wider G, Billeter M, Hornemann S, Glockshuber R, Wüthrich K (1998) Prion protein NMR structure and familial human spongiform encephalopathies. Proc Natl Acad Sci USA 95: 11667–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson CJ, Nilsson KP, Hornemann S, Heikenwalder M, Manco G, Schwarz P, Ott D, Rülicke T, Liberski PP, Julius C, Falsig J, Stitz L, Wüthrich K, Aguzzi A (2009) De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci USA 106: 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietnicki W, Petersen RB, Gambetti P, Surewicz WK (1998) Familial mutations and the thermodynamic stability of the recombinant human prion protein. J Biol Chem 273: 31048–31052 [DOI] [PubMed] [Google Scholar]
- Tahiri-Alaoui A, James W (2005) Rapid formation of amyloid from α-monomeric recombinant human PrP in vitro. Protein Sci 14: 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A, Rogers M, Borchelt DR, McKinley MP, Scott M, Serban D, Prusiner SB (1990) Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc Natl Acad Sci USA 87: 8262–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83: 79–90 [DOI] [PubMed] [Google Scholar]
- Terwilliger TC (2000) Maximum-likelihood density modification. Acta Crystallogr D 56: 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J (1999) Automated MAD and MIR structure solution. Acta Crystallogr D 55: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzi NL, Cancellotti E, Baybutt H, Blackford L, Bradford B, Plinston C, Coghill A, Hart P, Piccardo P, Barron RM, Manson JC (2008) Host PrP glycosylation: a major factor determining the outcome of prion infection. PLoS Biol 6: e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A (1997) MOLREP: an automated program for molecular replacement. J Appl Crystallogr 30: 1022–1025 [Google Scholar]
- Wüthrich K, Riek R (2001) Three-dimensional structures of prion proteins. Adv Protein Chem 57: 55–82 [DOI] [PubMed] [Google Scholar]
- Zahn R, Liu A, Lührs T, Riek R, von Schroetter C, López Garcia F, Billeter M, Calzolai L, Wider G, Wüthrich K (2000) NMR solution structure of the human prion protein. Proc Natl Acad Sci USA 97: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, von Schroetter C, Wüthrich K (1997) Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS Lett 417: 400–404 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Swietnicki W, Zagorski MG, Surewicz WK, Sönnichsen FD (2000) Solution structure of the E200K variant of human prion protein. Implications for the mechanism of pathogenesis in familial prion diseases. J Biol Chem 275: 33650–33654 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File