Abstract
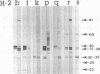
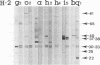
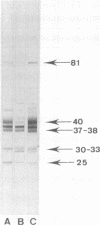
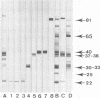
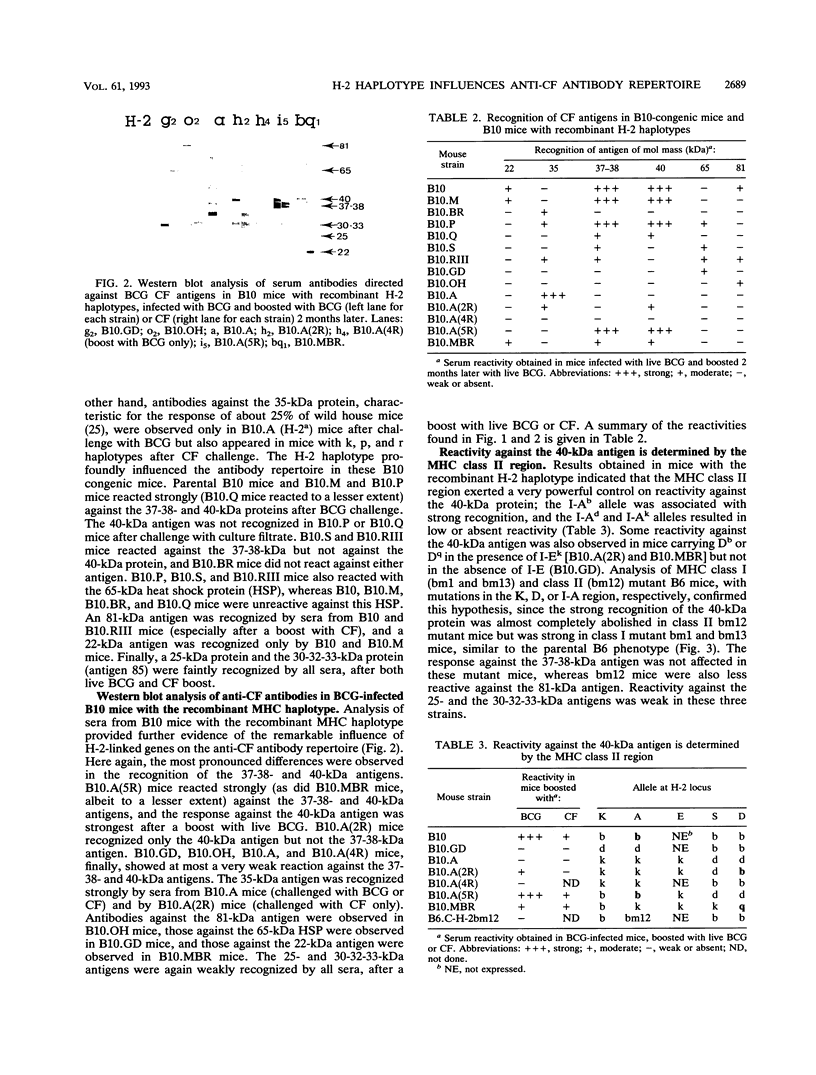
C57BL/10 and C57BL/6 mice (H-2b); B10 congenic mice with f, k, p, q, r, and s H-2 haplotypes; B10 mice with recombinant g2, o2, a, h2, h4, i5, and bq1 H-2 haplotypes; and B6 mice with major histocompatibility complex (MHC) mutant bm1 and bm13 (class I) and bm12 (class II) haplotypes were infected intravenously with 4 x 10(6) CFU of live Mycobacterium bovis BCG and examined by Western immunoblot analysis for serum antibodies against BCG culture filtrate antigens, following a boost injection with live BCG or with BCG culture filtrate. Parental B10 and B6 mice reacted very intensely with three culture filtrate protein bands with estimated molecular masses of 37, 38, and 40 kDa. Response against the 40-kDa protein was stronger following a boost injection with live BCG than following a boost with culture filtrate. Sera from mice with f, p, i5, bm1, and bm13 haplotypes reacted strongly, with both the 37-38- and 40-kDa antigens, and sera from mice with q and bq1 haplotypes showed a somewhat weaker reaction. Sera from mice with r, s, and bm12 haplotypes reacted against the 37-38-kDa antigen but not against the 40-kDa antigen, and sera from mice with the h2 haplotype reacted only with the 40-kDa antigen but not with the 37-38-kDa antigen. Sera from mice with the k, g2, o2, a, and h4 haplotypes showed, at most, a very weak reaction with the 37-38- and 40-kDa antigens. These results demonstrate that MHC genes profoundly affect the antibody repertoire used against culture filtrate antigens in mice infected with live M. bovis BCG. In particular, as shown in mice with the recombinant H-2 haplotype and in class II mutant bm12 mice, the I-A heterodimer controls the recognition of the immunodominant 40-kDa antigen. By using crossed immunoelectrophoresis, this 40-kDa antigen was identified as antigen 88 according to the reference system of Closs et al. for BCG antigens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeleye T. A., Colston M. J., Butler R., Jenner P. J. The antibody repertoire to proteins of Mycobacterium leprae. Genetic influences at the antigen and epitope level. J Immunol. 1991 Sep 15;147(6):1947–1953. [PubMed] [Google Scholar]
- Andersen A. B., Andersen P., Ljungqvist L. Structure and function of a 40,000-molecular-weight protein antigen of Mycobacterium tuberculosis. Infect Immun. 1992 Jun;60(6):2317–2323. doi: 10.1128/iai.60.6.2317-2323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossie A., Vitetta E. S. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell Immunol. 1991 Jun;135(1):95–104. doi: 10.1016/0008-8749(91)90257-c. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Ivanyi J. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology. 1990 Sep;71(1):113–119. [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Brett S., Orrell J. M., Swanson Beck J., Ivanyi J. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology. 1992 May;76(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Cogniaux J., De Schepper N., Blondiau M. L., Cornet B., Horal P., Vahlne A. Characterization of monoclonal antibodies against the p17 core protein of the human immunodeficiency virus 1. J Immunol Methods. 1990 Apr 17;128(2):165–175. doi: 10.1016/0022-1759(90)90207-c. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P., Soares R., Arala-Chaves M. Susceptibility to infection with Mycobacterium avium is paradoxically correlated with increased synthesis of specific anti-bacterial antibodies. Int Immunol. 1991 May;3(5):445–452. doi: 10.1093/intimm/3.5.445. [DOI] [PubMed] [Google Scholar]
- Fifis T., Costopoulos C., Radford A. J., Bacic A., Wood P. R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991 Mar;59(3):800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Forget A., Benoit J. C., Turcotte R., Gusew-Chartrand N. Enhancement activity of anti-mycobacterial sera in experimental Mycobacterium bovis (BCG) infection in mice. Infect Immun. 1976 May;13(5):1301–1306. doi: 10.1128/iai.13.5.1301-1306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Tse H. Y. Insights into immune-response gene function using an Ia mutant mouse strain. Crit Rev Immunol. 1987;7(3):169–191. [PubMed] [Google Scholar]
- Havlir D. V., Wallis R. S., Boom W. H., Daniel T. M., Chervenak K., Ellner J. J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991 Feb;59(2):665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Ljungqvist L., ten Berg R., Van Vooren J. P. Repertoires of antibodies to culture filtrate antigens in different mouse strains infected with Mycobacterium bovis BCG. Infect Immun. 1990 Jul;58(7):2192–2197. doi: 10.1128/iai.58.7.2192-2197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Lenoir C., van Vooren J. P. Antibody repertoire against culture filtrate antigens in wild house mice infected with Mycobacterium bovis BCG. Clin Exp Immunol. 1990 Nov;82(2):369–372. doi: 10.1111/j.1365-2249.1990.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Launois P., Huygen K., De Bruyn J., N'Diaye M., Diouf B., Sarthouj L., Grimaud J., Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991 Nov;86(2):286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. C., Rosenthal A. S., Passmore H. C., Hansen T. H. Selective loss of antigen-specific Ir gene function in IA mutant B6.C-H-2bm12 is an antigen presenting cell defect. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6406–6410. doi: 10.1073/pnas.78.10.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist L., Worsaae A., Heron I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB.B10 and CBA/J mice. Infect Immun. 1988 Aug;56(8):1994–1998. doi: 10.1128/iai.56.8.1994-1998.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliulo E., De Feo V., Stirpe A., Riva C., Scevola D. Enhanced in vitro phagocytic power of macrophages from PPD-stimulated skin sites in human subjects hypersensitive to PPD. Clin Exp Immunol. 1973 Jul;14(3):371–376. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Stélandre M., Bosseloir Y., De Bruyn J., Maes P., Content J. Cloning and sequence analysis of the gene encoding an NADP-dependent alcohol dehydrogenase in Mycobacterium bovis BCG. Gene. 1992 Nov 2;121(1):79–86. doi: 10.1016/0378-1119(92)90164-k. [DOI] [PubMed] [Google Scholar]
- Vordemeier H. M., Harris D. P., Roman E., Lathigra R., Moreno C., Ivanyi J. Identification of T cell stimulatory peptides from the 38-kDa protein of Mycobacterium tuberculosis. J Immunol. 1991 Aug 1;147(3):1023–1029. [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Hasløv K., Heron I., Bennedsen J. Allergenic and blastogenic reactivity of three antigens from Mycobacterium tuberculosis in sensitized guinea pigs. Infect Immun. 1987 Dec;55(12):2922–2927. doi: 10.1128/iai.55.12.2922-2927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal L. P., de Hoop J., Stukart M. J., Gleichmann H., Melvold R. W., Melief C. J. Nonresponsiveness to the male antigen H-Y in H-2 I-A-mutant B6.C-H-2bm12 is not caused by defective antigen presentation. J Immunol. 1983 Feb;130(2):655–660. [PubMed] [Google Scholar]