Abstract
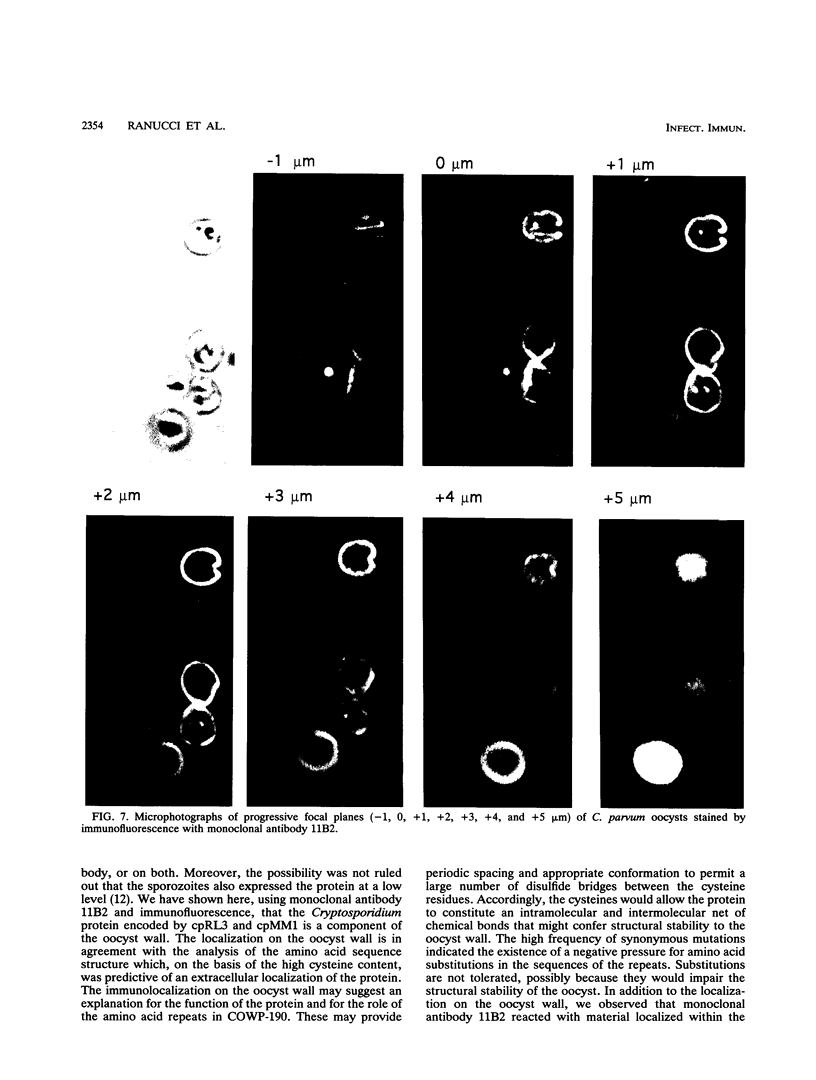
The oocyst wall is one of the components that permits cryptosporidia both to survive in the environment and to retain infectivity. With the aim of identifying Cryptosporidium proteins specifically expressed at the oocyst stage, we screened lambda gt11 genomic libraries of Cryptosporidium parvum with both an oocyst antiserum and a specific genetic probe. We isolated, from distinct libraries, two overlapping clones containing an open reading frame encoding a 1,252-amino-acid polypeptide. The analysis of the deduced amino acid sequence revealed unusually high contents of cysteine, proline, and histidine. The sequence was also characterized by two distinct amino acid motifs, each repeated several times. The DNA sequences coding for the amino acid repeats showed a high frequency of synonymous mutations, a result suggesting that the repeated motifs may be functionally and/or structurally important to the parasite. Antisera and monoclonal antibodies developed against a recombinant polypeptide encompassing the first 786 amino acids revealed that the corresponding protein in C. parvum had an apparent molecular weight of 190,000. Moreover, confocal microscopy analysis with immunofluorescence indicated that the protein was localized on the oocyst wall as a uniform stain and within the oocyst itself as bright granules in close association with the residual body.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnin A., Dubremetz J. F., Camerlynck P. Characterization and immunolocalization of an oocyst wall antigen of Cryptosporidium parvum (Protozoa: Apicomplexa). Parasitology. 1991 Oct;103(Pt 2):171–177. doi: 10.1017/s0031182000059448. [DOI] [PubMed] [Google Scholar]
- Casemore D. P., Sands R. L., Curry A. Cryptosporidium species a "new" human pathogen. J Clin Pathol. 1985 Dec;38(12):1321–1336. doi: 10.1136/jcp.38.12.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F. G., Vermund S. H. Human cryptosporidiosis. Crit Rev Microbiol. 1988;16(2):113–159. doi: 10.3109/10408418809104469. [DOI] [PubMed] [Google Scholar]
- Flanigan T. P., Aji T., Marshall R., Soave R., Aikawa M., Kaetzel C. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect Immun. 1991 Jan;59(1):234–239. doi: 10.1128/iai.59.1.234-239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff E. N., Smith P. D. Perspectives on gastrointestinal infections in AIDS. Gastroenterol Clin North Am. 1988 Sep;17(3):451–463. [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Kim K., Goozé L., Petersen C., Gut J., Nelson R. G. Isolation, sequence and molecular karyotype analysis of the actin gene of Cryptosporidium parvum. Mol Biochem Parasitol. 1992 Jan;50(1):105–113. doi: 10.1016/0166-6851(92)90248-i. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lally N. C., Baird G. D., McQuay S. J., Wright F., Oliver J. J. A 2359-base pair DNA fragment from Cryptosporidium parvum encoding a repetitive oocyst protein. Mol Biochem Parasitol. 1992 Nov;56(1):69–78. doi: 10.1016/0166-6851(92)90155-d. [DOI] [PubMed] [Google Scholar]
- Ludin C. M., Afifi S. A., Hasenan N., Maimunah A., Anuar A. K. Cryptosporidiosis among children with acute gastroenteritis in the pediatric ward in the General Hospital, Penang. Southeast Asian J Trop Med Public Health. 1991 Jun;22(2):200–202. [PubMed] [Google Scholar]
- Lumb R., Lanser J. A., O'Donoghue P. J. Electrophoretic and immunoblot analysis of Cryptosporidium oocysts. Immunol Cell Biol. 1988 Oct-Dec;66(Pt 5-6):369–376. doi: 10.1038/icb.1988.48. [DOI] [PubMed] [Google Scholar]
- McDonald V., Deer R. M., Nina J. M., Wright S., Chiodini P. L., McAdam K. P. Characteristics and specificity of hybridoma antibodies against oocyst antigens of Cryptosporidium parvum from man. Parasite Immunol. 1991 May;13(3):251–259. doi: 10.1111/j.1365-3024.1991.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Nina J. M., McDonald V., Dyson D. A., Catchpole J., Uni S., Iseki M., Chiodini P. L., McAdam K. P. Analysis of oocyst wall and sporozoite antigens from three Cryptosporidium species. Infect Immun. 1992 Apr;60(4):1509–1513. doi: 10.1128/iai.60.4.1509-1513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozio E., Gomez Morales M. A., Barbieri F. M., La Rosa G. Cryptosporidium: different behaviour in calves of isolates of human origin. Trans R Soc Trop Med Hyg. 1992 Nov-Dec;86(6):636–638. doi: 10.1016/0035-9203(92)90165-9. [DOI] [PubMed] [Google Scholar]
- Richardson A. J., Frankenberg R. A., Buck A. C., Selkon J. B., Colbourne J. S., Parsons J. W., Mayon-White R. T. An outbreak of waterborne cryptosporidiosis in Swindon and Oxfordshire. Epidemiol Infect. 1991 Dec;107(3):485–495. doi: 10.1017/s0950268800049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Hall J. R., Jennings M. W., Harris T. J., Marsh K., Newbold C. I., Tate V. E., Weatherall D. J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988 Sep 1;335(6185):79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Sterba F., Sulcová I. Závaznost výskytu kokcidií Cryptosporidium sp. v etiopatogenezi neonatálního diarhoického syndromu a enteritid telat na základe soubezného histopatologického a parazitologického vysetrení. Vet Med (Praha) 1989 Jan;34(1):13–26. [PubMed] [Google Scholar]
- Stüber D., Bannwarth W., Pink J. R., Meloen R. H., Matile H. New B cell epitopes in the Plasmodium falciparum malaria circumsporozoite protein. Eur J Immunol. 1990 Apr;20(4):819–824. doi: 10.1002/eji.1830200416. [DOI] [PubMed] [Google Scholar]
- Waldman E., Tzipori S., Forsyth J. R. Separation of Cryptosporidium species oocysts from feces by using a percoll discontinuous density gradient. J Clin Microbiol. 1986 Jan;23(1):199–200. doi: 10.1128/jcm.23.1.199-200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]