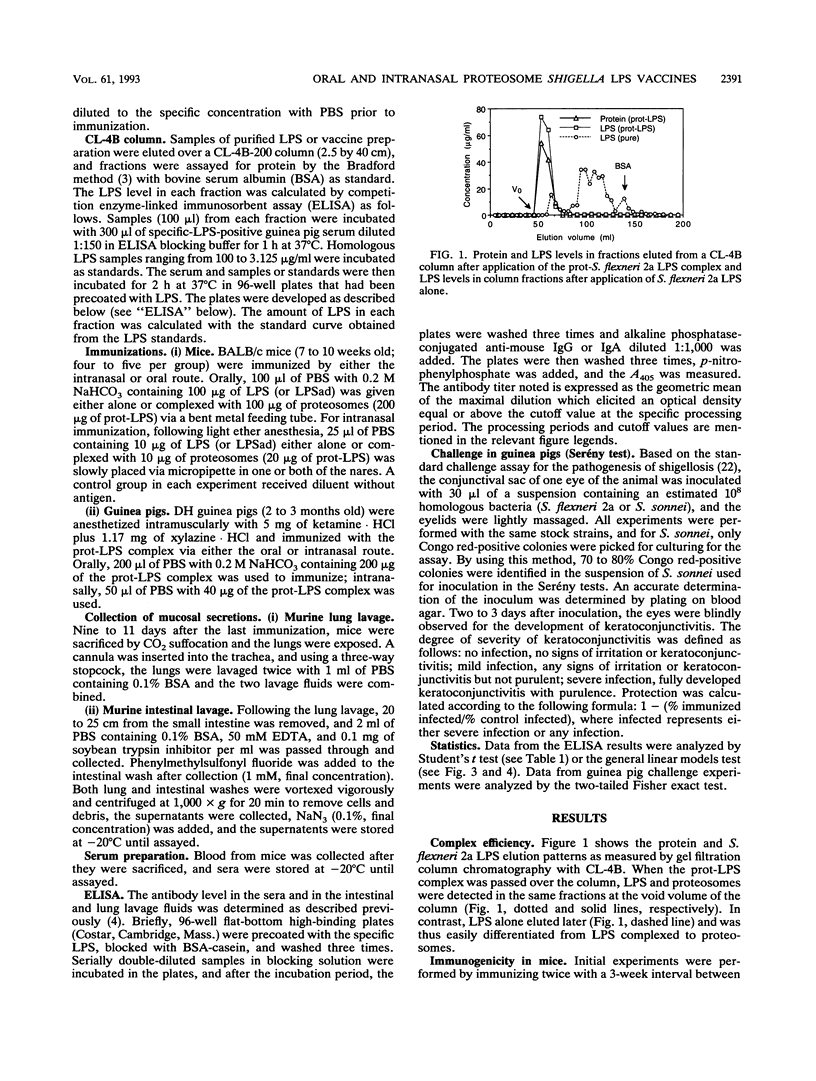
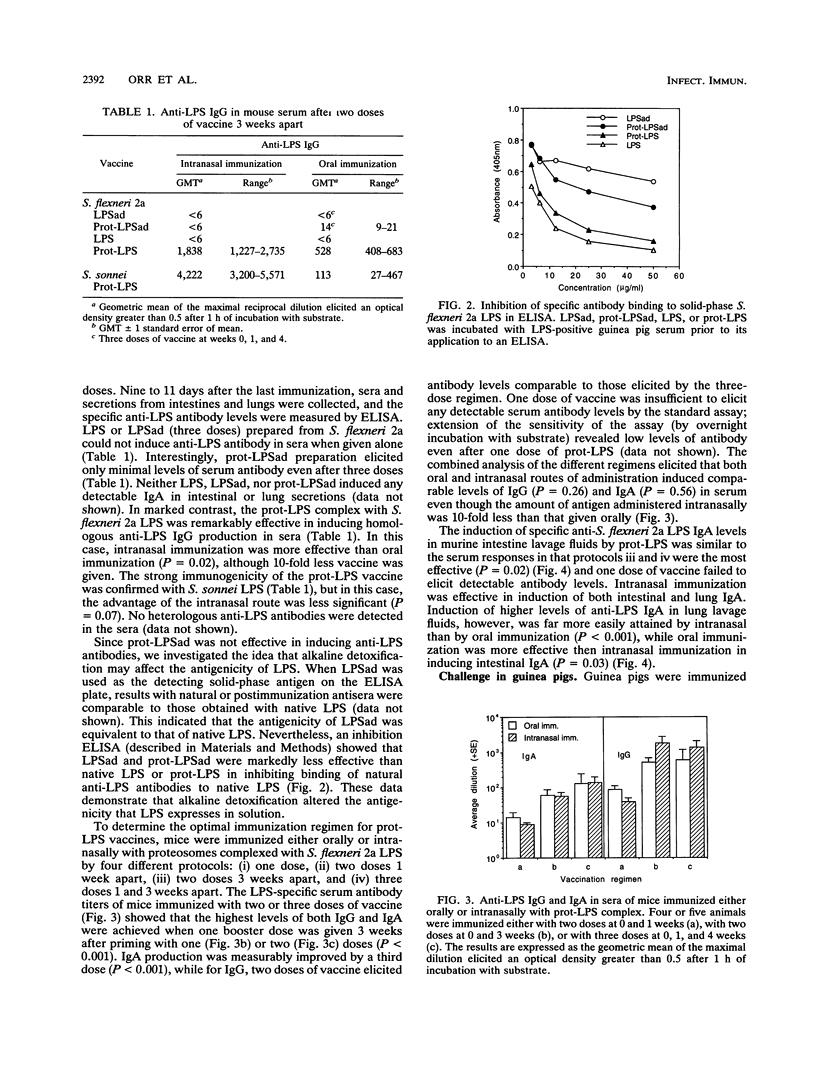
Abstract
Immunity against shigellosis has been shown to correlate with the presence of antibodies specific for Shigella lipopolysaccharide (LPS). We here propose a new candidate vaccine for shigellosis composed of purified Shigella flexneri 2a or Shigella sonnei LPS hydrophobically complexed with group C type 2b Neisseria meningitidis outer membrane protein proteosomes. Immunization of mice either orally or intranasally with this complex induced specific homologous anti-LPS antibodies in both intestinal and respiratory secretions as well as in sera. Strong anamnestic responses were found after two or three immunizations. LPS alone, alkaline-detoxified LPS, or alkaline-detoxified LPS complexed with proteosomes was not effective. Oral or intranasal immunization of guinea pigs with two or more doses of this proteosome-LPS vaccine elicited homologous protection against Shigella keratoconjunctivitis (Serény test). These data demonstrate that proteosomes can be used as an effective mucosal vaccine delivery system and that orally or intranasally administered acellular vaccines can protect against Shigella infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. E., Levine M. M., Clements M. L., Losonsky G., Herrington D., Berman S., Formal S. B. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987 Jun;155(6):1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen D., Green M. S., Block C., Rouach T., Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988 May;157(5):1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- Eldridge J. H., Meulbroek J. A., Staas J. K., Tice T. R., Gilley R. M. Vaccine-containing biodegradable microspheres specifically enter the gut-associated lymphoid tissue following oral administration and induce a disseminated mucosal immune response. Adv Exp Med Biol. 1989;251:191–202. doi: 10.1007/978-1-4757-2046-4_18. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Maenza R. M., Austin S., LaBrec E. H. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol Med. 1967 Jun;125(2):347–349. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- HIGGINS A. R., FLOYD T. M., KADER M. A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am J Trop Med Hyg. 1955 Mar;4(2):281–288. [PubMed] [Google Scholar]
- Hartman A. B., Powell C. J., Schultz C. L., Oaks E. V., Eckels K. H. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991 Nov;59(11):4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Van de Verg L., Formal S. B., Hale T. L., Tall B. D., Cryz S. J., Tramont E. C., Levine M. M. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990 Aug;8(4):353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Kotloff K. L., Herrington D. A., Hale T. L., Newland J. W., Van De Verg L., Cogan J. P., Snoy P. J., Sadoff J. C., Formal S. B., Levine M. M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992 Jun;60(6):2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramp W. J., Six H. R., Kasel J. A. Postimmunization clearance of liposome entrapped adenovirus type 5 hexon. Proc Soc Exp Biol Med. 1982 Jan;169(1):135–139. doi: 10.3181/00379727-169-41321. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., Ballou W. R., Smith L. F., Wirtz R. A., Zollinger W. D., Hockmeyer W. T. Proteosome-lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science. 1988 May 6;240(4853):800–802. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Meitert T., Pencu E., Ciudin L., Tonciu M. Vaccine strain Sh. flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen (Sh. flexneri T32-Istrati). Arch Roum Pathol Exp Microbiol. 1984 Jul-Dec;43(3-4):251–278. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Stein K. E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992 Jun;165 (Suppl 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Nencioni L., Villa L., Keren D. F., Lowell G. H., Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature. 1983 Nov 10;306(5939):184–186. doi: 10.1038/306184a0. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Villa L., De Magistris M. T., Romano M., Silvestri S., Boraschi D., Nencioni L. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J Immunol. 1986 Sep 1;137(5):1504–1510. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aizpurua H. J., Russell-Jones G. J. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988 Feb 1;167(2):440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]


