Abstract
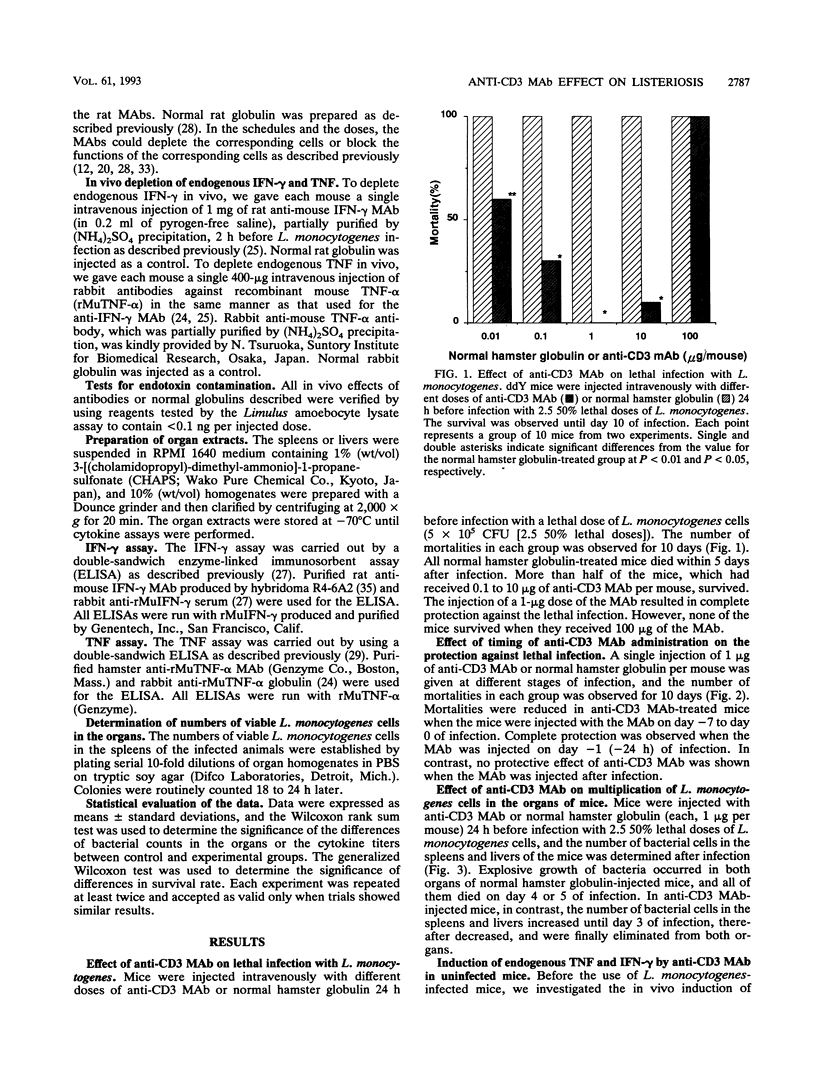
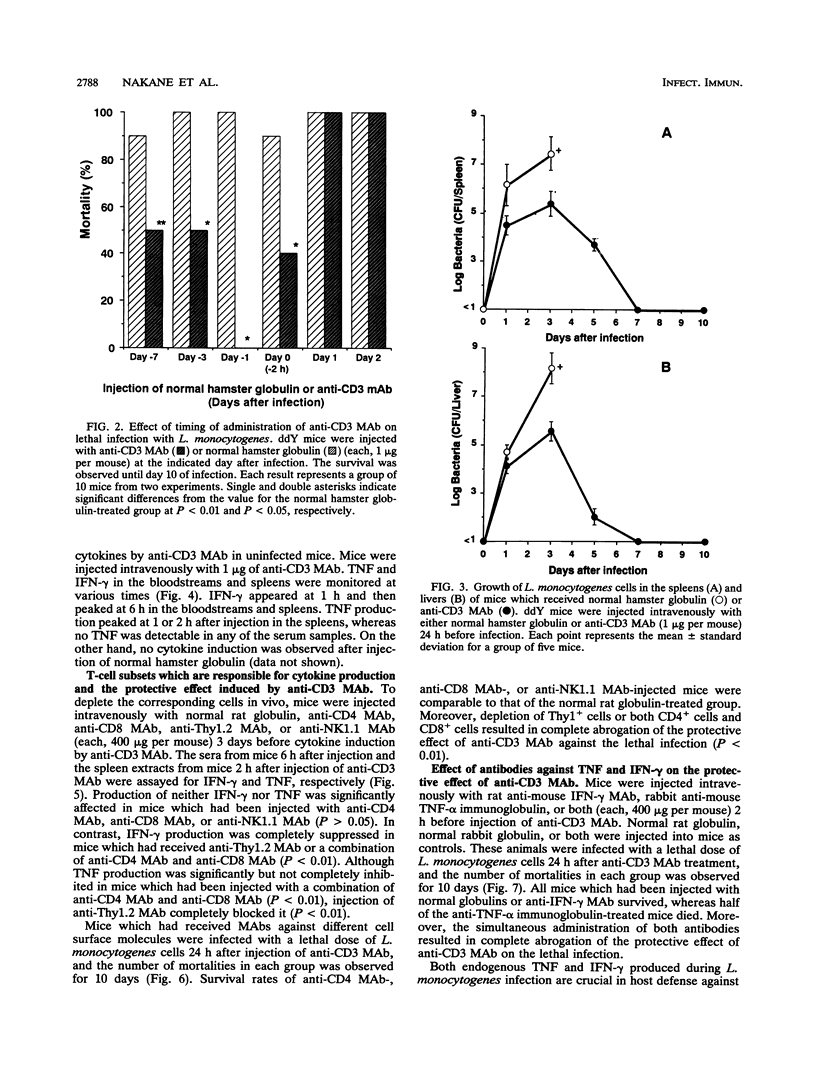
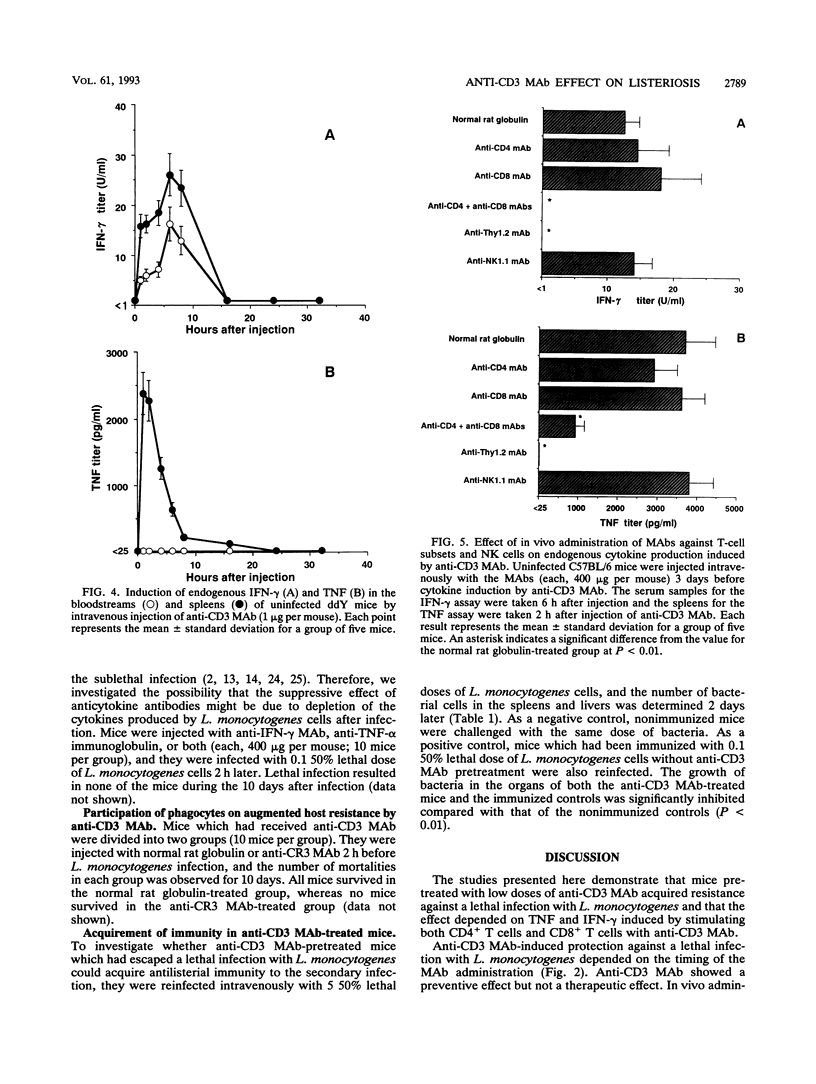
Mice were protected against a lethal infection with Listeria monocytogenes when treated with low doses of an anti-CD3 monoclonal antibody (MAb). Injection of anti-CD3 MAb induced rapid production of endogenous tumor necrosis factor (TNF) in the spleens and endogenous gamma interferon (IFN-gamma) in the bloodstreams and spleens of mice. Administration of anti-Thy1.2 MAb or a combination of anti-CD4 MAb and anti-CD8 MAb resulted in suppression of anti-CD3 MAb-induced endogenous cytokine production and antilisterial resistance. Alternatively, in vivo depletion of anti-CD3 MAb-induced TNF and IFN-gamma by the simultaneous administration of antibodies against TNF and IFN-gamma suppressed anti-CD3 MAb-induced antilisterial resistance. Moreover, injection of anti-complement receptor type 3 (Mac-1, CD11b) resulted in inhibition of anti-CD3 MAb-induced antilisterial resistance. These results suggest that the preventive effect of anti-CD3 MAb might be due to activation of phagocytes by TNF and IFN-gamma induced by stimulating CD4+ T cells and CD8+ T cells with the MAb. Furthermore, treatment with anti-CD3 MAb did not inhibit establishment of acquired resistance against secondary infection with L. monocytogenes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegre M., Vandenabeele P., Flamand V., Moser M., Leo O., Abramowicz D., Urbain J., Fiers W., Goldman M. Hypothermia and hypoglycemia induced by anti-CD3 monoclonal antibody in mice: role of tumor necrosis factor. Eur J Immunol. 1990 Mar;20(3):707–710. doi: 10.1002/eji.1830200337. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Resolution of primary murine listeriosis and acquired resistance to lethal secondary infection can be mediated predominantly by Thy-1+ CD4- CD8- cells. J Infect Dis. 1991 Nov;164(5):869–877. doi: 10.1093/infdis/164.5.869. [DOI] [PubMed] [Google Scholar]
- Ellenhorn J. D., Hirsch R., Schreiber H., Bluestone J. A. In vivo administration of anti-CD3 prevents malignant progressor tumor growth. Science. 1988 Oct 28;242(4878):569–571. doi: 10.1126/science.2902689. [DOI] [PubMed] [Google Scholar]
- Ferran C., Dy M., Sheehan K., Schreiber R., Grau G., Bluestone J., Bach J. F., Chatenoud L. Cascade modulation by anti-tumor necrosis factor monoclonal antibody of interferon-gamma, interleukin 3 and interleukin 6 release after triggering of the CD3/T cell receptor activation pathway. Eur J Immunol. 1991 Oct;21(10):2349–2353. doi: 10.1002/eji.1830211009. [DOI] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- Gregory S. H., Wing E. J., Tweardy D. J., Shadduck R. K., Lin H. S. Primary listerial infections are exacerbated in mice administered neutralizing antibody to macrophage colony-stimulating factor. J Immunol. 1992 Jul 1;149(1):188–193. [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A., Moldawer L. L., Helfgott D., Kilian P. L., Sehgal P. B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992 Mar 1;148(5):1486–1492. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Havell E. A., Sehgal P. B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991 Jan 15;146(2):756–761. [PubMed] [Google Scholar]
- Hirsch R., Eckhaus M., Auchincloss H., Jr, Sachs D. H., Bluestone J. A. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [PubMed] [Google Scholar]
- Hirsch R., Gress R. E., Pluznik D. H., Eckhaus M., Bluestone J. A. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989 Feb 1;142(3):737–743. [PubMed] [Google Scholar]
- Kast W. M., Bluestone J. A., Heemskerk M. H., Spaargaren J., Voordouw A. C., Ellenhorn J. D., Melief C. J. Treatment with monoclonal anti-CD3 antibody protects against lethal Sendai virus infection by induction of natural killer cells. J Immunol. 1990 Oct 1;145(7):2254–2259. [PubMed] [Google Scholar]
- Koo G. C., Dumont F. J., Tutt M., Hackett J., Jr, Kumar V. The NK-1.1(-) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986 Dec 15;137(12):3742–3747. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Yasuda I. Induction of alpha/beta interferon and gamma interferon in mice infected with Listeria monocytogenes during pregnancy. Infect Immun. 1985 Dec;50(3):877–880. doi: 10.1128/iai.50.3.877-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Chen Y., Minagawa T. Endogenous gamma interferon-independent host resistance against Listeria monocytogenes infection in CD4+ T cell- and asialo GM1+ cell-depleted mice. Infect Immun. 1991 Oct;59(10):3439–3445. doi: 10.1128/iai.59.10.3439-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Minagawa T. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992 Feb;60(2):523–528. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. W., Sheehan K. C., Brunt L. M., Dower S. K., Unanue E. R., Schreiber R. D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]


