Abstract
Objective:
To test the hypothesis that lower 25-hydroxyvitamin D [25(OH)D] levels are associated with a greater likelihood of cognitive impairment and risk of cognitive decline.
Methods:
We measured 25(OH)D and assessed cognitive function using the Modified Mini-Mental State Examination (3MS) and Trail Making Test Part B (Trails B) in a cohort of 1,604 men enrolled in the Osteoporotic Fractures in Men Study and followed them for an average of 4.6 years for changes in cognitive function.
Results:
In a model adjusted for age, season, and site, men with lower 25(OH)D levels seemed to have a higher odds of cognitive impairment, but the test for trend did not reach significance (impairment by 3MS: odds ratio [OR] 1.84, 95% confidence interval [CI] 0.81–4.19 for quartile [Q] 1; 1.41, 0.61–3.28 for Q2; and 1.18, 0.50–2.81 for Q3, compared with Q4 [referent group; p trend = 0.12]; and impairment by Trails B: OR 1.66, 95% CI 0.98–2.82 for Q1; 0.96, 0.54–1.69 for Q2; and 1.30, 0.76–2.22 for Q3, compared with Q4 [p trend = 0.12]). Adjustment for age and education further attenuated the relationships. There was a trend for an independent association between lower 25(OH)D levels and odds of cognitive decline by 3MS performance (multivariable OR 1.41, 95% CI 0.89–2.23 for Q1; 1.28, 0.84–1.95 for Q2; and 1.06, 0.70–1.62 for Q3, compared with Q4 [p = 0.10]), but no association with cognitive decline by Trails B.
Conclusion:
We found little evidence of independent associations between lower 25-hydroxyvitamin D level and baseline global and executive cognitive function or incident cognitive decline.
GLOSSARY
- 3MS
= Modified Mini-Mental State Examination;
- 25(OH)D
= 25-hydroxyvitamin D;
- BMI
= body mass index;
- CI
= confidence interval;
- IADL
= instrumental activities of daily living;
- MrOS
= Osteoporotic Fractures in Men;
- OR
= odds ratio;
- PASE
= Physical Activity Scale for the Elderly;
- Q
= quartile;
- Trails B
= Trail Making Test Part B.
The prevalence of 25 hydroxyvitamin D [25(OH)D] deficiency, defined as 25(OH)D level less than 20 ng/mL, is high, especially among the elderly, with 25% to 65% affected.1–6 While much research has focused on the adverse effect of 25(OH)D deficiency on bone health,5,7 associations between 25(OH)D deficiency and non–bone health outcomes, including hypertension,8 cardiovascular morbidity,9 diabetes,10,11 and cancer,12,13 have also been reported. In addition, there is a growing body of literature to support the role of vitamin D in brain function and development.14–25 Despite the experimental and animal evidence supporting an important role for vitamin D in mood and cognition, epidemiologic studies testing this hypothesis are scarce. Cross-sectional studies that examined the association between 25(OH)D levels and cognition were limited by small sample size,26–28 did not control for potential confounding factors,28 had suboptimal analytic methods to measure 25(OH)D levels,6,26–31 or reported conflicting results.30 To our knowledge, there are no prospective cohort studies examining the association between 25(OH)D level and cognitive decline. To test the hypothesis that lower 25(OH)D levels are associated with a greater likelihood of cognitive impairment and risk of cognitive decline, we measured 25(OH)D and assessed cognitive function using the Modified Mini-Mental State Examination (3MS) and Trail Making Test Part B (Trails B) in a cohort of 1,604 community-dwelling men aged 65 years or older who were enrolled in the Osteoporotic Fractures in Men (MrOS) Study and followed them prospectively for an average of 4.6 years for changes in cognitive function.
METHODS
Participants.
The MrOS Study is a multicenter, prospective study of risk factors for vertebral and nonvertebral fractures in older men. The design, measures, and recruitment methods have been described previously.32,33 Briefly, 5,995 men aged 65 years or older were recruited from March 2000 to April 2002 from the populations of Birmingham, Alabama; Minneapolis, Minnesota; the Monongahela Valley, near Pittsburgh, Pennsylvania; Palo Alto, California; Portland, Oregon; and San Diego, California.
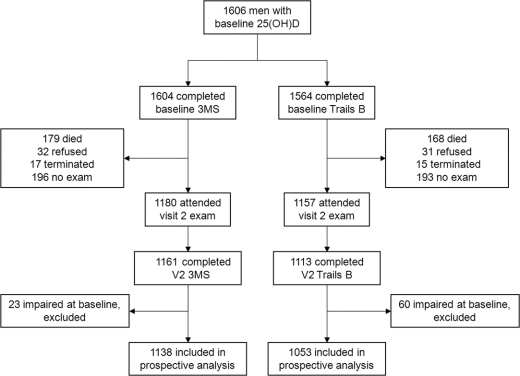
Men were excluded from the study if they could not walk without assistance, had bilateral hip replacements, did not live in or planned to move from the area surrounding the study site, or had a severe medical condition that would preclude participation in follow-up. Serum 25(OH)D measurements were obtained on specimens collected from a random sample of 1,606 participants at baseline examination. Of these, 1,604 participants (99.9%) had cognitive testing with 3MS, and of those, 1,564 participants (97.5%) had cognitive testing with Trails B at baseline examination and were included in the analyses examining the cross-sectional association between 25(OH)D level and odds of cognitive impairment. An average (SD) of 4.6 (0.4) years later, 1,376 men (96.6% of survivors) participated in a second visit including 196 men who completed a mailed questionnaire only and 1,180 men who also attended a second clinic examination during which they underwent cognitive testing.
Standard protocol approvals, registrations, and patient consents.
Approval was obtained from the institutional review boards of the participating institutions, and written informed consent was obtained from all study participants.
Vitamin D assays.
Fasting morning blood was collected, and serum was prepared immediately after phlebotomy and then stored at −70°C. All samples remained frozen until assay. Measures for 25(OH)D2 (ergocalciferol) and 25(OH)D3 (cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described.34 Deuterated stable isotope (d3–25-hydroxyvitamin D) was added to a 0.2-mL serum sample as internal standard. 25(OH)D2, 25(OH)D3, and the internal standard were extracted using acetonitrile precipitation. The extracts were then further purified online and analyzed by liquid chromatography–tandem mass spectrometry using multiple reaction monitoring. 25(OH)D2 and 25(OH)D3 were quantified and reported individually. The minimum detectable limit was 4 ng/mL for 25(OH)D2 and 2 ng/mL for 25(OH)D3. Duplicate pooled serum controls were included in every other assay run. Using the pooled serum, the interassay coefficient of variation (between assays) was 4.4% and the intra-assay coefficient of variation (within assay) was 4.9%. Total 25(OH)D was calculated by adding the 25(OH)D2 and 25(OH)D3 values. We used quartiles of the total vitamin D as the primary predictor. Because exposure to sunlight was expected to influence vitamin D levels, all analyses were adjusted for season and latitude of clinic site. Season of baseline visit was coded as winter (January–March), spring (April–June), summer (July–September), and fall (October–December).
Cognitive testing.
Cognitive function was assessed by a trained technician with the 3MS (primary outcome) and Trails B (secondary outcome) at baseline and at the follow-up examination. The 3MS is a test of global cognitive function, with scores ranging from 0 to 100, with higher scores representing better cognitive function.35 Trails B is a test of executive function. It assesses attention, concentration, psychomotor speed, cognitive shifting, and complex sequencing function by measuring the time required to connect a series of sequentially numbered and lettered circles. Shorter completion times indicate better performance, with test scores affected by age, education, and general intelligence.36 For cross-sectional analyses, prevalent cognitive impairment was defined as having a baseline 3MS score <8037 or a Trails B time greater than 1.5 SD above the mean (>226.5 seconds). For prospective analyses, incident cognitive impairment was defined as having a 3MS score <80 or a decline of 5 points or more37 on the follow-up 3MS (approximately 1 SD change), or having a change in Trails B completion time that was 1 SD or more above the sample's mean change in completion time for those without prevalent impairment at baseline (>50.7 seconds), between the baseline and follow-up examinations. Men with prevalent cognitive impairment at baseline, as defined by a given cognitive test, were excluded from longitudinal analyses examining the association between 25(OH)D level and risk of cognitive decline as defined by that test.
Other measures.
Demographic characteristics included age, education, and race. Lifestyle factors included alcohol consumption, smoking history, and physical activity (Physical Activity Scale for the Elderly [PASE] score).38 Medical history included self-reported comorbid conditions. Diabetes was determined by combining data on self-report, medication usage, and fasting blood glucose. The number of selected medical conditions was calculated, which included history of cardiovascular disease (myocardial infarction, congestive heart failure, angina), diabetes, stroke, hypertension, and chronic obstructive pulmonary disease. Quality-of-life measures included self-rated health and the 12-Item Short Form Health Survey mental component summary (MCS) score.39 Functional status was assessed from information on 5 instrumental activities of daily living (IADLs), which included walking 2 to 3 blocks on level ground, climbing 10 steps, preparing meals, doing heavy housework, and shopping for groceries or clothing.40 Physical measures performed included height (stadiometer) and weight (balance beam or digital scale).41 Height and weight were used to calculate body mass index (BMI) as weight in kilograms divided by the square of height in meters.
Statistical analysis.
Differences in baseline characteristics according to the quartile of 25(OH)D level were compared using χ2 tests for categorical variables, analysis of variance for continuous variables with normal distributions, and Kruskal-Wallis tests for variables with skewed distributions. To examine the association between baseline 25(OH)D level and odds of cognitive impairment and decline, we used logistic regression models. We expressed 25(OH)D level as quartiles (and used quartile 4 as a reference) and, in secondary analyses, as a dichotomous variable (≤19.9 ng/mL and >20 ng/mL) and as a continuous variable. Because the results of the secondary analyses were not different from those that expressed 25(OH)D as quartiles, only the results of the primary analyses are reported. Analyses were first minimally adjusted for age, clinic site, and season of blood draw. Next, these analyses were further adjusted for ethnicity and education. The final multivariable models included covariates that were significantly associated with 25(OH)D levels at baseline with a p < 0.1 or known risk factors for cognitive impairment in the MrOS cohort; age, clinic site, race/ethnicity, education, self-reported health status, IADL impairments, smoking, alcohol consumption, BMI, and physical activity were included in multivariable models. In addition, because lower 25(OH)D levels are associated with darker skin, we performed analyses limited to white men.
RESULTS
The mean (SD) baseline 3MS score was 93.2 (6.4), and the mean (SD) time to completion of Trails B was 136.1 (60.3) seconds. Compared with 1,564 men who completed Trails B examination and 3MS testing and had 25(OH)D levels, those who did not complete the baseline Trails B examination (n = 40) were on average older (76.3 vs 73.7 years, p = 0.008), were less likely to be white (80% vs 90%, p = 0.06), were less educated (52.5% vs 75.8% with some college education or beyond, p < 0.001), drank less alcohol (2.9 vs 4.6 drinks per week, p = 0.03), had lower MCS scores (52.1 vs 55.7, p = 0.02), and reported more medical conditions (16.7% vs 8.3% with ≥3 conditions, p = 0.03) and IADL impairments (7.5% vs 4.6% with ≥3 IADLs, p = 0.005).
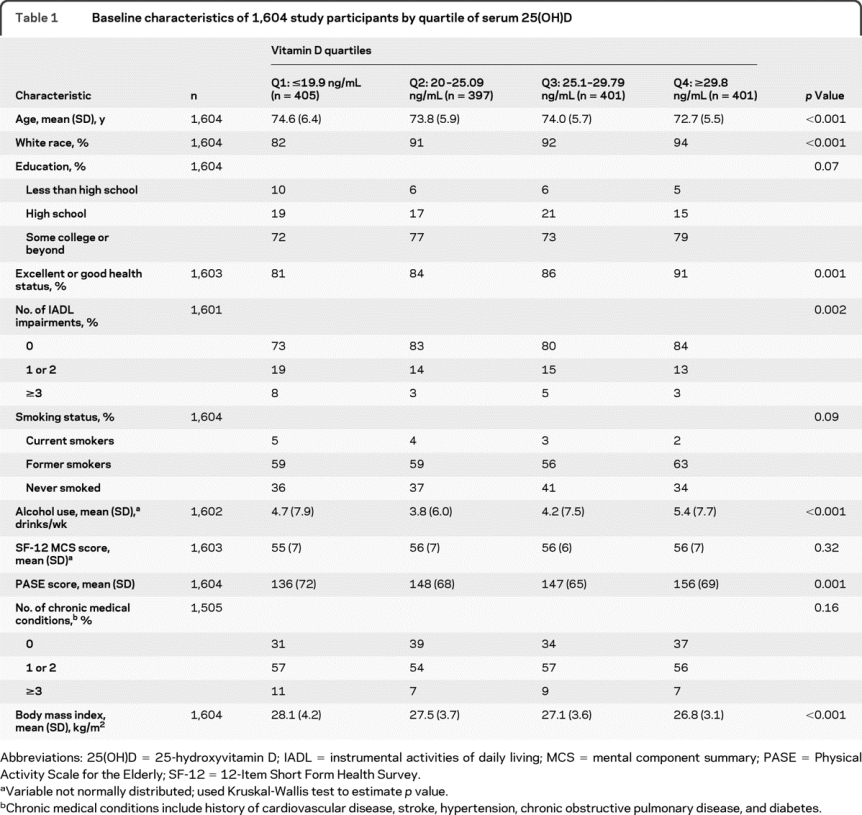
Participants in the lower quartiles of vitamin D level were older, had higher BMI, were less likely to be white, were less likely to report excellent or good health, were more likely to report IADL impairments, and had a lower physical activity level (table 1).
Table 1 Baseline characteristics of 1,604 study participants by quartile of serum 25(OH)D
25(OH)D levels and baseline cognitive impairment.
Fifty-five men (3.4%) were classified as “impaired” at baseline based on having a 3MS score <80, and 145 men (9.3%) were classified as impaired at baseline based on time to completion of Trails B greater than 226.5 seconds. In all, 179 men were classified as impaired by at least 1 of the definitions. Of these, 124 were classified as impaired by the Trials B criteria but not by the 3MS criteria, 22 were classified as impaired by the 3MS criteria but not by the Trails B criteria, 21 were classified as impaired by both criteria, and 12 were classified as impaired according to the 3MS but were missing data on Trails B.
In a model adjusted for age, season, and site, men with lower 25(OH)D levels had an increased odds of cognitive impairment at baseline as defined by 3MS testing compared with the referent group quartile 4: odds ratio (OR) 1.84 (95% CI 0.81–4.19) for quartile 1, 1.41 (0.61–3.28) for quartile 2, and 1.18 (0.50–2.81) for quartile 3; however, the results did not reach significance (p trend = 0.12; table 2). These results, indicating some evidence of a possible association between lower 25(OH)D level and cognitive impairment, were largely explained by the lower prevalence of white race and lower education levels in participants in the lower 25(OH)D quartiles. After adjustment for educational level and race, there seemed to be no difference in effect size across the quartiles. When only white participants were included, there was no association between 25(OH)D level and cognitive impairment by 3MS testing after adjusting for age, season, and site.
Table 2 Association between quartiles of 25(OH)D and odds of cognitive impairment at baseline
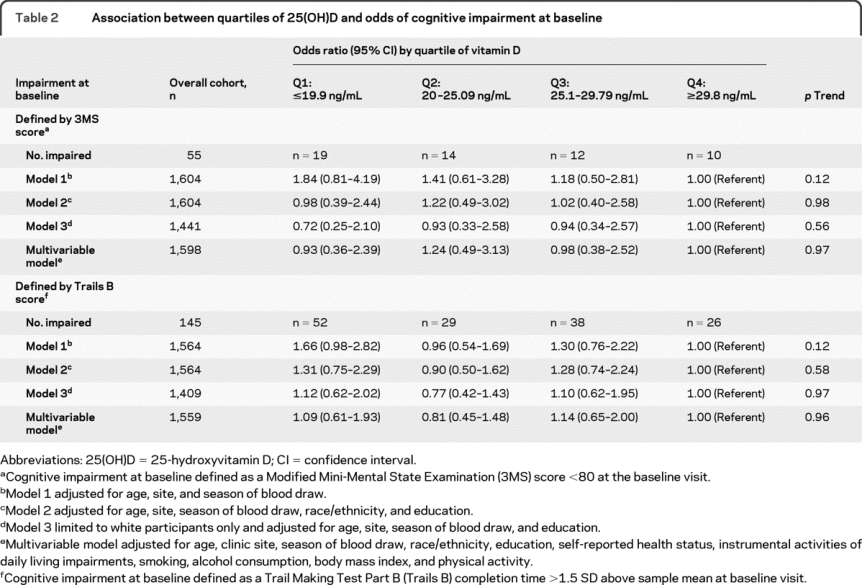
In addition, lower 25(OH)D level (<19.9 ng/mL) seemed to be associated with greater odds of baseline cognitive impairment as defined by Trails B testing compared with the referent group quartile 4 (adjusted for age, season, and site): OR 1.66 (95% CI 0.98–2.82) (p = 0.06 for comparison with quartile 4) for quartile 1, 0.96 (0.54–1.69) for quartile 2, and 1.30 (0.76–2.22) for quartile 3 (p trend = 0.12). However, there was no evidence of an independent association between 25(OH)D levels and baseline performance on the Trails B after adjustment for other covariates and limiting analysis to white participants.
25(OH)D levels and cognitive decline.
One thousand one hundred eighty men (73.6%) with baseline 25(OH)D measurement and 3MS testing attended the second clinic visit, of which 1,161 (72.4%) had 3MS data at visit 2. One thousand one hundred fifty-seven men (74.0%) with baseline 25(OH)D measurement and Trails B testing attended the second clinic visit, of which 1,113 (69.4%) had Trails B data at visit 2 (figure). Compared with those 443 men in the initial analysis subset who did not have follow-up cognitive measures, men who had visit 2 measurement of 3MS or Trails B were on average younger at baseline (72.7 vs 76.6 years, p < 0.001), were more educated (78.0% vs 68.0% reported having attended some college or beyond, p < 0.001), were more likely to be white (90.9% vs 87.1%, p = 0.03), were more physically active (PASE score 153.4 vs 129.3, p < 0.001), had fewer chronic medical conditions (6% vs 15% reported having ≥3 selected medical conditions, p < 0.001) and IADL impairments (2.6% vs 10.4% reported having ≥3 IADL impairments, p < 0.001), and had better self-reported health (89.6 vs 74.4 reported excellent or good health, p < 0.001). In addition, men with follow-up cognitive data drank approximately 0.6 more alcoholic beverages per week (p = 0.01) and smoked less by 2.4% (p < 0.001). Men who did not return for cognitive measures had lower baseline vitamin D levels by an average of 2.4 ng/mL (p < 0.001). Mean (SD) baseline 3MS and Trails B scores were 94.0 (5.2) and 126.8 (54.1) seconds for those who attended the follow-up cognitive examination, and 90.9 (8.4) for 3MS and 161.0 (68.5) seconds for those who did not attend the follow-up examination.
Figure Road map of 1,606 participants with baseline 25(OH)D levels
25(OH)D = 25-hydroxyvitamin D; 3MS = Modified Mini-Mental State Examination; Trails B = Trail Making Test Part B.
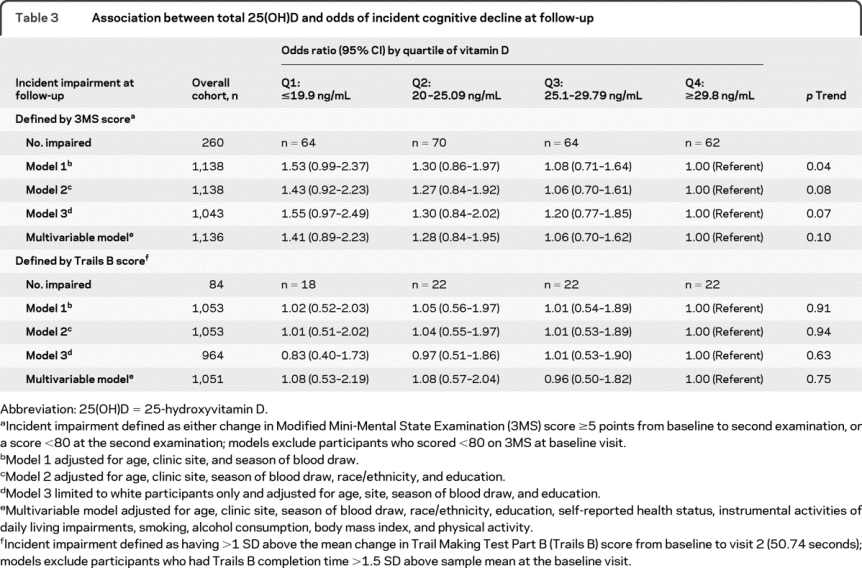
After excluding those who did not complete follow-up cognitive testing and those who were impaired at baseline by a given test (23 [2.0%] by 3MS and 60 [5.4%] by Trails B), 1,138 men by the 3MS and 1,053 men by the Trails B were included in analyses of incident cognitive decline. Two hundred sixty men (22.8%) had developed incident cognitive impairment at follow-up based on 3MS score <80 or decline of ≥5 points, and 84 men (8.0%) developed incident cognitive impairment as defined by the change in time to completion of Trails B >50.7 seconds.
In models adjusted for age, season, and site, there was evidence of an association between lower 25(OH)D levels and odds of incident cognitive decline as defined by the 3MS performance: OR 1.53 (95% CI 0.99–2.37) for quartile 1, 1.30 (0.86–1.97) for quartile 2, and 1.08 (0.71–1.64) for quartile 3, compared with quartile 4 (p trend = 0.04; table 3). Additional adjustment for race and education only slightly attenuated the magnitude of the association, but the test for trend did not reach significance (p trend = 0.08). Findings were similar in the analysis limited to white men and after multivariable adjustment. There was no evidence of an association between 25(OH)D level and cognitive decline by performance on Trails B.
Table 3 Association between total 25(OH)D and odds of incident cognitive decline at follow-up
DISCUSSION
In this study of community-dwelling older men, we found little evidence of independent associations between lower 25(OH)D levels and baseline impairment of global cognitive or executive function or cognitive decline as assessed by 3MS and Trails B. With the exception of an association between 25(OH)D levels and cognitive decline as assessed by repeated 3MS examinations that reached borderline significance, crude associations between 25(OH)D levels and cognitive impairment and decline were largely explained by potential confounding factors such as race and educational level.
The only study28 that found a correlation between lower vitamin D levels and performance on the 3MS was a small retrospective chart review, and no adjustment for other covariates was performed. In a study conducted in elders receiving home health services, correlation between 25(OH)D and Mini-Mental State Examination score (a measure of global cognitive function) approached significance, whereas the correlation between 25(OH)D and tests assessing executive function (Trails A and B, digit symbol coding, digit span, and matrix reasoning) was significant after adjustment for confounders.6 A European study conducted in elderly men revealed an association between 25(OH)D and performance on the Digit Symbol Substitution Test, which measures psychomotor speed and visual scanning.29 Conflicting results from the Third National Health and Nutrition Examination Survey study reported that psychometric measures were not associated with 25(OH)D level in the adolescent and adult groups, and elderly participants aged 60 years or older in the highest 25(OH)D quintile had the worst performance on a learning and memory task (p = 0.02) after adjustment for age, sex, race/ethnicity, and activity.30 A study that examined an association between 25(OH)D level and mood disorder and cognition31 found an association between 25(OH)D level <20 ng/mL with an active mood disorder, as well as poor performance on the Short Blessed Test and higher Clinical Dementia Rating score, after adjustment for age, race, gender, and season of vitamin D determination, but no association with performance on the 3MS. Another case-control study of subjects with secondary hyperparathyroidism without kidney disease found an association between secondary hyperparathyroidism and poor performance on 3 cognitive tests that tested working memory capacity, speed of information processing, and language compared with normal controls, whereas lower levels of vitamin D were not associated with cognitive performance.26
Although there was an association between lower vitamin D levels and incident cognitive decline by the 3MS, its magnitude was somewhat attenuated by adjustment for age, site, and season of blood draw, and it no longer reached the level of significance after further covariate adjustment. Because the finding was of borderline significance, further large prospective studies are needed to test this hypothesis.
The study had a number of strengths, including prospective design, comprehensive measures of the cohort baseline characteristics, and analytical method used to quantify 25(OH)D level, but it also had several limitations. The participants were mostly healthy, white, elderly, community-dwelling men; therefore, the findings might not be generalizable to other populations. Because there was a trend for a higher risk of cognitive decline as assessed by the 3MS, the study might have been underpowered to detect an association because of healthy participant population and low prevalence of cognitive impairment. The excluded participants who did not have cognitive testing or did not participate in the follow-up were older, were frailer, and had lower baseline vitamin D levels and cognitive function, leaving healthy men in the cohort. Because a validated measure of depressive symptoms was not available at baseline, we did not include this factor in the multivariate models. Although widely accepted measures of cognition in older people were used, no uniform definition of cognitive impairment or decline exists for Trails B testing. For example, because there was no Trails A measurement, we are unable to discern whether worsening Trails B performance indicates worsening executive function or slower performance.
We did not find an independent association between vitamin D level and cognitive performance in the cohort of community-dwelling elderly men at baseline. There was a trend for an association between lower 25OH(D) level and decline in global cognitive function as measured by performance on the 3MS. Further studies that include women and a more comprehensive battery of neuropsychiatric testing are needed to further evaluate whether vitamin D deficiency is an independent determinant of age-related changes in cognitive function.
AUTHOR CONTRIBUTIONS
Ms. Paudel performed the statistical analyses and is independent of any commercial funder.
COINVESTIGATORS
Coinvestigators in the MrOS Study Research Group: Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): S.R. Cummings (Principal Investigator), D.C. Bauer (Coinvestigator), D.M. Black (Coinvestigator), P.M. Cawthon (Coinvestigator), M.C. Nevitt (Coinvestigator), K.L. Stone (Coinvestigator), R. Fullman (Project Director), R. Benard, T. Blackwell, A. Chau, L. Christianson, L. Concepcion, J. Diehl, S. Ewing, M. Farrell, C. Fox, S. Hoffland, J. Ireland, M. Jaime-Chavez, E. Kwan, S.L. Harrison, W. Liu, L.Y. Lui, A. Mills, L. Nusgarten, L. Palermo, N. Parimi, L. Perreault, J. Schneider, R. Scott, D. Tanaka, C. Yeung; Administrative Center (Oregon Health & Science University): E. Orwoll (Principal Investigator), K. Phipps (Coinvestigator), L. Marshall (Coinvestigator), J. Babich Blank (Project Director), L. Lambert, C. Nielson, Y. Wang, C. Petersen, M. Powell; University of Alabama, Birmingham: C.E. Lewis (Principal Investigator), J. Shikany (Coinvestigator), P. Johnson (Project Director), N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, C. Atkins, C. Collier, J. Smith, C. Sassaman; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (Coinvestigator), N. Nelson (Clinic Coordinator), P. Van Coevering (Program Director), S. Fillhouer (Project Director), R. Andrews, C. Bowie, M. Forseth, R. Gran, F. Imker-Witte, S. Luthi, K. Moen, N. Muehlbauer, M. Paudel, M. Slindee, S. Ziesche; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (Coinvestigator), K. Kent, N. Ellsworth, A. Krauss, R. Gupta, S. Hartley, M. Bowers; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (Coinvestigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), T. Dam (Coinvestigator), M.L. Carrion-Petersen (Project Director), P. Miller, N. Kamantigue, K. Marksbury, M. Stephens, Z. Torres.
DISCLOSURE
Dr. Slinin is a full-time employee of the US Department of Veterans Affairs. Ms. Paudel reports no disclosures. Dr. Taylor is a full-time research employee of the U.S. Department of Veterans Affairs. Dr. Fink receives research support from the VA RR&D [B5027R (PI)], the NIH [NIA U01 AG030644 (Coinvestigator), NIAMS 2 U01 AR045614-08A1 (Coinvestigator), and NIDDK R01 DK063300-01A2 (Coinvestigator)], and the National Kidney Foundation (Coinvestigator). Dr. Ishani receives research support from the NIH [1R21DK076780 (PI)], the VA [CSR&D Merit Review IIR 03-295 (PI) and 2 VISN 23 Awards (PI and Co-PI)], and the National Kidney Foundation (Co-PI)]. Dr. Canales has received funding for travel for non–industry-sponsored activities. Dr. Yaffe has served on scientific advisory boards for Novartis and Pfizer Inc.; has received honoraria and funding for travel for non–industry-sponsored activities; serves as an Associate Editor for the American Journal of Geriatric Psychiatry; has received honoraria from Posit Science, the American Academy of Neurology, and Novartis; receives research support from the NIH [R01-AG010897–22 (PI), NIA R01 AG026720 (Co-PI), NIA R01 DK069406 (PI), R01 AG021918 (PI), and NIA R21 DK070713 (PI)] and the US Department of Defense [W81XWH-05-2–0094 (PI) and K24 AG031155 (PI)], the National Alliance for Research on Schizophrenia and Depression, and an anonymous foundation. Dr. Barrett-Connor serves on scientific advisory boards for Amgen, Amylin Pharmaceuticals, and Merck Serono; has received funding for travel from Amgen, Eli Lilly and Company, Merck Serono, Pfizer Inc., Roche, Proctor & Gamble Pharmaceuticals, and Amylin Pharmaceuticals; serves on editorial boards for the Journal of Clinical Endocrinology & Metabolism and Menopause; receives royalties from publishing Control of Communicable Diseases in Man (Abram S. Benenson, James Chin, eds. Washington, DC: The American Public Health Association; 1985); has received honoraria (gifted to his university) from Amgen, Eli Lilly and Company, Merck Serono, Pfizer Inc., and Roche; served on a speakers' bureau for Solvay Pharmaceuticals, Inc.; and receives research support from Amgen, Boehringer Ingelheim, Merck Serono, Arena Pharmaceuticals, Inc., Roche, Pfizer Inc., and the NIH [NIA AG07181 (PI), NIA AG028507 (PI), and DK31801 (PI)]. Dr. Orwoll has served on scientific advisory boards for Merck Serono, Eli Lilly and Company, Les Laboratoires Servier, and Zelos Therapeutics, Inc.; has received funding for travel from Endo Pharmaceuticals, Merck Serono, The Scripps Institute, and Eli Lilly and Company; serves on the editorial board of Bone; has received speaker honoraria from Merck Serono and for non–industry-sponsored lectures; serves as a consultant to Merck Serono, Eli Lilly and Company, Les Laboratoires Servier, and Zelos Therapeutics, Inc.; and receives research support from Amgen, Pfizer Inc., Eli Lilly and Company, Novartis, Zelos Therapeutics, Inc., Imaging Therapeutics, Inc., Solvay Pharmaceuticals, Inc., the NIH [NHLBI 1 R01 HL070838 (PI), NIAMS U01-AR45647 (PI), NIA U01-AG027810 (PI), NCRR UL1 RR024140 (PI), NIDCR 1 R01 DE014386 (PI), NIAMS AR 049439 (PI), and NIAMS 1 R01 AR051124 (Contributor)], and the Osteogenesis Imperfecta Foundation (PI). Dr. Shikany receives research support from Kraft Foods Inc., Frito-Lay Inc., General Mills Bell Institute of Health and Nutrition, Kaiser Permanente, the USDA [36-12093–034-76190 (PI)], and the NIH [NHLBI 1 U01 HL79171 (PI), NCI CA37429 (PI), NHLBI NO1 HC48047 (Co-PI), NHLBI 115-9338-01-02 (Coinvestigator), NIAMS 1 U01 AR 45632-08A1 (Coinvestigator), 5 R01 HL70837 (Coinvestigator), NCI N01 CN75022 (Coinvestigator), NHLBI 1 R01 HL080477 (Coinvestigator), NHLBI NO1 HC48047 (Coinvestigator), NCI subproject of 3U54CA118948 subproject (Coleader), and NCI 5P60MD00502-04 (PI)]. Dr. LeBlanc receives research support from the NIH [K23-RR020049 (PI)] and the Medical Research Foundation (PI). Dr. Cauley serves as a consultant to Novartis and receives research support as follows: [U01 AG012546-15] (Co-PI) NIA; 44-240-7160-5 (PI) Subcontract through Boston University; [2803212] (PI) subcontract through California Pacific Medical Center; [U01 AR045654-10] (PI) NIH; [N01-WH-7-4318] (PI) NHLBI; [R01 AG027576-24] (PI) NIH; [Y481844] (PI) subcontract through University of Arizona; [R01 AG026463-02] (PI) NIA; [P60 AR054731-02] (Co-PI) NIAMS; [R01 AG028050-02] (Co-PI) NIA; [HHSN26720070002] (PI) NICHD; and [WFUHS11200] (PI) subcontract through Wake Forest. Dr. Ensrud has received research support from Bionovo and receives research support from the NIH [AG05394 (PI), AR45614 (PI), and HL070847 (PI)] and through subcontracts at California Pacific Medical Center Research Institute (NIH AG026720), UC-Davis (NIH AR052000), Boston University (NIH AG18037), and the Minneapolis Medical Research Foundation.
Address correspondence and reprint requests to Dr. Yelena Slinin, One Veterans Dr. (111J), Minneapolis, MN 55417 slini001@umn.edu
Editorial, page 13
See also pages 18 and 27
e-Pub ahead of print on November 25, 2009, at www.neurology.org.
Study funding: The Osteoporotic Fractures in Men Study is supported by the NIH (the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research) under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
Disclosure: Author disclosures are provided at the end of the article.
Received April 17, 2009. Accepted in final form August 3, 2009.
REFERENCES
- 1.Gloth FM III, Gundberg CM, Hollis BW, Haddad JG Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA 1995;274:1683–1686. [DOI] [PubMed] [Google Scholar]
- 2.Goldray D, Mizrahi-Sasson E, Merdler C, et al. Vitamin D deficiency in elderly patients in a general hospital. J Am Geriatr Soc 1989;37:589–592. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med 1998;338:777–783. [DOI] [PubMed] [Google Scholar]
- 4.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis 2005;15:S5–S101. [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 6.Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci 2009;64:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257–2264. [DOI] [PubMed] [Google Scholar]
- 8.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007;49:1063–1069. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer TE, White NH, Hough S, et al. Alterations in circulating vitamin D metabolites in the young insulin-dependent diabetic. J Clin Endocrinol Metab 1981;53:1154–1159. [DOI] [PubMed] [Google Scholar]
- 11.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10:185–197. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med 2007;4:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol 2009;19:84–88. [DOI] [PubMed] [Google Scholar]
- 14.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005;29:21–30. [DOI] [PubMed] [Google Scholar]
- 15.Baas D, Prufer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia 2000;31:59–68. [DOI] [PubMed] [Google Scholar]
- 16.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat 1999;16:135–145. [DOI] [PubMed] [Google Scholar]
- 17.Magrassi L, Bono F, Milanesi G, Butti G. Vitamin D receptor expression in human brain tumors. J Neurosurg Sci 1992;36:27–30. [PubMed] [Google Scholar]
- 18.Stumpf WE, O'Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain: an autoradiographic study. Histochemistry 1987;87:393–406. [DOI] [PubMed] [Google Scholar]
- 19.Veenstra TD, Prufer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res 1998;804:193–205. [DOI] [PubMed] [Google Scholar]
- 20.McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 2004;89–90:557–560. [DOI] [PubMed]
- 21.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641–653. [DOI] [PubMed] [Google Scholar]
- 22.Ibi M, Sawada H, Nakanishi M, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 2001;40:761–771. [DOI] [PubMed] [Google Scholar]
- 23.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001;21:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neveu I, Naveilhan P, Jehan F, et al. 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 1994;24:70–76. [DOI] [PubMed] [Google Scholar]
- 25.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport 2004;15:1271–1274. [DOI] [PubMed] [Google Scholar]
- 26.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: the Tromso study. J Neurol 2006;253:464–470. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins CH, Birge SJ, Sheline YI, Morris JC. Vitamin D deficiency is associated with worse cognitive performance and lower bone density in older African Americans. J Natl Med Assoc 2009;101:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 2007;460:202–205. [DOI] [PubMed] [Google Scholar]
- 29.Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry 2009;80:722–729. [DOI] [PubMed] [Google Scholar]
- 30.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology 2007;29:49–54. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 2006;14:1032–1040. [DOI] [PubMed] [Google Scholar]
- 32.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials 2005;26:557–568. [DOI] [PubMed] [Google Scholar]
- 33.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) Study: a large observational study of the determinants of fracture in older men. Contemp Clin Trials 2005;26:569–585. [DOI] [PubMed] [Google Scholar]
- 34.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 2006;91:3055–3061. [DOI] [PubMed] [Google Scholar]
- 35.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 36.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276. [Google Scholar]
- 37.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 38.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–162. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scores, 3rd ed. Lincoln, RI: QualityMetric, Inc.; 1998. [Google Scholar]
- 40.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1 1987;(21):1–115. [PubMed] [Google Scholar]
- 41.Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 1983;26:1346–1353. [DOI] [PubMed] [Google Scholar]