Abstract
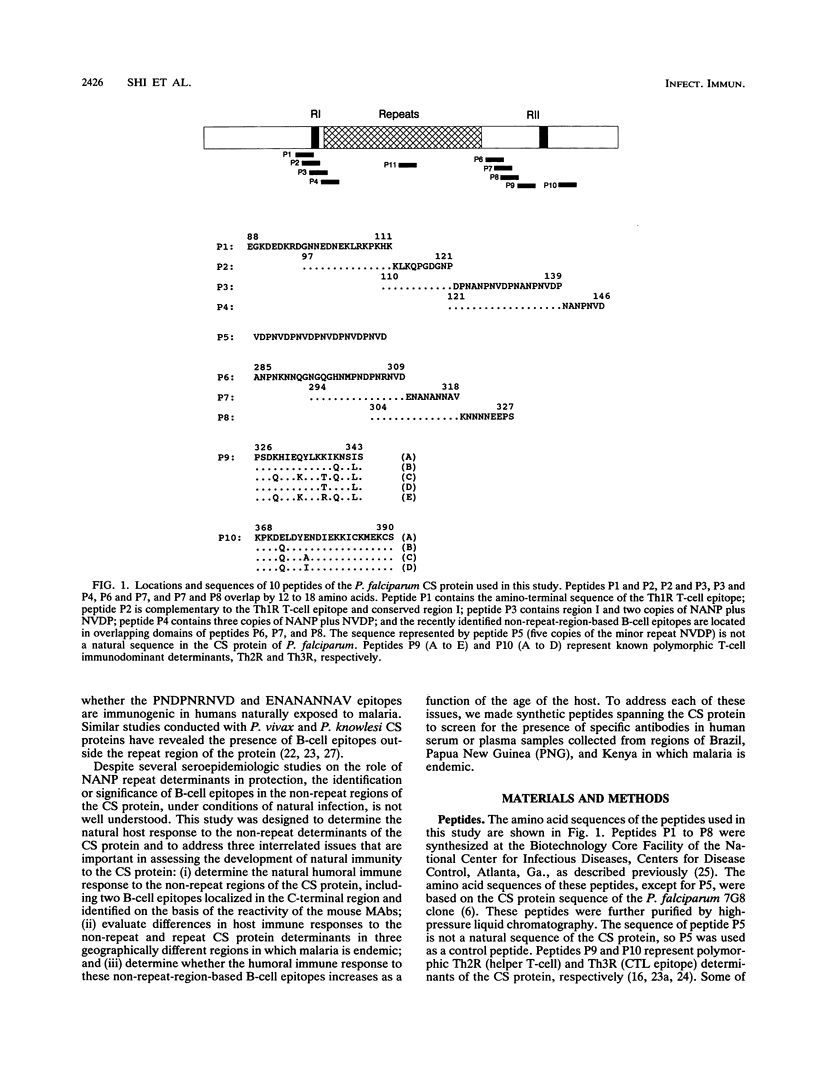
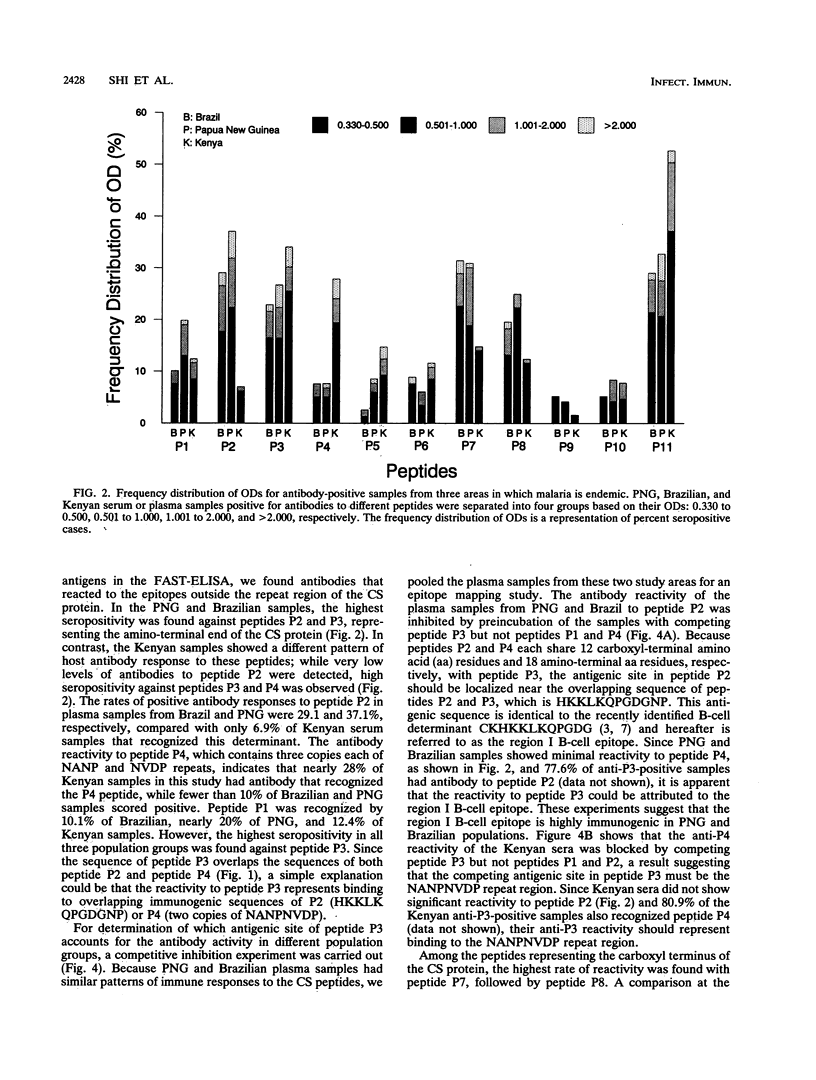
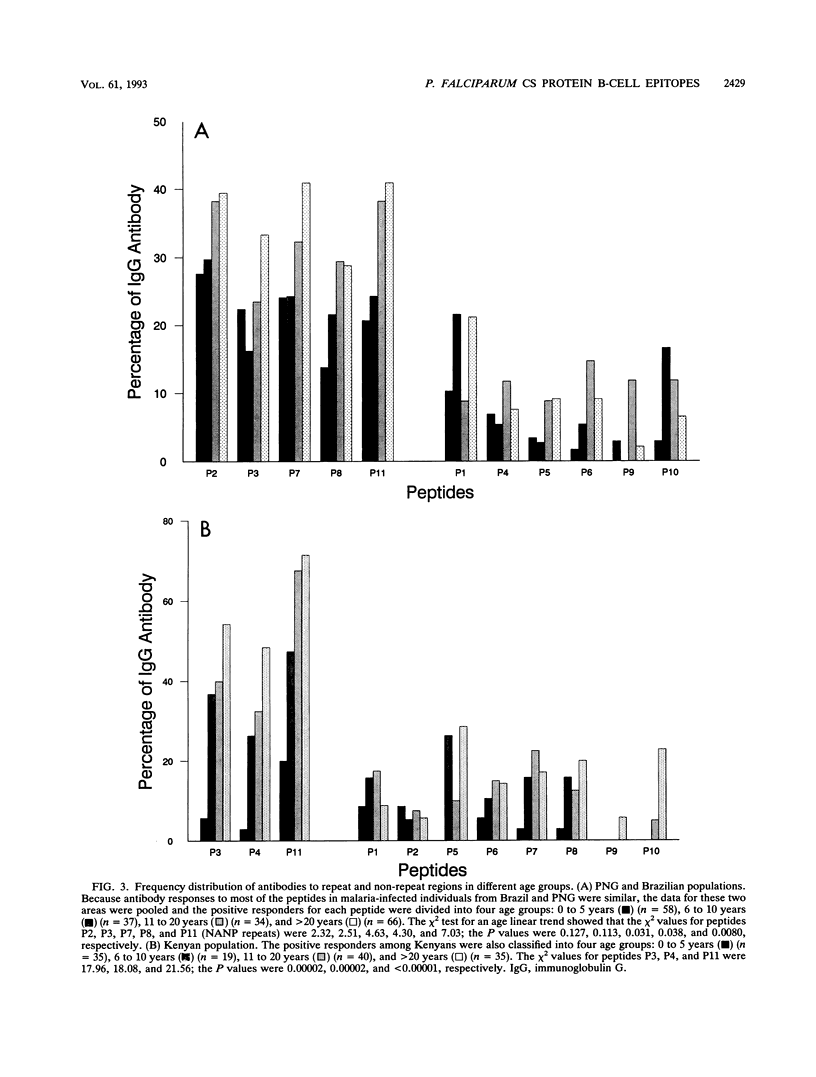
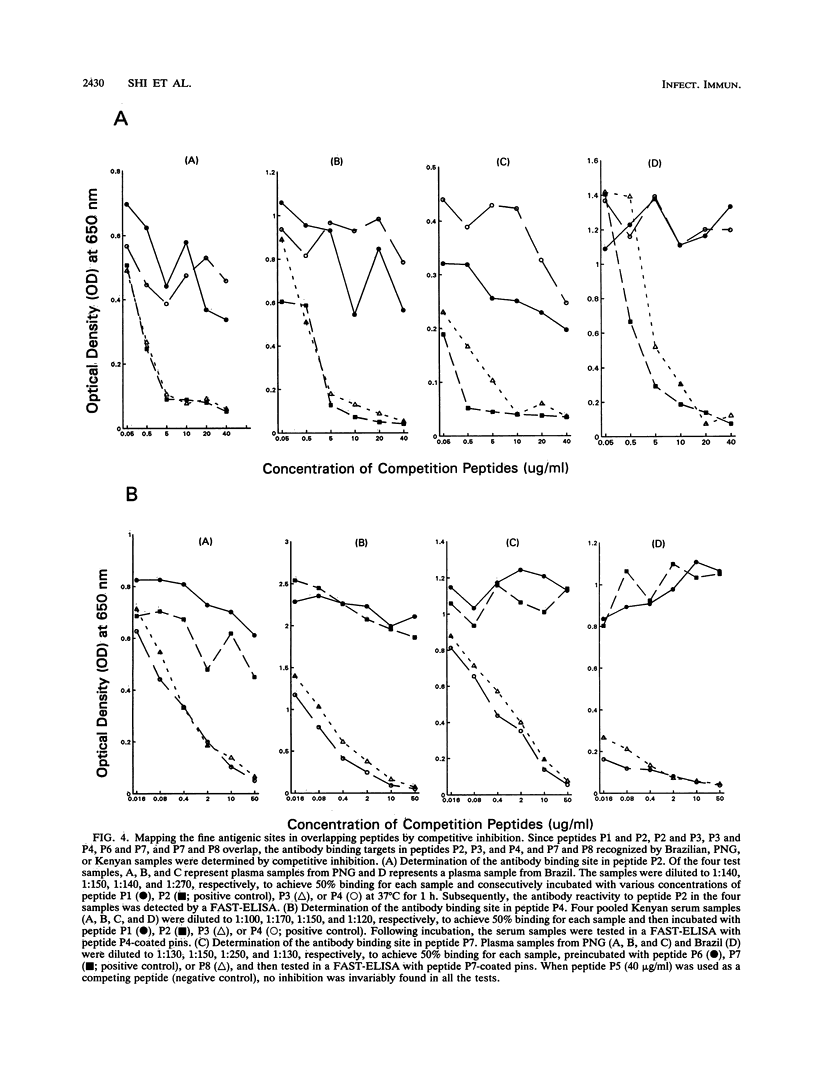
Synthetic peptides and human serum or plasma samples from regions of Brazil, Papua New Guinea, and Kenya in which malaria is endemic were used to identify B-cell epitopes localized outside the repeat region of the circumsporozoite (CS) protein of the human malaria parasite Plasmodium falciparum. In agreement with recent observations, our results confirm the presence of two non-repeat-region-based B-cell epitopes of the CS protein. Of these two epitopes, only the region I epitope (KPKHKKLKQPGDGNP) was previously shown to be recognized by human sera. In this study, we show that human immune sera from malarious regions recognize another B-cell epitope, ENANANNAV, that resides carboxyl to the repeat region. The present study reveals that (i) the repeat-sequence (NANP)-based B-cell epitope of the CS protein is an immunogenic but not immunodominant epitope; (ii) the natural expression of antibody responses to the two non-repeat-region-based B-cell epitopes of the CS protein varies in different populations in which malaria is endemic; (iii) although the host immune responses to the non-repeat-region-based B-cell epitopes increase as a function of host age, this increase is not statistically significant for the region I epitope but is significant for the other epitope; and (iv) the Th1R T-cell site but not the Th2R or Th3R T-cell site induces an antibody response in the human host. This study confirms the immunogenic potential of non-repeat-region-based B-cell epitopes and suggests that antibody pressures may also contribute to the maintenance of the antigenic diversity of the CS protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Bates M. D., Tam J. P., Hollingdale M. R. Synthetic peptides from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium knowlesi recognize the human hepatoma cell line HepG2-A16 in vitro. J Exp Med. 1986 Dec 1;164(6):1915–1922. doi: 10.1084/jem.164.6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Wirtz R. A., Brabin B. J., Battistutta D., Cattani J. A., Maizels R. M., Alpers M. P. Differential antibody responses to Plasmodium falciparum and P. vivax circumsporozoite proteins in a human population. J Clin Microbiol. 1989 Jun;27(6):1346–1351. doi: 10.1128/jcm.27.6.1346-1351.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C., Dupont A., Akue J. P., Kaufmann M. H., Verdini A. S., Pessi A., Del Giudice G. Natural antibodies against three distinct and defined antigens of Plasmodium falciparum in residents of a mesoendemic area in Gabon. Am J Trop Med Hyg. 1988 Aug;39(2):150–156. doi: 10.4269/ajtmh.1988.39.150. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cheng Q., Mazier D., Berbiguier N., Cooper J. A., Engers H. D., Chizzolini C., Verdini A. S., Bonelli F., Pessi A. Immunogenicity of a non-repetitive sequence of Plasmodium falciparum circumsporozoite protein in man and mice. Immunology. 1988 Feb;63(2):187–191. [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Felix A. M., Gillessen D., Heimer E. P., Just M., Pink J. R., Sinigaglia F., Stürchler D., Takacs B., Trzeciak A. Assessment in humans of a synthetic peptide-based vaccine against the sporozoite stage of the human malaria parasite, Plasmodium falciparum. J Immunol. 1988 Jan 15;140(2):626–633. [PubMed] [Google Scholar]
- Fries L. F., Gordon D. M., Schneider I., Beier J. C., Long G. W., Gross M., Que J. U., Cryz S. J., Sadoff J. C. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect Immun. 1992 May;60(5):1834–1839. doi: 10.1128/iai.60.5.1834-1839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. Development and optimization of the FAST-ELISA for detecting antibodies to Schistosoma mansoni. J Immunol Methods. 1986 Sep 27;92(2):167–176. doi: 10.1016/0022-1759(86)90162-6. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Wistar R., Jr, Ballou W. R., Hollingdale M. R., Wirtz R. A., Schneider I., Marwoto H. A., Hockmeyer W. T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med. 1986 Sep 4;315(10):601–606. doi: 10.1056/NEJM198609043151001. [DOI] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Lockyer M. J., Marsh K., Newbold C. I. Wild isolates of Plasmodium falciparum show extensive polymorphism in T cell epitopes of the circumsporozoite protein. Mol Biochem Parasitol. 1989 Dec;37(2):275–280. doi: 10.1016/0166-6851(89)90159-x. [DOI] [PubMed] [Google Scholar]
- Marsh K., Hayes R. H., Carson D. C., Otoo L., Shenton F., Byass P., Zavala F., Greenwood B. M. Anti-sporozoite antibodies and immunity to malaria in a rural Gambian population. Trans R Soc Trop Med Hyg. 1988;82(4):532–537. doi: 10.1016/0035-9203(88)90495-6. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Wheeler J. G., Blackman M. J., Bennett S., Takacs B., Schönfeld H. J., Holder A. A., Greenwood B. M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992 May;14(3):321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Romero P., Heimer E. P., Herrera S., Felix A. M., Nussenzweig R. S., Zavala F. Antigenic analysis of the repeat domain of the circumsporozoite protein of Plasmodium vivax. J Immunol. 1987 Sep 1;139(5):1679–1682. [PubMed] [Google Scholar]
- Sharma S., Gwadz R. W., Schlesinger D. H., Godson G. N. Immunogenicity of the repetitive and nonrepetitive peptide regions of the divergent CS protein of Plasmodium knowlesi. J Immunol. 1986 Jul 1;137(1):357–361. [PubMed] [Google Scholar]
- Shi Y. P., Alpers M. P., Povoa M. M., Lal A. A. Diversity in the immunodominant determinants of the circumsporozoite protein of Plasmodium falciparum parasites from malaria-endemic regions of Papua New Guinea and Brazil. Am J Trop Med Hyg. 1992 Dec;47(6):844–851. doi: 10.4269/ajtmh.1992.47.844. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bannwarth W., Pink J. R., Meloen R. H., Matile H. New B cell epitopes in the Plasmodium falciparum malaria circumsporozoite protein. Eur J Immunol. 1990 Apr;20(4):819–824. doi: 10.1002/eji.1830200416. [DOI] [PubMed] [Google Scholar]
- Vergara U., Gwadz R., Schlesinger D., Nussenzweig V., Ferreira A. Multiple non-repeated epitopes on the circumsporozoite protein of Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Mar;14(3):283–292. doi: 10.1016/0166-6851(85)90056-8. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]


