Abstract
The DNA of living cells is highly compacted. Inherent in this spatial constraint is the need for cells to organize individual genetic loci so as to facilitate orderly retrieval of information. Complex genetic regulatory mechanisms are crucial to all organisms, and it is becoming increasingly evident that spatial organization of genes is one very important mode of regulation for many groups of genes. In eukaryotic nuclei, it appears not only that DNA is organized in three-dimensional space but also that this organization is dynamic and interactive with the transcriptional state of the genes. Spatial organization occurs throughout evolution and with genes transcribed by all classes of RNA polymerases in all eukaryotic nuclei, from yeast to human. There is an increasing body of work examining the ways in which this organization and consequent regulation are accomplished. In this review, we discuss the diverse strategies that cells use to preferentially localize various classes of genes.
It has long been realized that DNA is often organized in a manner that contributes to the regulated and efficient expression of gene products. Even so, the fact that most collections of co-regulated genes, or “regulons”, are not co-linear has led to the tacit assumption that co-regulation of linearly scattered genes is achieved by diffusible transcription factors and other regulators. This assumption of diffusible, location-independent regulation is consistent with the fact that the linear arrangement of most genes in chromosomes is not tightly conserved, even when the sequences of the genes themselves are. A growing body of work indicates, however, that preferential three-dimensional positioning of many genes in eukaryotic nuclei is part of their transcriptional programming and, at least in some cases, facilitates use of their RNA transcripts.
Operons and other linear organizational strategies
In bacteria, it is common to have all or part of a regulon made as a single transcription unit, a polycistronic operon. The operon was the earliest genetic regulatory system to have its physical DNA arrangement elucidated in the study of the lac operon, which controls lactose utilization in Escherichia coli (Jacob et al. 1960). The prokaryotic operon exemplifies how cells use linear organization to achieve regulation in one dimension and is perhaps the simplest example of spatial regulation of gene expression.
Although it was thought for some time that only bacteria and archaea contain operons, it is now known that some eukaryotes also have genomic regions that fit the classical definition of an operon. The recent completion of the genome sequence of the trypanosome Leishmania major reveals global arrangement of genes in polycistronic clusters of various sizes (Ivens et al. 2005). There are several examples of operons in other metazoans such as flatworms and certain primitive chordates (Ganot et al. 2004), but the best studied example of operons in eukaryotes remains the nematode Caenorhabditis elegans, the first eukaryotic organism in which extensive operons were discovered (Spieth et al. 1993). It is estimated that approximately 15% of C. elegans genes are present in operons (Blumenthal et al. 2002; Blumenthal and Gleason 2003). Unlike with prokaryotic operons, though, the products of the individual genes encoded by most of the operons in C. elegans are mostly not functionally related. Thus, it has been suggested that C. elegans operons are evolutionarily distinct from those present in bacteria and may have arisen, not for purposes of co-regulation as with prokaryotic operons, but from a need either to select for a smaller genome or to confer a more optimal spatial arrangement for the genes themselves. That being said, the genome of C. elegans does contain a few polycistronic transcripts whose component genes do encode related protein products (Clark et al. 1994; Huang et al. 1994; Page 1997; Treinin et al. 1998). In these instances, there is an argument to be made in favor of preferential localization of gene products for co-regulation. In some cases, it has been suggested that C. elegans operons serve purposes of co-regulation in response to a global signal (Blumenthal and Gleason 2003), and in fact, there is emerging evidence consistent with this idea (Baugh et al. 2009). Thus, in the case of C. elegans, the same type of gene structure might have arisen for different needs, whether for pure spatial compaction or for regulation of gene expression.
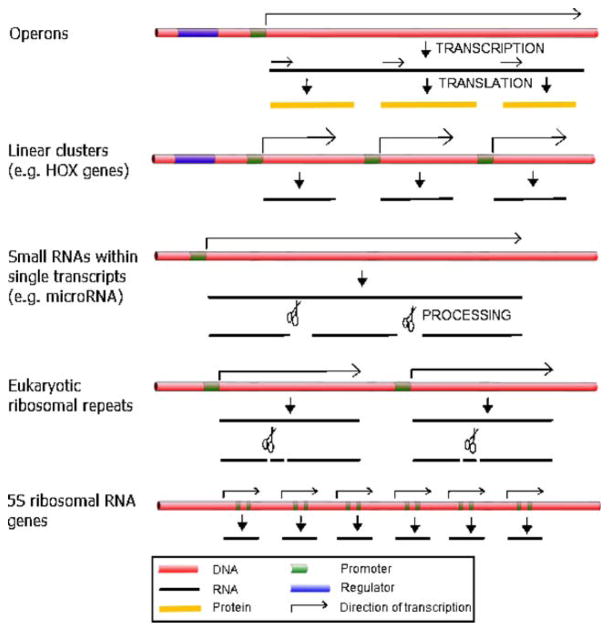
In addition to operons, there are other varieties of linear clusters of genes in eukaryotes (Fig. 1). Some have probably arisen originally by gene duplication, but in many cases, the linear arrangements appear to benefit from regional regulatory signals that control substantial distances on the linear chromosomes. Homeotic genes, and in particular the HOX gene clusters, provide one example. First discovered in Drosophila, HOX genes encode several transcription factors responsible for establishing the pattern of development along the anterior–posterior axis. They are linearly positioned within the clusters in the order in which they are developmentally expressed (Lewis 1978; Kaufman et al. 1980). HOX genes were subsequently discovered in metazoans (Akam 1989; Duboule and Dolle 1989; Graham et al. 1989), and their clustering has been preserved throughout evolution, although the reasons for this conservation are not fully understood (reviewed in Kappen and Ruddle 1993; Mann 1997; Kmita and Duboule 2003; Garcia-Fernandez 2005; Lappin et al. 2006). There are also several non-HOX homeotic genes that are arranged in linear clusters, and these too have evolutionary conservation in higher metazoans (reviewed in Holland 2001). Another well-studied linear grouping is the mammalian globin genes (Proudfoot et al. 1980; Shen et al. 2001; Cao and Moi 2002; Liang et al. 2008; Noordermeer and de Laat 2008; Palstra et al. 2008). As with the HOX clusters, they are arranged linearly in the order in which they are expressed and are particularly interesting in that there is not only linear but also spatial coordination (discussed below).
Fig. 1.
Methods of linear gene organization. Eukaryotic cells have developed a variety of ways to arrange genetic information on the linear map to regulate gene expression. From top to bottom: Operons, which are transcribed as a single polycistronic transcript under control of an upstream operator; Linear clusters, such as the HOX genes, which are under control of a common regulator; Small RNAs, such as microRNAs, which are transcribed as a polycistronic unit and then processed into smaller RNAs; the Pol I-transcribed ribosomal repeats, transcribed in eukaryotes as a 35S transcript and then processed into 18S, 5.8S, and 28S rRNAs; and the small Pol III-transcribed 5S genes, which in most eukaryotes are present in tandemly repeated linear clusters
An emerging body of work shows that many small noncoding RNAs are expressed as polycistronic units and then cut up into their functional components. In particular, these small functional RNAs include the small nucleolar (sno) RNAs and microRNAs (Tycowski and Steitz 2001; Lee et al. 2002). In yeast, while most snoRNAs are encoded by dispersed monocistronic genes, there are also five polycistronic clusters of two to seven snoRNA genes; the precursor transcripts are then processed by RNase III family members (Chanfreau et al. 1998; Lowe and Eddy 1999; Qu et al. 1999). While some snoRNAs in yeast and most in mammals are intron-encoded, in plants, most snoRNAs are polycistronic. In Arabadopsis thaliana, the majority of snoRNA genes are present in clusters transcribed from a single promoter and then processed (Leader et al. 1997). There are also some intron-encoded clusters of snoRNAs in plants, particularly in the rice genome (Liang et al. 2002). In addition, there have been dicistronic transfer RNA (tRNA)–snoRNA genes found in both Arabadopsis and in rice, whose precursor transcripts are processed by RNase Z (Kruszka et al. 2003). This could lead to an even higher degree of regulation between the component tRNA and snoRNA products. Likewise, microRNAs are present in clusters even more extensively in metazoans. At least 40% of microRNAs in humans have been shown to be present in clusters with pairwise distances of less than 3,000 nucleotides (Altuvia et al. 2005), and while the functional relevance of these clusters is not entirely clear, at least one recent study suggests that some components of micro-RNA clusters do in fact have functional associations with each other (Kim et al. 2009).
Linear and spatial organization of ribosomal genes
One type of DNA sequence that is found as linear groupings in nearly all life forms is the transcription unit encoding the ribosomal RNA (rRNA) subunits (reviewed in Haeusler and Engelke 2006). The rDNA is transcribed as a single polycistronic unit and then processed into its component RNAs while being assembled into pre-ribosomal particles with protein subunits. In prokaryotes, the mature RNA components are the 16S, 23S, and 5S rRNAs. In eukaryotes, RNA polymerase I (Pol I) transcribes the rDNA polycistronic unit, which is then processed into the 18S, 28S, and 5.8S mature ribosomal RNAs. The rDNA transcription unit is typically found as many tandem linear repeats in genomes in all phyla of life—approximately 150–200 copies in Saccharomyces cerevisiae and about 400 copies in human. In the case of S. cerevisiae, a single tandem array of rRNA genes is located on the right arm of chromosome XII. In other metazoans, the ribosomal genes are also present as clusters, although there is typically not one single cluster but rather a few chromosomal locations. For example, the human tandem repeat clusters are located on the five acrocentric chromosomes (Henderson et al. 1972).
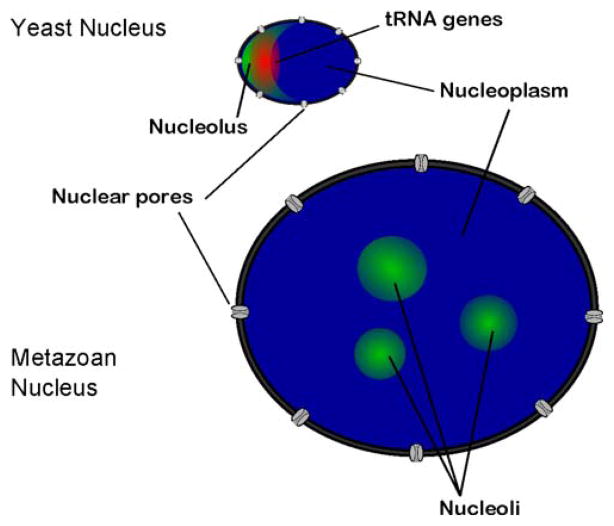
Whether present in the linear genome map as a single cluster or as multiple clusters, the ribosomal gene arrays act as the organization points of dense nuclear subcompartments termed nucleoli, the location of rRNA transcription, processing, and assembly into pre-ribosomal nucleoprotein particles (reviewed in Pederson 1998; Gerbi et al. 2003; Prieto and McStay 2005; Boisvert et al. 2007). Because of this role of the ribosomal clusters, they are often termed nucleolar organizer regions. Yeast contain a single nucleolus visible by fluorescence in situ hybridization (FISH) microscopy or electron microscopy as a large crescent-shaped structure at one end of the nucleus, with other species containing varying numbers of nucleoli (Fig. 2). The nucleolus exemplifies one way in which cells have developed preferential spatial positioning of genes of like function in order to maximize efficiency of cellular processes. By concentrating rRNA genes all in one place within the nucleus, the machinery needed for their transcription, processing, and assembly into ribosomes can be localized to a distinct nuclear subdomain, resembling a “factory” that Henry Ford would have envied. The framework (RNA) moves along an assembly line while the appropriate components (proteins) are loaded on at the right time and the necessary finishing steps (processing) are carried out. While localization at the nucleolus may not be essential (Oakes et al. 1993; Oakes et al. 1998), concentration and organization of various components there could facilitate timely and efficient incorporation, rather than if the components were dispersed throughout the nucleus. This sort of “assembly line” efficiency may be particularly important for ribosome biosynthesis since it is a massive and highly complex effort that often occupies over half the RNA synthetic expenditure of the cell. The substructures associated with the rDNA Pol I transcription units and pre-ribosome assembly have been examined extensively for several decades (reviewed in Puvion-Dutilleul et al. 1991; Schwarzacher and Wachtler 1991; Scheer and Weisenberger 1994; Olson and Dundr 2005; Hernandez-Verdun 2006).
Fig. 2.
Comparison of yeast and metazoan nucleoli. The eukaryotic nucleolus is defined by the Pol I-transcribed ribosomal cluster. In yeast, the ribosomal cluster is located on the linear map in one group on chromosome XII; consequently, the yeast nucleolus can be visualized by FISH microscopy as a single crescent-shaped structure, typically localized to one side of the nucleus. In yeast, the tRNA genes can be visualized by FISH microscopy as a single cluster localized to the nucleolus. Metazoans generally have multiple clusters of ribosomal genes; thus, metazoan nuclei usually have several nucleoli spread throughout the nucleus. The metazoan nucleus is generally several times larger than the yeast nucleus
There is one type of rRNA gene that is not part of the large Pol I transcripts in eukaryotic nuclei: the 5S rRNA gene. The 5S genes are transcribed by RNA polymerase III (Pol III) and are organized differently from the other rRNA genes, although there are distinct similarities. In some cases, notably in S. cerevisiae, the 5S genes are interspersed within the Pol I-transcribed ribosomal tandem arrays (Bell et al. 1977), but in most eukaryotes, they are arranged in various numbers of tandem repeat clusters that are linearly separate from the large ribosomal clusters. In some cases, they are present in a single cluster, as in the chicken genome (Daniels and Delany 2003), and in other cases, 5S rRNA gene types that are expressed at distinctive times in development are found in separate clusters, as with the oocyte type vs. somatic genes in Xenopus (Harper et al. 1983). Linear clustering of the 5S genes might facilitate the same types of regulatory benefits that are seen in the clustering of the large ribosomal clusters. Placement of the 5S genes away from the other ribosomal genes in higher eukaryotes may serve further regulatory roles.
In a smaller number of cases, such as in Schizocaccharomyces pombe (Mao et al. 1982) or in Neurospora crassa (Metzenberg et al. 1985), the 5S genes are more dispersed throughout the linear map. In at least one case, in the non-conventional dimorphic yeast Yarrowia lipolytica, while the many of the 5S genes are scattered throughout the genome, nearly half of the 5S genes appear to be present in dicistronic tRNA-5S gene clusters (Acker et al. 2008). This unique type of linear cluster could potentially allow very tightly regulated transcription between the tRNA and the 5S RNA transcripts. There is also evidence that 5S genes that are otherwise clustered can be retrotransposed and that these copies are scattered throughout the genome, some of which are expressed (Drouin 2000). In these select cases, a further level of organization might come into play for spatial regulation of expression. This would not be much of a surprise, as a substantial body of work suggests that in metazoans with separate 5S gene clusters, there is a high degree of spatial organization throughout evolution. Since the 5S genes of S. cerevisiae are within the large ribosomal cluster, they are necessarily nucleolar. Additionally, FISH and electron microscopy have shown nucleolar localization of the transcribed 5S gene clusters in other organisms as well (reviewed in Haeusler and Engelke 2006). Thus, the three-dimensional localization of 5S genes to the nucleolus, whether arranged in linear clusters or scattered across the genome, might be a component of coordinating the overall regulation of ribosomal processing and assembly.
Yeast tRNA genes: co-localization of many linearly dispersed loci
The ribosomal RNA genes are not the only class of genes that are thought to be localized to the nucleolus. Recently, it has been shown in S. cerevisiae that the 274 tRNA genes, which are Pol III transcription units scattered throughout the linear map of the 16 chromosomes, are preferentially localized to the nucleolus (Thompson et al. 2003). At the time of this finding, there was some knowledge in the field about global positioning of specific regions of the yeast genome—centromeres, telomeres, and the silent mating loci are all localized to the nuclear periphery (reviewed in Gasser 2001; Loidl 2003). The finding that tRNA genes dispersed throughout the genome were localized to a single nuclear substructure was a somewhat astonishing observation, though it had been foreshadowed by earlier findings that components of the pre-tRNA processing pathway are found there. Imaging by FISH had shown that pre-tRNA transcripts are localized primarily to the nucleolus in S. cerevisiae, and some early tRNA processing enzymes in S. cerevisiae—specifically the endoribonuclease, RNase P, and the tRNA isopentenyltransferase, Mod5—are also nucleolar (Bertrand et al. 1998; Tolerico et al. 1999). In retrospect, the concentration of tRNA genes and early processing machinery to nucleolus makes a certain amount of logistical sense since it is the site of massive synthesis of the other non-messenger RNAs involved in translation—5S, 5.8S, and the large ribosomal RNAs. Synthesis of these RNAs is co-regulated under various conditions with tRNAs (Briand et al. 2001), and spatial coordination of these biosynthetic pathways could provide possibilities for co-regulation, in addition to an assembly line for tRNAs, as well as ribosome synthesis and transport.
The spatial organization of tRNA genes is not likely to be a static situation. Although the majority of the tRNA genes appears to remain associated with the nucleolus throughout the cell cycle in yeast, even during late mitotic division (Haeusler et al. 2008), individual genes could vary. In fact, individual tRNA genes do dissociate into the nucleoplasm if transcription by Pol III is interrupted (Hull et al. 1994; Thompson et al. 2003). Disruption of nucleolar architecture also disperses tRNA genes and pre-tRNA transcripts throughout the nucleoplasm (Wang et al. 2005b), and thus individual tRNA genes likely become transiently dissociated from the cluster during division of the replicated nucleoli, even while the bulk of the genes seems to remain associated with the nucleolus. It is also possible that the tRNA genes can be either transiently or for long periods dissociated from the nucleolus in response to more dominant positioning imperatives from neighboring genes. No pattern of which other genes surround tRNA genes has yet made itself obvious, although genes that exist very near tRNA genes might need to be adapted to the environment. In general, transcription promoters for Pol II tend to be severely underrepresented within 500 base pairs of tRNA genes (Bolton and Boeke 2003), yet the Ty retrotransposon elements have developed a strong preference for inserting near tRNA genes, by at least two different mechanisms (Chalker and Sandmeyer 1990; Chalker and Sandmeyer 1992; Devine and Boeke 1996). This suggests not only that the retrotransposons have adapted but also that there is some selective advantage to the Ty or the host cell in this genomic arrangement. There is evidence that the proximity to the tRNA gene influences expression of the Ty element (Hull et al. 1994), consistent with a transcriptional regulatory adaptation.
The potential for condensation by Pol III complexes in mammals
The tRNA genes in metazoans can be found in single or multiple copies, but little is known about their expression or localization. There is some recent information about which families of tRNA genes are actively transcribed in humans and in mice (Lowe and Eddy 1997; Dittmar et al. 2006; Coughlin et al. 2009), but we still know relatively little about their localization. While there is no direct evidence of clustering of Pol III-transcribed genes outside of S. cerevisiae, it would seem possible that global organization by clustering of this type of transcription unit is not evolutionarily restricted to a single species. This hypothesis is supported by data that the clustering of tRNA genes in yeast appears to be mediated by at least one protein complex that is highly conserved throughout evolution. Chromatin immunoprecipitation followed by hybridization to high-resolution oligonucleotide microarrays revealed that the multi-subunit protein complex condensin is present throughout the cell cycle over all tRNA genes and over a small number of other sites bound by Pol III transcription factors across the entire S. cerevisiae genome (D’Ambrosio et al. 2008). Additionally, FISH microscopy of S. cerevisiae nuclei in cells containing temperature-sensitive alleles of all five subunits of condensin shows a dispersal or gross mislocalization of tRNA genes away from the nucleolus (Haeusler et al. 2008). Co-immunoprecipitation experiments further show association of condensin with a DNA-mediated complex of the general transcription factors TFIIIC and TFIIIB, although not with Pol III itself, suggesting that a potentially direct interaction of condensin with the tRNA gene transcription complex may be mediating the clustering of tRNA genes to the nucleolus.
The involvement of condensin in the dynamic positioning of chromosomal loci, while not immediately intuitive, is not overly surprising. Condensin is a member of the structural maintenance of chromosomes (SMC) family of protein complexes, whose components have a high degree of structural and functional conservation throughout evolution (reviewed in Cobbe and Heck 2000; Hirano 2002, 2006; Jessberger 2002; Huang et al. 2005; Losada and Hirano 2005; Uhlmann and Hopfner 2006). At least three distinct eukaryotic SMC complexes evolved from a single prokaryotic SMC complex, whose structure is remarkably similar to its eukaryotic counterparts. SMC complexes are thought to associate directly with DNA to mediate various activities such as chromosome condensation and cohesion and to be essential for processes such as replication and repair. Most studies on the condensin complex in the decade and a half since its identification have focused on its functions during mitosis and meiosis. There is, however, emerging evidence for a more widespread role for condensin during interphase in various eukaryotic models (reviewed in Legagneux et al. 2004; Hirano 2005; Tsang et al. 2007b). Specific examples come from studies on gene regulation and transcriptional control in Drosophila (Lupo et al. 2001; Dej et al. 2004; Cobbe et al. 2006) and maintenance of genome structure in yeast (Tsang et al. 2007a). Thus, it would not be surprising if, as the eukaryotic genome grew larger and the job of organizing the genome became more complex, cells evolved alternate functions outside of mitosis and meiosis for condensin in the localization of genes during interphase. Additionally, the presence of condensin at tRNA genes located at the nucleolus is consistent with data from several groups showing that condensin is highly enriched at the rDNA cluster in both budding yeast and in fission yeast and is required to maintain proper compaction of the rDNA cluster during interphase (Freeman et al. 2000; Lavoie et al. 2004; Wang et al. 2005a; Tsang et al. 2007a; Nakazawa et al. 2008; Wang and Strunnikov 2008).
The involvement of highly conserved protein complexes at tRNA gene clusters brings into question whether clustering of Pol III elements occurs in higher eukaryotes. While the 5S and tRNA genes encode the most abundant gene products of Pol III-transcribed genes, there are other, far more abundant DNA elements containing tRNA-class Pol III promoters. In many organisms, particularly vertebrates, the most abundant Pol III elements are the short interspersed nuclear elements (SINEs; reviewed in Okada 1991; Deininger and Batzer 2002; Belancio et al. 2008). Some of the better-studied SINEs include the five major families of mouse SINEs, which are retrotransposons derived from pre-tRNA and 7SL RNA and make up about 7% of the murine genome (Waterston et al. 2002). In humans, the predominant SINEs are the Alu elements, which arose from 7SL RNA transcripts and are thought to comprise at least 10% of the human genome (Lander et al. 2001). Since the discovery of SINEs 40 years ago, various hypotheses have been advanced for possible evolutionary advantages conferred by these “junk” sequences in the large genomes of higher eukaryotes. In many cases, they have been proposed to serve as regulatory sequences, both positive and negative (Saffer and Thurston 1989; Tomilin et al. 1990; Saksela and Baltimore 1993; Thorey et al. 1993; Ferrigno et al. 2001). In support of this idea, it has been shown that mouse B2 SINE transcripts can directly bind to Pol II and negatively regulate Pol II transcription (Allen et al. 2004; Espinoza et al. 2004). Newer studies reveal that human Alu transcripts can also bind to Pol II and repress its transcriptional activity (Mariner et al. 2008; Yakovchuk et al. 2009). Additionally, B2 SINE DNA can act as a chromatin “boundary element” (Lunyak et al. 2007), a block to propagation of nucleosome-mediated chromatin regulation, similar to the function of tRNA genes and even partially assembled Pol III transcription complexes as boundary elements in yeast (Donze et al. 1999; Donze and Kamakaka 2001; Simms et al. 2008).
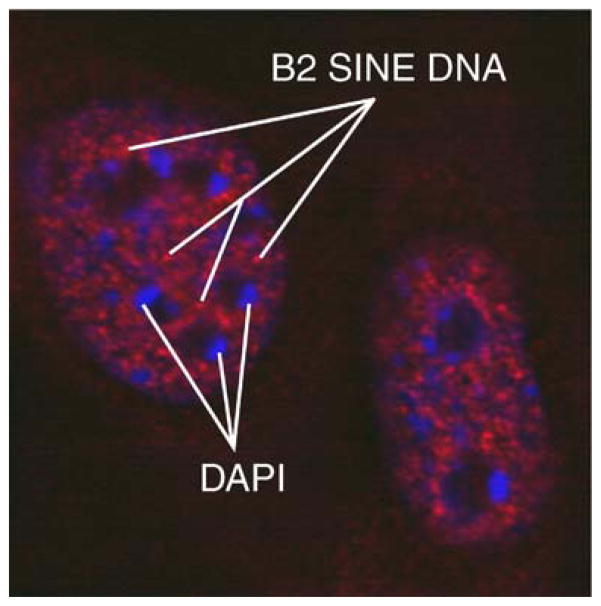
In vitro, SINEs can be transcribed efficiently by tRNA gene-like complexes, although they are not normally expressed at significant levels inside cells (Carey et al. 1986; Jang and Latchman 1989; Kim et al. 1995). It is not known if the SINEs—or a significant percentage of them—have complexes similar to the tRNA gene complexes (TFIIIC + TFIIIB + Pol III) associated with them in vivo. If they do have similar complexes, it may very well be possible that, as with yeast, they are similarly able through clustering to serve as chromosomal compaction and organizational signals. This would be consistent with at least one study showing clustering of SINE elements in human lymphocyte nuclei (Kaplan et al. 1993). Since mammalian genomes are up to a hundred times larger than those of yeast, but have only two or three times as many bona fide tRNA genes, one could surmise that SINE elements might serve the compaction function that tRNA genes assume in yeast, even without making stable, functional transcripts. Furthermore, since metazoans have at most a few nucleoli, the potential compaction function of SINE elements is likely to be independent of localization to any specific nuclear subcompartment (Fig. 3).
Fig. 3.
FISH microscopy showing distribution of mouse B2 SINE elements. Mouse embryonic fibroblasts were fixed in 1% paraformaldehyde and adhered to slides. Fluorescent oligonucleotides complementary to B2 SINEs hybridized to genomic DNA. There appears to be speckled signal of B2 elements throughout the nucleoplasm, suggesting clusters, but this signal appears not to be preferentially associated with either the nucleoli or the nuclear periphery. Red B2 SINE DNA, blue DAPI stain of AT-rich heterochromatin
Transcription factories and chromosome territories
The nucleolus can be thought of as a specialized version of what has been termed a “transcription factory” (reviewed in Pombo and Cook 1996; Pombo et al. 2000; Bartlett et al. 2006; Faro-Trindade and Cook 2006; Sexton et al. 2007; Carter et al. 2008). Just as localization of Pol I and Pol III transcription to the nucleolus serves to regulate coordinated cellular processes, so too has it been suggested that some actively elongating RNA polymerase II (Pol II) complexes are localized to factories of Pol II transcription. These were initially identified as foci of nascent transcription and later found to contain high local levels of Pol II (Jackson et al. 1993; Wansink et al. 1993; Iborra et al. 1996). Since such a significant amount of the genome is encoded by Pol II, spatially coordinated transcription by Pol II might also have a significant impact on the three-dimensional organization of the nucleus, similar to how clustering and localization of tRNA and rRNA genes organize the yeast genome. Indeed, while the details of transcription factory formation are not entirely clear, it appears that these factories are formed not only by genes from linearly distant regions of the same chromosome, as initially proposed, but also by genes on different chromosomes (Osborne et al. 2004; Osborne et al. 2007). Consequently, there is the possibility of a high degree of spatial organization resulting from Pol II factory formation. Nevertheless, the factory model is likely to be an oversimplified look at how genes come together, and the functional reasons for how and why various genes come together are only starting to be understood (Brown et al. 2008).
The co-localization of genes from different chromosomes is, on the surface, at odds with the idea that in most higher eukaryotes the interphase genome is widely thought to be arranged into chromosome territories (reviewed in Cremer and Cremer 2001; Parada and Misteli 2002; Parada et al. 2004; Gilbert et al. 2005; Cremer et al. 2006; Meaburn and Misteli 2007). A territory is a distinct spatial region of the nucleus in which a chromosome is contained during interphase. Visualization of chromosomes in nuclei from various species, by “painting” each chromosome with distinct fluorescent probes, shows individual chromosomes occupying distinct subnuclear sections that can be easily distinguished from one another. Nevertheless, as distinct as territories may appear, within the established territory model, there are examples of individual genomic loci that have been found well outside their expected territories, even for genes that are generally localized to their home territories (Volpi et al. 2000; Mahy et al. 2002a; Chambeyron and Bickmore 2004). Given the necessarily dynamic nature of the nucleus, though, it should not come as much of a surprise that genes can position and reposition themselves, even within the otherwise established territory model.
The majority of higher eukaryotes are now thought to have chromosome territories, although these have not been as clearly delineated in S. cerevisiae, despite the recent demonstration of ordered gene positions in yeast (Berger et al. 2008). The yeast genome is comparatively much smaller than those of higher eukaryotes, so it is possible that its chromosome organization evolved around a different format. It has been proposed that the yeast genome undertakes a Rabl-type organization, with centromeres and telomeres at opposite poles of the nucleus (Bystricky et al. 2004; Bystricky et al. 2005). The existence of chromosome territories or other global organization scheme in a particular organism does not preclude the possibility of organization by Pol III elements (tRNA genes or SINEs), although it probably means that any resulting condensation is a local, rather than broadly interchromosomal, phenomenon.
A model for interactions between chromosome territories was initially proposed and termed the interchromosome domain (ICD) model (Cremer et al. 1993). The basis for this model was that transcriptionally active regions of the genome must be readily accessible to the nuclear machinery that are localized to the interchromosomal regions between territories. Individual genes can thus be strategically positioned at the interface of two or more territories to allow for maximum regulation and energetic favorability. Individual chromatin fibers would occasionally loop out into the ICD, where rare chromosomal contacts between loci would occur; some have called these contacts “chromosome kissing” (Kleckner and Weiner 1993; Cavalli 2007; de Laat 2007). While this paradigm seems compelling, newer data indicate that there is a much higher degree of interaction between the territories than the ICD model suggests (reviewed in Sachs et al. 2000; Chubb et al. 2002; Hlatky et al. 2002; Holley et al. 2002; Spilianakis et al. 2005).
In order to revise the ICD model and attempt a better picture of how chromosome territories interact, Branco and Pombo developed a novel cryo-FISH method that preserves chromatin structure while providing very high microscopic resolution (Branco and Pombo 2006). They proposed a new model for interaction between territories that they call the interchromosomal network model (ICN). This model lays out a much more plastic arrangement of territories, where they are still localized to distinct regions of the nucleus but are much freer to intermingle with each other at their boundaries. The ICN model implies that significant interchromosomal contacts would drive the shape of territories in the nuclei of metazoans, and indeed, active transcription does affect the nature of interchromosomal contacts (Branco and Pombo 2006). In fact, there are a variety of factors that shape territories. In particular, the overall architecture of territories has been shown to change, often drastically, in response to developmental cues in cells (Kuroda et al. 2004; Stadler et al. 2004; Wegel and Shaw 2005; Bartova and Kozubek 2006). Altered epigenetic marks such as methylation can also instigate changes in nuclear organization by reorganizing chromosome territories (Matarazzo et al. 2007). We should therefore look at territories as pliable, rather than rigid or impenetrable structures, especially in light of data that transcriptional machinery is shown to have the ability to access the interior of chromosome territories (Mahy et al. 2002b). In fact, most transcriptional factors are quite capable of accessing the interiors of territories. Thus, the idea that active genes are mainly positioned at the surface of chromosome territories is likely to be an oversimplification.
Positions of individual genes, in addition to overall territory architecture, can also change quite drastically in response to differentiation, developmental signals, or other changes in their transcriptional state. Much work has been done on spatial positioning of the human globin genes, in addition to their linear organization (Brown et al. 2001; Tolhuis et al. 2002; Brown et al. 2006; Ragoczy et al. 2006; Zhou et al. 2006). More recently, it was demonstrated that active globin genes become clustered and localize to nuclear speckles (Brown et al. 2008), similar to how active tRNA genes cluster and localize at nucleoli in yeast. Studies on the oncogenes, bcr, abl, and c-myc show that they change positions relative to each other in response to cell cycle or developmental cues (Neves et al. 1999; Bartova et al. 2000), suggesting that spatial positioning of developmentally important genes aids in the differentiation processes of the cell. Activity-dependent repositioning has been shown with other human genes (Lanctot et al. 2007; Meaburn and Misteli 2008), and ligand binding to nuclear receptors can activate specific interactions between genes, which appear to be important for ligand-induced transcriptional regulation (Hu et al. 2008). Repositioning of one gene can also bring along with it adjacent, functionally unrelated genes (Zink et al. 2004), similar to how individual yeast tRNA genes might become positioned away from the nucleolus in response to more dominant localization signals from adjacent loci. Functionally distinct alleles of the same gene can, at least in one example, occupy different positions within the nucleus (Takizawa et al. 2008). Recently it has been shown possible to construct a map of the three-dimensional organization of the human interphase genome in relation to the transcriptome, thus tying together global genomic structure and function (Goetze et al. 2007). Indeed, there is an emerging body of work suggesting that functional interactions across chromosomes can drive gene localization (Rajapakse et al. 2009).
Positioning of genes as a component of regulation
Several studies, particularly in yeast and flies, have provided further evidence and mechanistic insight as to how individual genes can become dramatically repositioned based on gene activity. Multiple studies in S. cerevisiae have shown large-scale relocalization of gene positions when cells are induced under certain conditions. When cells are treated with alpha-factor, the FIG 2 gene becomes highly localized to the nuclear periphery, specifically toward the side of mating projection (Casolari et al. 2005). The SUC2 gene, which encodes a sucrose invertase, is mobile within the nucleus when repressed in glucose media, but when cells are grown in the absence of glucose to activate SUC2, the gene becomes tightly localized to the nuclear periphery (Sarma et al. 2007). Localization of genes to the periphery is often accompanied by physical and genetic connection to the nuclear pore complex (Casolari et al. 2004, 2005; Cabal et al. 2006). Additionally, there appears to be some pathway dependence on gene dynamics. Transcriptional activation of the subtelomeric gene HXK1 by growth on a non-glucose carbon source has been shown to relocate it to the nuclear pore complex (Taddei et al. 2006); however, when the same gene is activated via an alternative pathway, using the VP16 activator, nuclear pore association is eliminated. This result would suggest that a single gene would need to be differentially positioned within the nucleus in order to be regulated by different pathways. Recent work in Drosophila indicates that the hsp70 gene cluster is anchored to the nuclear periphery, and proteins that are involved in retaining the hsp70 cluster at the periphery are also implicated in its transcriptional regulation (Kurshakova et al. 2007). Additional work in Drosophila suggests a potential link between dosage compensation and localization of the X-chromosome to the nuclear pore complex (Mendjan et al. 2006).
Several studies in yeast demonstrate that artificial tethering of genes to the nuclear periphery can alter the expression of those genes or other regulatory genes. Tethering the yeast INO1 locus to the nuclear periphery activates the INO1 gene itself and can additionally promote either silencing or activation of other regulatory genes (Brickner and Walter 2004). Doing the same to the HXK1 gene also promotes its own transcriptional activation (Taddei et al. 2006). Tethering genes to the nuclear periphery can also be sufficient to activate an artificial promoter (Menon et al. 2005). These data appear to be contrary to the original view that the nuclear periphery is a transcriptionally silenced domain in yeast.
In addition to these observations from yeast, several intriguing studies on artificial tethering of loci have been done recently in human cells. It has been shown that a gene can be artificially targeted to the periphery and is subsequently able to recruit its transcriptional machinery (Kumaran and Spector 2008). Other work shows repression of certain loci when artificially targeted to the nuclear lamina (Reddy et al. 2008), while another report indicates that when specific chromosomes are tethered to the nuclear periphery, it is possible to change the expression patterns of certain genes to varying degrees (Finlan et al. 2008). This latter finding could have intriguing effects on human health, as aberrant gene positioning of selected marker genes may be a diagnostic tool to identify diseased alleles (e.g., Meaburn and Misteli 2008).
In addition to the relatively recently discovered phenomenon of activation at the yeast nuclear periphery, there can be a type of transcriptional “memory” at the periphery (reviewed in Ahmed and Brickner 2007). Upon activation, the INO1 and GAL1 genes are recruited to the periphery, but when repressed, they continue to remain associated with the periphery for several generations before returning to their previous location in the nucleoplasm. Studies with the GAL1 gene show that retention at the periphery allows the cells to turn these genes back on after reactivation more rapidly than nucleoplasmic GAL1 loci. Brickner and colleagues propose that cells have a mechanism to identify recently repressed genes and suggest that this is a novel type of epigenetic memory for the cell (Brickner 2009). Rapid reactivation of both GAL1 and INO1 genes and retention of either locus at the periphery are dependent on the histone variant H2A.Z (Brickner et al. 2007). In the case of the GAL1 gene, rapid reactivation is also dependent on the SWI/SNF chromatin remodeling complex and the Gal1 protein itself (Kundu et al. 2007; Zacharioudakis et al. 2007). We can compare these requirements of specific nuclear factors in the efficient expression and localization of these loci to the requirement of condensin in positioning tRNA genes to the nucleolus, where pre-tRNA synthesis and initial processing might be readily coordinated with 5S and other rRNA biosynthetic pathways.
Concluding remarks
Computers use a tool called a cache for temporary storage of data that will likely be accessed again in the near future. Access to that data from the cache is easier or faster than from where it was originally located. In other words, once a specific memory location is accessed, that location or nearby locations can be made easier to access in the short term. We can relate this to the idea of epigenetic transcriptional memory. The nuclear periphery might act as a sort of “cache” for the GAL1 and INO1 genes, and retention of these genes in the cache of the periphery allows for faster gene activation than if those genes needed to be accessed from their original location, the nucleoplasm.
Indeed, the overall idea that cells use preferential positioning of genetic loci to regulate expression and use of gene products can be seen as one of efficient information retrieval. For thousands of years, library science has dealt with the problem of storing large numbers of documents so that they may be found and accessed readily. Computers eventually made it possible to store information in numbers that were previously beyond human limits, and the subsequent science of information retrieval allowed the creation of models to facilitate orderly and efficient access of information. The prompt recovery of genetic material from within the three-dimensional space of our nuclei is a problem not entirely different from this. As an example, the placement of tRNA genes at the nucleolus facilitates orderly retrieval of those “documents” by those who are looking for them, i.e., the tRNA processing and assembly machineries. The yeast genome contains in total 6,000 genes, and humans are thought to contain 20,000–25,000 genes by most recent estimates. The rapid recovery of this large amount of information from as compact and complex of a space as the nucleus necessitates cells to develop “models” of their own for searching and using this data.
Acknowledgments
The authors thank Tom Misteli, Tom Blumenthal, and Jason Brickner for critical reading of the manuscript and members of the Engelke lab for useful discussion. This work was supported by NIH grant GM082875 to DRE and by the NIH University of Michigan Genetics Predoctoral Training Grant (T32 GM07544) to DAP.
References
- Acker J, Ozanne C, Kachouri-Lafond R, et al. Dicistronic tRNA-5S rRNA genes in Yarrowia lipolytica: an alternative TFIIIA-independent way for expression of 5S rRNA genes. Nucleic Acids Res. 2008;36:5832–5844. doi: 10.1093/nar/gkn549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Brickner JH. Regulation and epigenetic control of transcription at the nuclear periphery. Trends Genet. 2007;23:396–402. doi: 10.1016/j.tig.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989;57:347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol. 2004;11:816–821. doi: 10.1038/nsmb813. [DOI] [PubMed] [Google Scholar]
- Altuvia Y, Landgraf P, Lithwick G, et al. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J, Blagojevic J, Carter D, et al. Specialized transcription factories. Biochem Soc Symp. 2006;73:67–75. doi: 10.1042/bss0730067. [DOI] [PubMed] [Google Scholar]
- Bartova E, Kozubek S. Nuclear architecture in the light of gene expression and cell differentiation studies. Biol Cell. 2006;98:323–336. doi: 10.1042/BC20050099. [DOI] [PubMed] [Google Scholar]
- Bartova E, Kozubek S, Kozubek M, et al. The influence of the cell cycle, differentiation and irradiation on the nuclear location of the abl, bcr and c-myc genes in human leukemic cells. Leuk Res. 2000;24:233–241. doi: 10.1016/s0145-2126(99)00174-5. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Demodena J, Sternberg PW. RNA Pol II accumulates at promoters of growth genes during developmental arrest. Science. 2009;324:92–94. doi: 10.1126/science.1169628. [DOI] [PubMed] [Google Scholar]
- Belancio VP, Hedges DJ, Deininger P. Mammalian non-LTR retrotransposons: for better or worse, in sickness and in health. Genome Res. 2008;18:343–358. doi: 10.1101/gr.5558208. [DOI] [PubMed] [Google Scholar]
- Bell GI, DeGennaro LJ, Gelfand DH, et al. Ribosomal RNA genes of Saccharomyces cerevisiae. I. Physical map of the repeating unit and location of the regions coding for 5S, 5.8S, 18S, and 25S ribosomal RNAs. J Biol Chem. 1977;252:8118–8125. [PubMed] [Google Scholar]
- Berger AB, Cabal GG, Fabre E, et al. High-resolution statistical mapping reveals gene territories in live yeast. Nat Methods. 2008;5:1031–1037. doi: 10.1038/nmeth.1266. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Houser-Scott F, Kendall A, Singer RH, Engelke DR. Nucleolar localization of early tRNA processing. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T, Gleason KS. Caenorhabditis elegans operons: form and function. Nat Rev Genet. 2003;4:112–120. doi: 10.1038/nrg995. [DOI] [PubMed] [Google Scholar]
- Blumenthal T, Evans D, Link CD, et al. A global analysis of Caenorhabditis elegans operons. Nature. 2002;417:851–854. doi: 10.1038/nature00831. [DOI] [PubMed] [Google Scholar]
- Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- Bolton EC, Boeke JD. Transcriptional interactions between yeast tRNA genes, flanking genes and Ty elements: a genomic point of view. Genome Res. 2003;13:254–263. doi: 10.1101/gr.612203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006;4:e138. doi: 10.1371/journal.pbio.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand JF, Navarro F, Gadal O, Thuriaux P. Cross talk between tRNA and rRNA synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:189–195. doi: 10.1128/MCB.21.1.189-195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH. Transcriptional memory at the nuclear periphery. Curr Opin Cell Biol. 2009;21:127–133. doi: 10.1016/j.ceb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:e342. doi: 10.1371/journal.pbio.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner DG, Cajigas I, Fondufe-Mittendorf Y, et al. H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol. 2007;5:e81. doi: 10.1371/journal.pbio.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Amoils S, Horn JM, et al. Expression of alpha- and beta-globin genes occurs within different nuclear domains in haemopoietic cells. Nat Cell Biol. 2001;3:602–606. doi: 10.1038/35078577. [DOI] [PubMed] [Google Scholar]
- Brown JM, Leach J, Reittie JE, et al. Coregulated human globin genes are frequently in spatial proximity when active. J Cell Biol. 2006;172:177–187. doi: 10.1083/jcb.200507073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Green J, das Neves RP, et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J Cell Biol. 2008;182:1083–1097. doi: 10.1083/jcb.200803174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky K, Heun P, Gehlen L, Langowski J, Gasser SM. Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc Natl Acad Sci U S A. 2004;101:16495–16500. doi: 10.1073/pnas.0402766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky K, Laroche T, van Houwe G, Blaszczyk M, Gasser SM. Chromosome looping in yeast: telomere pairing and coordinated movement reflect anchoring efficiency and territorial organization. J Cell Biol. 2005;168:375–387. doi: 10.1083/jcb.200409091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal GG, Genovesio A, Rodriguez-Navarro S, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- Cao A, Moi P. Regulation of the globin genes. Pediatr Res. 2002;51:415–421. doi: 10.1203/00006450-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Carey MF, Singh K, Botchan M, Cozzarelli NR. Induction of specific transcription by RNA polymerase III in transformed cells. Mol Cell Biol. 1986;6:3068–3076. doi: 10.1128/mcb.6.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DR, Eskiw C, Cook PR. Transcription factories. Biochem Soc Trans. 2008;36:585–589. doi: 10.1042/BST0360585. [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, et al. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Drubin DA, Rando OJ, Silver PA. Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev. 2005;19:1188–1198. doi: 10.1101/gad.1307205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G. Chromosome kissing. Curr Opin Genet Dev. 2007;17:443–450. doi: 10.1016/j.gde.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Chalker DL, Sandmeyer SB. Transfer RNA genes are genomic targets for de Novo transposition of the yeast retrotransposon Ty3. Genetics. 1990;126:837–850. doi: 10.1093/genetics/126.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker DL, Sandmeyer SB. Ty3 integrates within the region of RNA polymerase III transcription initiation. Genes Dev. 1992;6:117–128. doi: 10.1101/gad.6.1.117. [DOI] [PubMed] [Google Scholar]
- Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanfreau G, Legrain P, Jacquier A. Yeast RNase III as a key processing enzyme in small nucleolar RNAs metabolism. J Mol Biol. 1998;284:975–988. doi: 10.1006/jmbi.1998.2237. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–445. doi: 10.1016/s0960-9822(02)00695-4. [DOI] [PubMed] [Google Scholar]
- Clark SG, Lu X, Horvitz HR. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994;137:987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbe N, Heck MM. Review: SMCs in the world of chromosome biology—from prokaryotes to higher eukaryotes. J Struct Biol. 2000;129:123–143. doi: 10.1006/jsbi.2000.4255. [DOI] [PubMed] [Google Scholar]
- Cobbe N, Savvidou E, Heck MM. Diverse mitotic and interphase functions of condensins in Drosophila. Genetics. 2006;172:991–1008. doi: 10.1534/genetics.105.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin DJ, Babak T, Nihranz C, Hughes TR, Engelke DR. Prediction and verification of mouse tRNA gene families. RNA Biol. 2009:6. doi: 10.4161/rna.6.2.8050. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- Cremer T, Kurz A, Zirbel R, et al. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer M, Dietzel S, et al. Chromosome territories—a functional nuclear landscape. Curr Opin Cell Biol. 2006;18:307–316. doi: 10.1016/j.ceb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio C, Schmidt CK, Katou Y, et al. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–2227. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels LM, Delany ME. Molecular and cytogenetic organization of the 5S ribosomal DNA array in chicken (Gallus gallus) Chromosome Res. 2003;11:305–317. doi: 10.1023/a:1024008522122. [DOI] [PubMed] [Google Scholar]
- de Laat W. Long-range DNA contacts: romance in the nucleus? Curr Opin Cell Biol. 2007;19:317–320. doi: 10.1016/j.ceb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Deininger PL, Batzer MA. Mammalian retroelements. Genome Res. 2002;12:1455–1465. doi: 10.1101/gr.282402. [DOI] [PubMed] [Google Scholar]
- Dej KJ, Ahn C, Orr-Weaver TL. Mutations in the Drosophila condensin subunit dCAP-G: defining the role of condensin for chromosome condensation in mitosis and gene expression in interphase. Genetics. 2004;168:895–906. doi: 10.1534/genetics.104.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine SE, Boeke JD. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 1996;10:620–633. doi: 10.1101/gad.10.5.620. [DOI] [PubMed] [Google Scholar]
- Dittmar KA, Goodenbour JM, Pan T. Tissue-specific differences in human transfer RNA expression. PLoS Genet. 2006;2:e221. doi: 10.1371/journal.pgen.0020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Kamakaka RT. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. Embo J. 2001;20:520–531. doi: 10.1093/emboj/20.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Adams CR, Rine J, Kamakaka RT. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin G. Expressed retrotransposed 5S rRNA genes in the mouse and rat genomes. Genome. 2000;43:213–215. doi: 10.1139/g99-100. [DOI] [PubMed] [Google Scholar]
- Duboule D, Dolle P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. Embo J. 1989;8:1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA. B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol. 2004;11:822–829. doi: 10.1038/nsmb812. [DOI] [PubMed] [Google Scholar]
- Faro-Trindade I, Cook PR. Transcription factories: structures conserved during differentiation and evolution. Biochem Soc Trans. 2006;34:1133–1137. doi: 10.1042/BST0341133. [DOI] [PubMed] [Google Scholar]
- Ferrigno O, Virolle T, Djabari Z, et al. Transposable B2 SINE elements can provide mobile RNA polymerase II promoters. Nat Genet. 2001;28:77–81. doi: 10.1038/ng0501-77. [DOI] [PubMed] [Google Scholar]
- Finlan LE, Sproul D, Thomson I, et al. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L, Aragon-Alcaide L, Strunnikov A. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J Cell Biol. 2000;149:811–824. doi: 10.1083/jcb.149.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot P, Kallesoe T, Reinhardt R, Chourrout D, Thompson EM. Spliced-leader RNA trans splicing in a chordate, Oikopleura dioica, with a compact genome. Mol Cell Biol. 2004;24:7795–7805. doi: 10.1128/MCB.24.17.7795-7805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6:881–892. doi: 10.1038/nrg1723. [DOI] [PubMed] [Google Scholar]
- Gasser SM. Positions of potential: nuclear organization and gene expression. Cell. 2001;104:639–642. doi: 10.1016/s0092-8674(01)00259-8. [DOI] [PubMed] [Google Scholar]
- Gerbi SA, Borovjagin AV, Lange TS. The nucleolus: a site of ribonucleoprotein maturation. Curr Opin Cell Biol. 2003;15:318–325. doi: 10.1016/s0955-0674(03)00049-8. [DOI] [PubMed] [Google Scholar]
- Gilbert N, Gilchrist S, Bickmore WA. Chromatin organization in the mammalian nucleus. Int Rev Cytol. 2005;242:283–336. doi: 10.1016/S0074-7696(04)42007-5. [DOI] [PubMed] [Google Scholar]
- Goetze S, Mateos-Langerak J, Gierman HJ, et al. The three-dimensional structure of human interphase chromosomes is related to the transcriptome map. Mol Cell Biol. 2007;27:4475–4487. doi: 10.1128/MCB.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Papalopulu N, Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Engelke DR. Spatial organization of transcription by RNA polymerase III. Nucleic Acids Res. 2006;34:4826–4836. doi: 10.1093/nar/gkl656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Pratt-Hyatt M, Good PD, Gipson TA, Engelke DR. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 2008;22:2204–2214. doi: 10.1101/gad.1675908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper ME, Price J, Korn LJ. Chromosomal mapping of Xenopus 5S genes: somatic-type versus oocyte-type. Nucleic Acids Res. 1983;11:2313–2323. doi: 10.1093/nar/11.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AS, Warburton D, Atwood KC. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci U S A. 1972;69:3394–3398. doi: 10.1073/pnas.69.11.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Verdun D. The nucleolus: a model for the organization of nuclear functions. Histochem Cell Biol. 2006;126:135–148. doi: 10.1007/s00418-006-0212-3. [DOI] [PubMed] [Google Scholar]
- Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. [DOI] [PubMed] [Google Scholar]
- Hirano T. Condensins: organizing and segregating the genome. Curr Biol. 2005;15:R265–275. doi: 10.1016/j.cub.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- Hlatky L, Sachs RK, Vazquez M, Cornforth MN. Radiation-induced chromosome aberrations: insights gained from biophysical modeling. Bioessays. 2002;24:714–723. doi: 10.1002/bies.10126. [DOI] [PubMed] [Google Scholar]
- Holland PW. Beyond the Hox: how widespread is homeobox gene clustering? J Anat. 2001;199:13–23. doi: 10.1046/j.1469-7580.2001.19910013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley WR, Mian IS, Park SJ, Rydberg B, Chatterjee A. A model for interphase chromosomes and evaluation of radiation-induced aberrations. Radiat Res. 2002;158:568–580. doi: 10.1667/0033-7587(2002)158[0568:amfica]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hu Q, Kwon YS, Nunez E, et al. Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc Natl Acad Sci U S A. 2008;105:19199–19204. doi: 10.1073/pnas.0810634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Tzou P, Sternberg PW. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell. 1994;5:395–411. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CE, Milutinovich M, Koshland D. Rings, bracelet or snaps: fashionable alternatives for Smc complexes. Philos Trans R Soc Lond B Biol Sci. 2005;360:537–542. doi: 10.1098/rstb.2004.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MW, Erickson J, Johnston M, Engelke DR. tRNA genes as transcriptional repressor elements. Mol Cell Biol. 1994;14:1266–1277. doi: 10.1128/mcb.14.2.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra FJ, Pombo A, Jackson DA, Cook PR. Active RNA polymerases are localized within discrete transcription “factories” in human nuclei. J Cell Sci. 1996;109(Pt 6):1427–1436. doi: 10.1242/jcs.109.6.1427. [DOI] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, Hassan AB, Errington RJ, Cook PR. Visualization of focal sites of transcription within human nuclei. Embo J. 1993;12:1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Perrin D, Sanchez C, Monod J. Operon: a group of genes with the expression coordinated by an operator. C R Hebd Seances Acad Sci. 1960;250:1727–1729. [PubMed] [Google Scholar]
- Jang KL, Latchman DS. HSV infection induces increased transcription of Alu repeated sequences by RNA polymerase III. FEBS Lett. 1989;258:255–258. doi: 10.1016/0014-5793(89)81667-9. [DOI] [PubMed] [Google Scholar]
- Jessberger R. The many functions of SMC proteins in chromosome dynamics. Nat Rev Mol Cell Biol. 2002;3:767–778. doi: 10.1038/nrm930. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Murray J, Sylvester JE, et al. The topographic organization of repetitive DNA in the human nucleolus. Genomics. 1993;15:123–132. doi: 10.1006/geno.1993.1020. [DOI] [PubMed] [Google Scholar]
- Kappen C, Ruddle FH. Evolution of a regulatory gene family: HOM/HOX genes. Curr Opin Genet Dev. 1993;3:931–938. doi: 10.1016/0959-437x(93)90016-i. [DOI] [PubMed] [Google Scholar]
- Kaufman TC, Lewis R, Wakimoto B. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: the homoeotic gene complex in polytene chromosome interval 84a-B. Genetics. 1980;94:115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kass DH, Deininger PL. Transcription and processing of the rodent ID repeat family in germline and somatic cells. Nucleic Acids Res. 1995;23:2245–2251. doi: 10.1093/nar/23.12.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Yu J, Han TS, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N, Weiner BM. Potential advantages of unstable interactions for pairing of chromosomes in meiotic, somatic, and premeiotic cells. Cold Spring Harb Symp Quant Biol. 1993;58:553–565. doi: 10.1101/sqb.1993.058.01.062. [DOI] [PubMed] [Google Scholar]
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- Kruszka K, Barneche F, Guyot R, et al. Plant dicistronic tRNA-snoRNA genes: a new mode of expression of the small nucleolar RNAs processed by RNase Z. Embo J. 2003;22:621–632. doi: 10.1093/emboj/cdg040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Horn PJ, Peterson CL. SWI/SNF is required for transcriptional memory at the yeast GAL gene cluster. Genes Dev. 2007;21:997–1004. doi: 10.1101/gad.1506607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Tanabe H, Yoshida K, et al. Alteration of chromosome positioning during adipocyte differentiation. J Cell Sci. 2004;117:5897–5903. doi: 10.1242/jcs.01508. [DOI] [PubMed] [Google Scholar]
- Kurshakova MM, Krasnov AN, Kopytova DV, et al. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. Embo J. 2007;26:4956–4965. doi: 10.1038/sj.emboj.7601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8:104–115. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lappin TR, Grier DG, Thompson A, Halliday HL. HOX genes: seductive science, mysterious mechanisms. Ulster Med J. 2006;75:23–31. [PMC free article] [PubMed] [Google Scholar]
- Lavoie BD, Hogan E, Koshland D. In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 2004;18:76–87. doi: 10.1101/gad.1150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader DJ, Clark GP, Watters J, et al. Clusters of multiple different small nucleolar RNA genes in plants are expressed as and processed from polycistronic pre-snoRNAs. Embo J. 1997;16:5742–5751. doi: 10.1093/emboj/16.18.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. Embo J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legagneux V, Cubizolles F, Watrin E. Multiple roles of condensins: a complex story. Biol Cell. 2004;96:201–213. doi: 10.1016/j.biolcel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Liang D, Zhou H, Zhang P, et al. A novel gene organization: intronic snoRNA gene clusters from Oryza sativa. Nucleic Acids Res. 2002;30:3262–3272. doi: 10.1093/nar/gkf426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Moghimi B, Yang TP, Strouboulis J, Bungert J. Locus control region mediated regulation of adult beta-globin gene expression. J Cell Biochem. 2008;105:9–16. doi: 10.1002/jcb.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl J. Chromosomes of the budding yeast Saccharomyces cerevisiae. Int Rev Cytol. 2003;222:141–196. doi: 10.1016/s0074-7696(02)22014-8. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. A computational screen for methylation guide snoRNAs in yeast. Science. 1999;283:1168–1171. doi: 10.1126/science.283.5405.1168. [DOI] [PubMed] [Google Scholar]
- Lunyak VV, Prefontaine GG, Nunez E, et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science. 2007;317:248–251. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- Lupo R, Breiling A, Bianchi ME, Orlando V. Drosophila chromosome condensation proteins topoisomerase II and barren colocalize with polycomb and maintain Fab-7 PRE silencing. Mol Cell. 2001;7:127–136. doi: 10.1016/s1097-2765(01)00161-7. [DOI] [PubMed] [Google Scholar]
- Mahy NL, Perry PE, Bickmore WA. Gene density and transcription influence the localization of chromatin outside of chromosome territories detectable by FISH. J Cell Biol. 2002a;159:753–763. doi: 10.1083/jcb.200207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy NL, Perry PE, Gilchrist S, Baldock RA, Bickmore WA. Spatial organization of active and inactive genes and noncoding DNA within chromosome territories. J Cell Biol. 2002b;157:579–589. doi: 10.1083/jcb.200111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RS. Why are Hox genes clustered? Bioessays. 1997;19:661–664. doi: 10.1002/bies.950190804. [DOI] [PubMed] [Google Scholar]
- Mao J, Appel B, Schaack J, et al. The 5S RNA genes of Schizosaccharomyces pombe. Nucleic Acids Res. 1982;10:487–500. doi: 10.1093/nar/10.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner PD, Walters RD, Espinoza CA, et al. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Matarazzo MR, Boyle S, D’Esposito M, Bickmore WA. Chromosome territory reorganization in a human disease with altered DNA methylation. Proc Natl Acad Sci U S A. 2007;104:16546–16551. doi: 10.1073/pnas.0702924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–781. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- Meaburn KJ, Misteli T. Locus-specific and activity-independent gene repositioning during early tumorigenesis. J Cell Biol. 2008;180:39–50. doi: 10.1083/jcb.200708204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendjan S, Taipale M, Kind J, et al. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell. 2006;21:811–823. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Menon BB, Sarma NJ, Pasula S, et al. Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc Natl Acad Sci U S A. 2005;102:5749–5754. doi: 10.1073/pnas.0501768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzenberg RL, Stevens JN, Selker EU, Morzycka-Wroblewska E. Identification and chromosomal distribution of 5S rRNA genes in Neurospora crassa. Proc Natl Acad Sci U S A. 1985;82:2067–2071. doi: 10.1073/pnas.82.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa N, Nakamura T, Kokubu A, et al. Dissection of the essential steps for condensin accumulation at kinetochores and rDNAs during fission yeast mitosis. J Cell Biol. 2008;180:1115–1131. doi: 10.1083/jcb.200708170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves H, Ramos C, da Silva MG, Parreira A, Parreira L. The nuclear topography of ABL, BCR, PML, and RARalpha genes: evidence for gene proximity in specific phases of the cell cycle and stages of hematopoietic differentiation. Blood. 1999;93:1197–1207. [PubMed] [Google Scholar]
- Noordermeer D, de Laat W. Joining the loops: beta-globin gene regulation. IUBMB Life. 2008;60:824–833. doi: 10.1002/iub.129. [DOI] [PubMed] [Google Scholar]
- Oakes M, Nogi Y, Clark MW, Nomura M. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol Cell Biol. 1993;13:2441–2455. doi: 10.1128/mcb.13.4.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes M, Aris JP, Brockenbrough JS, et al. Mutational analysis of the structure and localization of the nucleolus in the yeast Saccharomyces cerevisiae. J Cell Biol. 1998;143:23–34. doi: 10.1083/jcb.143.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N. SINEs. Curr Opin Genet Dev. 1991;1:498–504. doi: 10.1016/s0959-437x(05)80198-4. [DOI] [PubMed] [Google Scholar]
- Olson MO, Dundr M. The moving parts of the nucleolus. Histochem Cell Biol. 2005;123:203–216. doi: 10.1007/s00418-005-0754-9. [DOI] [PubMed] [Google Scholar]
- Osborne CS, Chakalova L, Brown KE, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- Osborne CS, Chakalova L, Mitchell JA, et al. Myc dynamically and preferentially relocates to a transcription factory occupied by Igh. PLoS Biol. 2007;5:e192. doi: 10.1371/journal.pbio.0050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AP. Cyclophilin and protein disulfide isomerase genes are co-transcribed in a functionally related manner in Caenorhabditis elegans. DNA Cell Biol. 1997;16:1335–1343. doi: 10.1089/dna.1997.16.1335. [DOI] [PubMed] [Google Scholar]
- Palstra RJ, de Laat W, Grosveld F. Beta-globin regulation and long-range interactions. Adv Genet. 2008;61:107–142. doi: 10.1016/S0065-2660(07)00004-1. [DOI] [PubMed] [Google Scholar]
- Parada L, Misteli T. Chromosome positioning in the interphase nucleus. Trends Cell Biol. 2002;12:425–432. doi: 10.1016/s0962-8924(02)02351-6. [DOI] [PubMed] [Google Scholar]
- Parada LA, McQueen PG, Misteli T. Tissue-specific spatial organization of genomes. Genome Biol. 2004;5:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo A, Cook PR. The localization of sites containing nascent RNA and splicing factors. Exp Cell Res. 1996;229:201–203. doi: 10.1006/excr.1996.0360. [DOI] [PubMed] [Google Scholar]
- Pombo A, Jones E, Iborra FJ, et al. Specialized transcription factories within mammalian nuclei. Crit Rev Eukaryot Gene Expr. 2000;10:21–29. [PubMed] [Google Scholar]
- Prieto JL, McStay B. Nucleolar biogenesis: the first small steps. Biochem Soc Trans. 2005;33:1441–1443. doi: 10.1042/BST0331441. [DOI] [PubMed] [Google Scholar]
- Proudfoot NJ, Shander MH, Manley JL, Gefter ML, Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980;209:1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F, Bachellerie JP, Puvion E. Nucleolar organization of HeLa cells as studied by in situ hybridization. Chromosoma. 1991;100:395–409. doi: 10.1007/BF00337518. [DOI] [PubMed] [Google Scholar]
- Qu LH, Henras A, Lu YJ, et al. Seven novel methylation guide small nucleolar RNAs are processed from a common polycistronic transcript by Rat1p and RNase III in yeast. Mol Cell Biol. 1999;19:1144–1158. doi: 10.1128/mcb.19.2.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragoczy T, Bender MA, Telling A, Byron R, Groudine M. The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation. Genes Dev. 2006;20:1447–1457. doi: 10.1101/gad.1419506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse I, Perlman MD, Scalzo D, et al. The emergence of lineage-specific chromosomal topologies from coordinate gene regulation. Proc Natl Acad Sci U S A. 2009;106:6679–6684. doi: 10.1073/pnas.0900986106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Sachs RK, Levy D, Chen AM, et al. Random breakage and reunion chromosome aberration formation model; an interaction-distance version based on chromatin geometry. Int J Radiat Biol. 2000;76:1579–1588. doi: 10.1080/09553000050201064. [DOI] [PubMed] [Google Scholar]
- Saffer JD, Thurston SJ. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989;9:355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela K, Baltimore D. Negative regulation of immunoglobulin kappa light-chain gene transcription by a short sequence homologous to the murine B1 repetitive element. Mol Cell Biol. 1993;13:3698–3705. doi: 10.1128/mcb.13.6.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma NJ, Haley TM, Barbara KE, et al. Glucose-responsive regulators of gene expression in Saccharomyces cerevisiae function at the nuclear periphery via a reverse recruitment mechanism. Genetics. 2007;175:1127–1135. doi: 10.1534/genetics.106.068932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U, Weisenberger D. The nucleolus. Curr Opin Cell Biol. 1994;6:354–359. doi: 10.1016/0955-0674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Schwarzacher HG, Wachtler F. The functional significance of nucleolar structures. Ann Genet. 1991;34:151–160. [PubMed] [Google Scholar]
- Sexton T, Umlauf D, Kurukuti S, Fraser P. The role of transcription factories in large-scale structure and dynamics of interphase chromatin. Semin Cell Dev Biol. 2007;18:691–697. doi: 10.1016/j.semcdb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Shen W, Liu DP, Liang CC. The regulatory network controlling beta-globin gene switching. Mol Biol Rep. 2001;28:175–183. doi: 10.1023/a:1015226103934. [DOI] [PubMed] [Google Scholar]
- Simms TA, Dugas SL, Gremillion JC, et al. TFIIIC binding sites function as both heterochromatin barriers and chromatin insulators in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:2078–2086. doi: 10.1128/EC.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J, Brooke G, Kuersten S, Lea K, Blumenthal T. Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell. 1993;73:521–532. doi: 10.1016/0092-8674(93)90139-h. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- Stadler S, Schnapp V, Mayer R, et al. The architecture of chicken chromosome territories changes during differentiation. BMC Cell Biol. 2004;5:44. doi: 10.1186/1471-2121-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, et al. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–778. doi: 10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Gudla PR, Guo L, Lockett S, Misteli T. Allele-specific nuclear positioning of the monoallelically expressed marker GFAP. Genes Dev. 2008;22:489–498. doi: 10.1101/gad.1634608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Haeusler RA, Good PD, Engelke DR. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorey IS, Cecena G, Reynolds W, Oshima RG. Alu sequence involvement in transcriptional insulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993;13:6742–6751. doi: 10.1128/mcb.13.11.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolerico LH, Benko AL, Aris JP, et al. Saccharomyces cerevisiae Mod5p-II contains sequences antagonistic for nuclear and cytosolic locations. Genetics. 1999;151:57–75. doi: 10.1093/genetics/151.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Tomilin NV, Iguchi-Ariga SM, Ariga H. Transcription and replication silencer element is present within conserved region of human Alu repeats interacting with nuclear protein. FEBS Lett. 1990;263:69–72. doi: 10.1016/0014-5793(90)80707-p. [DOI] [PubMed] [Google Scholar]
- Treinin M, Gillo B, Liebman L, Chalfie M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci U S A. 1998;95:15492–15495. doi: 10.1073/pnas.95.26.15492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CK, Li H, Zheng XS. Nutrient starvation promotes condensin loading to maintain rDNA stability. Embo J. 2007a;26:448–458. doi: 10.1038/sj.emboj.7601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CK, Wei Y, Zheng XF. Compacting DNA during the interphase: condensin maintains rDNA integrity. Cell Cycle. 2007b;6:2213–2218. doi: 10.4161/cc.6.18.4733. [DOI] [PubMed] [Google Scholar]
- Tycowski KT, Steitz JA. Non-coding snoRNA host genes in Drosophila: expression strategies for modification guide snoR-NAs. Eur J Cell Biol. 2001;80:119–125. doi: 10.1078/0171-9335-00150. [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Hopfner KP. Chromosome biology: the crux of the ring. Curr Biol. 2006;16:R102–105. doi: 10.1016/j.cub.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Volpi EV, Chevret E, Jones T, et al. Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci. 2000;113(Pt 9):1565–1576. doi: 10.1242/jcs.113.9.1565. [DOI] [PubMed] [Google Scholar]
- Wang BD, Strunnikov A. Transcriptional homogenization of rDNA repeats in the episome-based nucleolus induces genome-wide changes in the chromosomal distribution of condensin. Plasmid. 2008;59:45–53. doi: 10.1016/j.plasmid.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BD, Eyre D, Basrai M, Lichten M, Strunnikov A. Condensin binding at distinct and specific chromosomal sites in the Saccharomyces cerevisiae genome. Mol Cell Biol. 2005a;25:7216–7225. doi: 10.1128/MCB.25.16.7216-7225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Haeusler RA, Good PD, et al. Silencing near tRNA genes requires nucleolar localization. J Biol Chem. 2005b;280:8637–8639. doi: 10.1074/jbc.C500017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink DG, Schul W, van der Kraan I, et al. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993;122:283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Wegel E, Shaw P. Gene activation and deactivation related changes in the three-dimensional structure of chromatin. Chromosoma. 2005;114:331–337. doi: 10.1007/s00412-005-0015-7. [DOI] [PubMed] [Google Scholar]
- Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci U S A. 2009;106:5569–5574. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharioudakis I, Gligoris T, Tzamarias D. A yeast catabolic enzyme controls transcriptional memory. Curr Biol. 2007;17:2041–2046. doi: 10.1016/j.cub.2007.10.044. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Xin L, Song W, et al. Active chromatin hub of the mouse alpha-globin locus forms in a transcription factory of clustered housekeeping genes. Mol Cell Biol. 2006;26:5096–5105. doi: 10.1128/MCB.02454-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D, Amaral MD, Englmann A, et al. Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J Cell Biol. 2004;166:815–825. doi: 10.1083/jcb.200404107. [DOI] [PMC free article] [PubMed] [Google Scholar]