Abstract
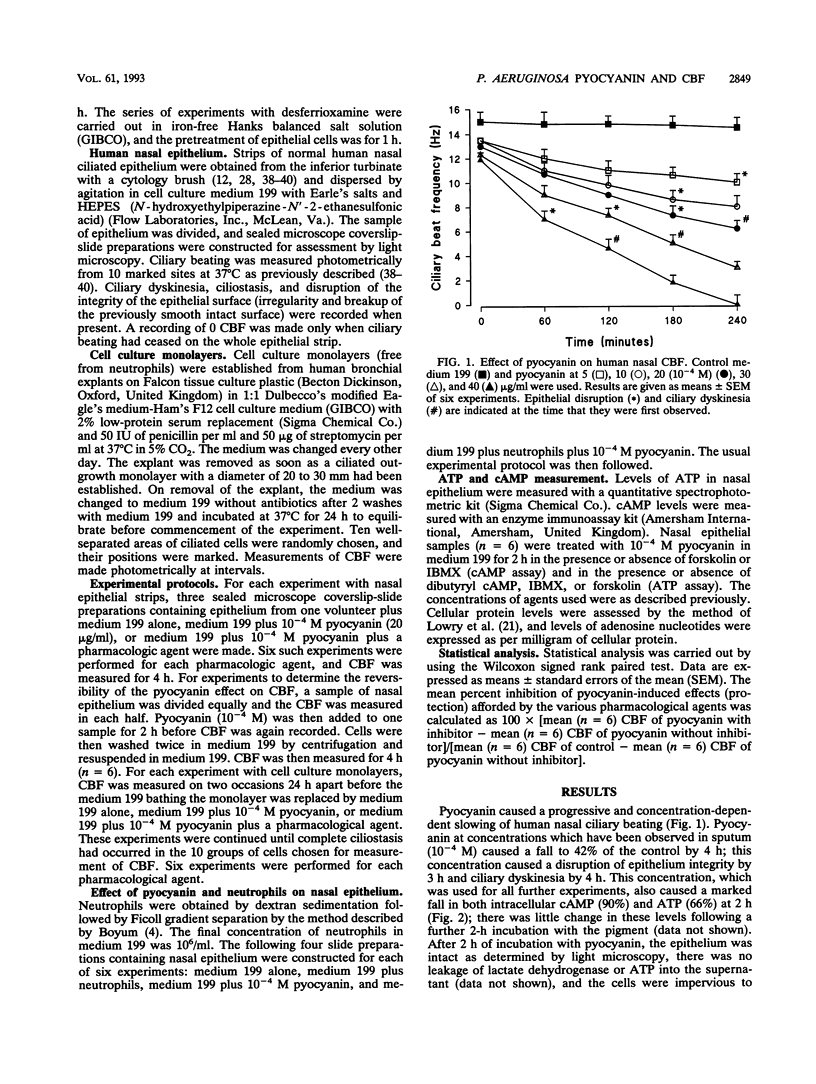
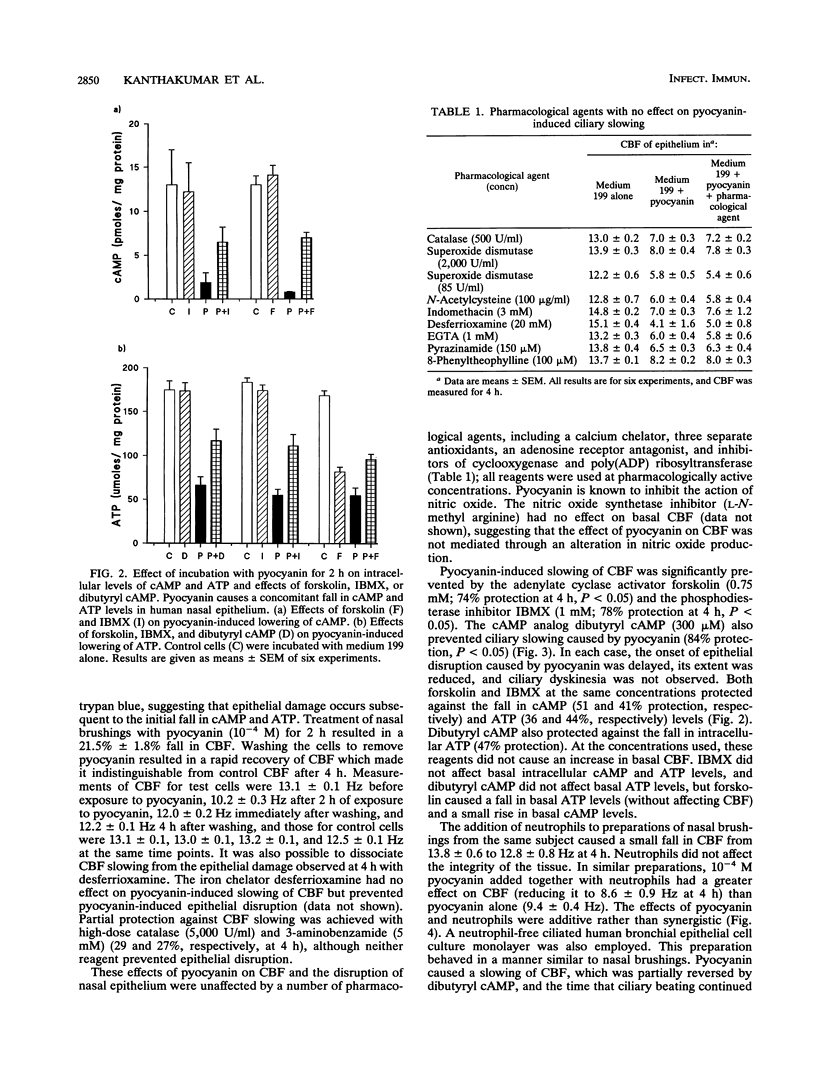
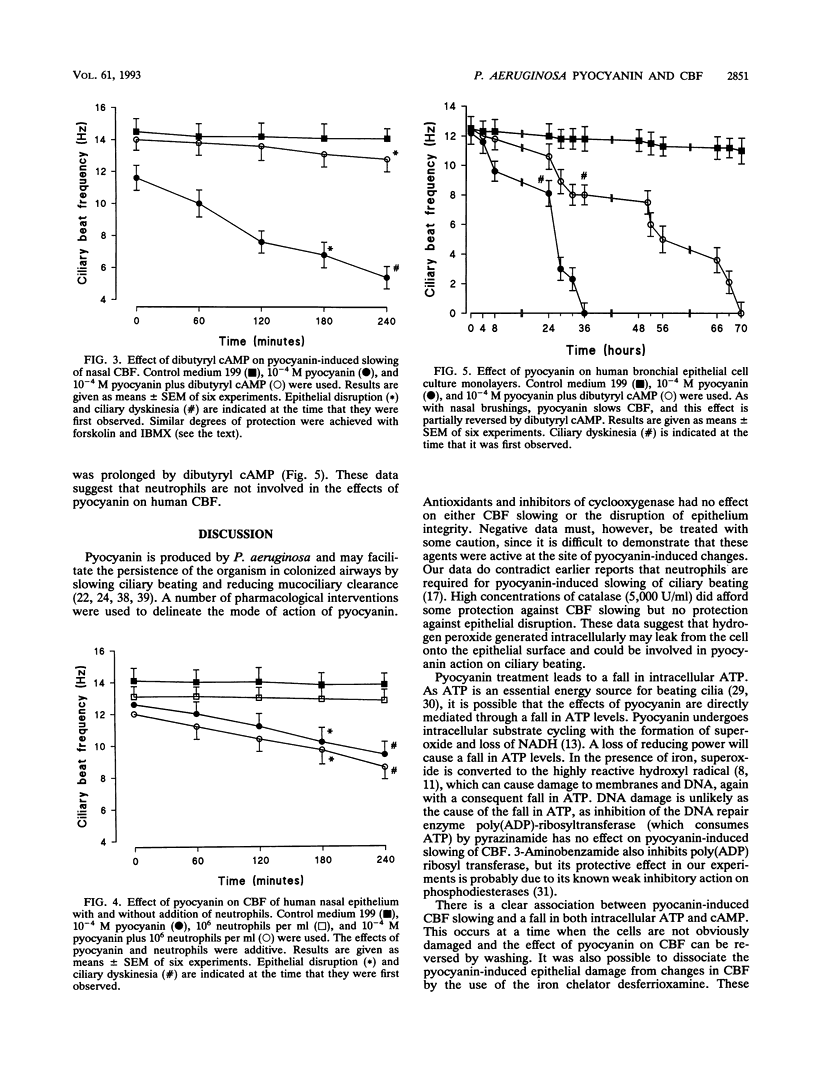
Pyocyanin is a blue redox active pigment produced by Pseudomonas aeruginosa. It is present at concentrations of up to 10(-4) M in sputa from patients with cystic fibrosis and bronchiectasis who are heavily colonized with this organism. Pyocyanin, at physiologically relevant concentrations, slows human nasal ciliary beat frequency (CBF) in vitro and leads to disruption of the epithelium. Pyocyanin-induced slowing of CBF after 2 h was associated with a significant fall in intracellular cyclic AMP (cAMP) (90%) and ATP (66%) and was reversible after the pyocyanin was removed by washing. These effects were not mediated through interaction with neutrophils. The pyocyanin-induced fall in CBF was not affected by EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid], pyrazinamide, 8-phenyltheophylline, indomethacin, or antioxidants, including catalase (500 U/ml), superoxide dismutase, and N-acetylcysteine. Ciliary slowing was, however, prevented (> 70%) by isobutylmethylxanthine and forskolin, both of which increase intracellular cAMP, and also by the cAMP analog, dibutyryl cAMP. There was also a concomitant protection against the fall in both cAMP and ATP. These agents also delayed the onset of epithelial disruption associated with pyocyanin treatment. In contrast, treatment with the iron chelator desferrioxamine prevented epithelial disruption, although it had no effect on pyocyanin-induced slowing of CBF. It appears that ciliary slowing can be dissociated from epithelial disruption and that the effects of pyocyanin on CBF are associated with a fall in both intracellular cAMP and ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amitani R., Wilson R., Rutman A., Read R., Ward C., Burnett D., Stockley R. A., Cole P. J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Jan;4(1):26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- Armstrong A. V., Stewart-Tull D. E., Roberts J. S. Characterisation of the Pseudomonas aeruginosa factor that inhibits mouse-liver mitochondrial respiration. J Med Microbiol. 1971 May;4(2):249–262. doi: 10.1099/00222615-4-2-249. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Di Benedetto G., Manara-Shediac F. S., Mehta A. Effect of cyclic AMP on ciliary activity of human respiratory epithelium. Eur Respir J. 1991 Jul;4(7):789–795. [PubMed] [Google Scholar]
- Drost E., Lannan S., Bridgeman M. M., Brown D., Selby C., Donaldson K., MacNee W. Lack of effect of N-acetylcysteine on the release of oxygen radicals from neutrophils and alveolar macrophages. Eur Respir J. 1991 Jun;4(6):723–729. [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Fawthrop D. J., Boobis A. R., Davies D. S. Mechanisms of cell death. Arch Toxicol. 1991;65(6):437–444. doi: 10.1007/BF01977355. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980 Jan;141(1):156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett D. J., Charniga L., Bean K., Ohman D. E., Cohen M. S. Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect Immun. 1992 Feb;60(2):328–336. doi: 10.1128/iai.60.2.328-336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Hartman P. E., Hartman Z., Young V. M. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal Biochem. 1979 May;95(1):19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansley A. B., Sanderson M. J., Dirksen E. R. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol. 1992 Aug;263(2 Pt 1):L232–L242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Sutkowski E. M., Seamon K. B. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989 Nov;10(11):442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- Munro N. C., Barker A., Rutman A., Taylor G., Watson D., McDonald-Gibson W. J., Towart R., Taylor W. A., Wilson R., Cole P. J. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol (1985) 1989 Jul;67(1):316–323. doi: 10.1152/jappl.1989.67.1.316. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. Biology of Pseudomonas aeruginosa in relation to pulmonary infection in cystic fibrosis. J R Soc Med. 1986;79 (Suppl 12):13–18. [PMC free article] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Ras G. J., Anderson R., Taylor G. W., Savage J. E., Van Niekerk E., Wilson R., Cole P. J. Proinflammatory interactions of pyocyanin and 1-hydroxyphenazine with human neutrophils in vitro. J Infect Dis. 1990 Jul;162(1):178–185. doi: 10.1093/infdis/162.1.178. [DOI] [PubMed] [Google Scholar]
- Riva C. M., Morganroth M. L., Ljungman A. G., Schoeneich S. O., Marks R. M., Todd R. F., 3rd, Ward P. A., Boxer L. A. Iloprost inhibits neutrophil-induced lung injury and neutrophil adherence to endothelial monolayers. Am J Respir Cell Mol Biol. 1990 Oct;3(4):301–309. doi: 10.1165/ajrcmb/3.4.301. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Satir P., Sleigh M. A. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- Satir P. The role of axonemal components in ciliary motility. Comp Biochem Physiol A Comp Physiol. 1989;94(2):351–357. doi: 10.1016/0300-9629(89)90558-6. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Starke P. E., Hoek J. B., Farber J. L. Calcium-dependent and calcium-independent mechanisms of irreversible cell injury in cultured hepatocytes. J Biol Chem. 1986 Mar 5;261(7):3006–3012. [PubMed] [Google Scholar]
- Tamaoki J., Kanemura T., Sakai N., Isono K., Kobayashi K., Takizawa T. Endothelin stimulates ciliary beat frequency and chloride secretion in canine cultured tracheal epithelium. Am J Respir Cell Mol Biol. 1991 May;4(5):426–431. doi: 10.1165/ajrcmb/4.5.426. [DOI] [PubMed] [Google Scholar]
- Tamaoki J., Kondo M., Takizawa T. Adenosine-mediated cyclic AMP-dependent inhibition of ciliary activity in rabbit tracheal epithelium. Am Rev Respir Dis. 1989 Feb;139(2):441–445. doi: 10.1164/ajrccm/139.2.441. [DOI] [PubMed] [Google Scholar]
- Tamaoki J., Kondo M., Takizawa T. Effect of cAMP on ciliary function in rabbit tracheal epithelial cells. J Appl Physiol (1985) 1989 Mar;66(3):1035–1039. doi: 10.1152/jappl.1989.66.3.1035. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Loi R., Rendell N. B., Taylor G. W. Nitric oxide is inactivated by the bacterial pigment pyocyanin. Biochem J. 1990 Mar 15;266(3):921–923. [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Watson D., Rutman A., Taylor G. W., Cole P. J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988 Sep;56(9):2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


