Abstract
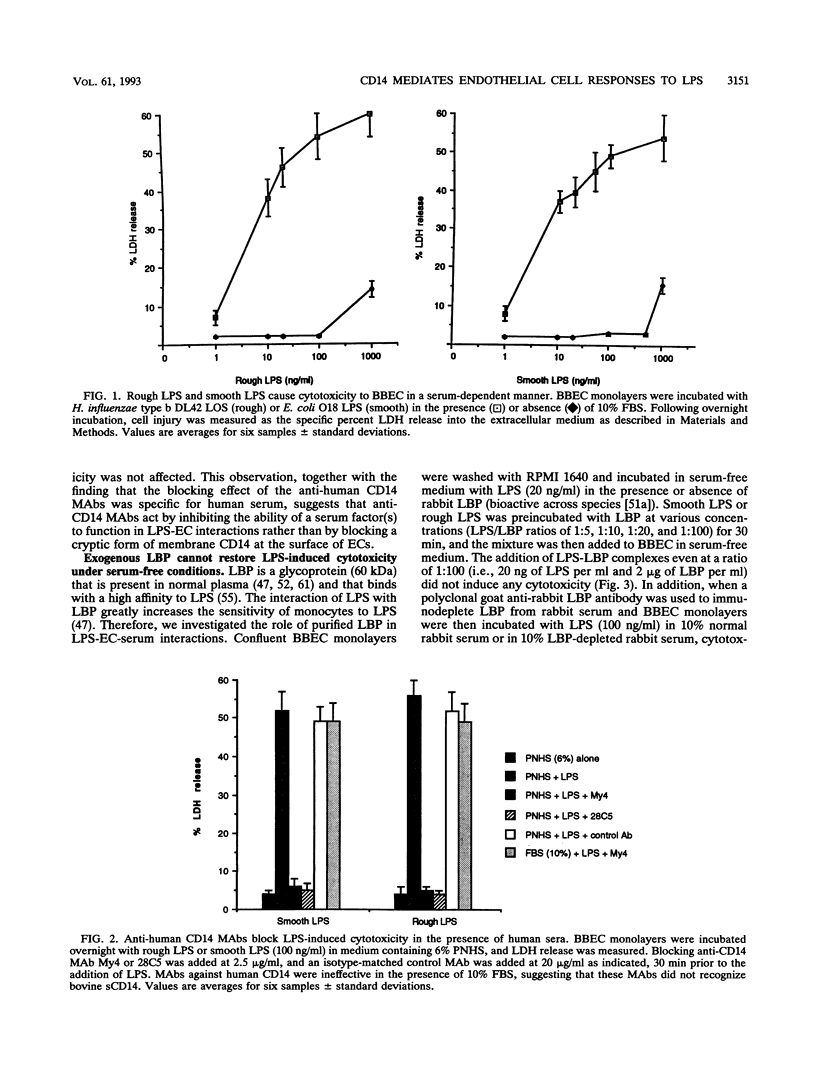
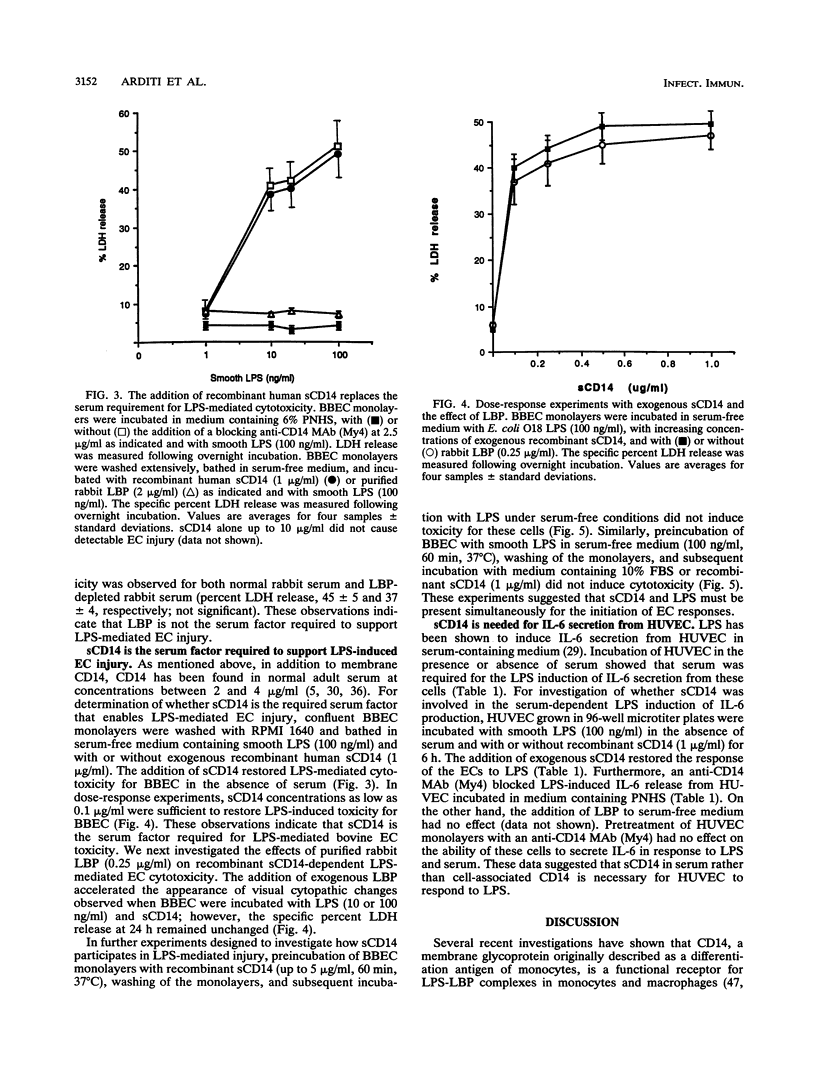
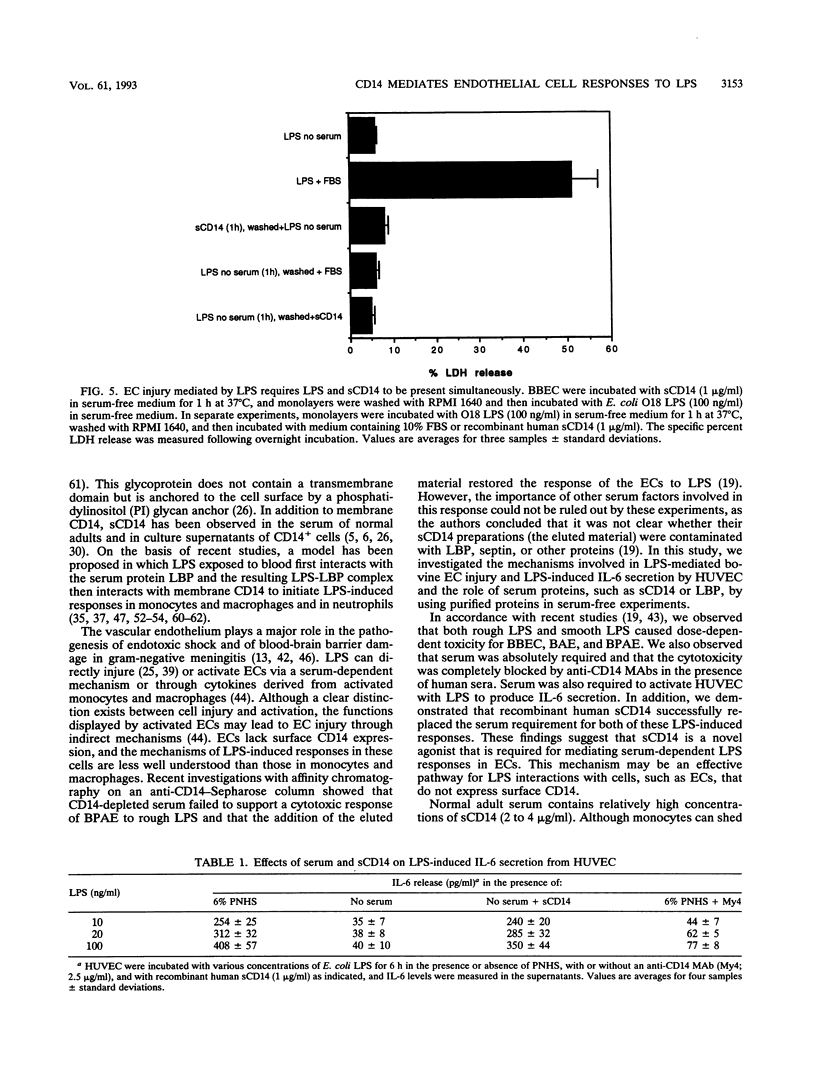
Vascular endothelial cell (EC) injury by lipopolysaccharides (LPS) plays a major role in the pathogenesis of gram-negative bacterial sepsis and endotoxic shock. The studies described here were performed to define further the molecular mechanisms involved in the EC responses to LPS. We showed that serum was required for LPS-mediated cytotoxicity for bovine brain microvessel, pulmonary, and aortic ECs and that anti-human CD14 antibodies completely blocked LPS-mediated cytotoxicity for ECs in the presence of human serum. The addition of a recombinant soluble form of human CD14 to serum-free medium restored the LPS-mediated cytotoxicity, whereas the addition of LPS binding protein (LBP), a serum protein that potentiates LPS-induced responses to monocytes, had no effect. A similar dependency on serum or recombinant soluble CD14 (under serum-free conditions) was observed for LPS-induced secretion of interleukin-6 by human umbilical vein ECs. These findings indicate that soluble CD14 is required for LPS-mediated EC responses independently of LPB, suggesting that serum soluble CD14 represents a naturally occurring agonist for EC responses to LPS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Ball E. D., Graziano R. F., Shen L., Fanger M. W. Monoclonal antibodies to novel myeloid antigens reveal human neutrophil heterogeneity. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5374–5378. doi: 10.1073/pnas.79.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger C., Ulevitch R. J., Dayer J. M. Modulation of endotoxic activity of lipopolysaccharide by high-density lipoprotein. Pathobiology. 1991;59(6):378–383. doi: 10.1159/000163681. [DOI] [PubMed] [Google Scholar]
- Bazil V., Baudys M., Hilgert I., Stefanová I., Low M. G., Zbrozek J., Horejsí V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989 Jul;26(7):657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Bazil V., Strominger J. L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991 Sep 1;147(5):1567–1574. [PubMed] [Google Scholar]
- Beekhuizen H., Blokland I., Corsèl-van Tilburg A. J., Koning F., van Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol. 1991 Dec 1;147(11):3761–3767. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle A. M., Jayaram Y., Hogg N. Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol. 1990 Aug;81(2):339–345. doi: 10.1111/j.1365-2249.1990.tb03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier C., Haeffner-Cavaillon N., Caroff M., Kazatchkine M. D. Binding sites for endotoxins (lipopolysaccharides) on human monocytes. J Immunol. 1991 Sep 15;147(6):1899–1904. [PubMed] [Google Scholar]
- Couturier C., Jahns G., Kazatchkine M. D., Haeffner-Cavaillon N. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-alpha by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1992 Jun;22(6):1461–1466. doi: 10.1002/eji.1830220619. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Cross A. S., Sadoff J. C., Fürer E. Synthesis and characterization of Escherichia coli O18 O-polysaccharide conjugate vaccines. Infect Immun. 1990 Feb;58(2):373–377. doi: 10.1128/iai.58.2.373-377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S. H., Haas R., Jentoft N., Rosenberry T. L., Tartakoff A. M. The glycophospholipid anchor of Thy-1. Biosynthetic labeling experiments with wild-type and class E Thy-1 negative lymphomas. J Biol Chem. 1987 Apr 5;262(10):4728–4732. [PubMed] [Google Scholar]
- Ferrero E., Hsieh C. L., Francke U., Goyert S. M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990 Jul 1;145(1):331–336. [PubMed] [Google Scholar]
- Ferrero E., Jiao D., Tsuberi B. Z., Tesio L., Rong G. W., Haziot A., Goyert S. M. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey E. A., Miller D. S., Jahr T. G., Sundan A., Bazil V., Espevik T., Finlay B. B., Wright S. D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992 Dec 1;176(6):1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz I. E., Warren J., Estrada C., Roberts E., Krause D. N. Long-term serial cultivation of arterial and capillary endothelium from adult bovine brain. In Vitro Cell Dev Biol. 1985 Mar;21(3 Pt 1):172–180. doi: 10.1007/BF02621355. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E. M., Seremetis S. V., Winchester R. J., Silver J., Mattison A. C. Biochemistry and expression of myelomonocytic antigens. J Immunol. 1986 Dec 15;137(12):3909–3914. [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E., Rettig W. J., Yenamandra A. K., Obata F., Le Beau M. M. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988 Jan 29;239(4839):497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Shively J. E., Silver J. Biochemical characterization of a second family of human Ia molecules, HLA-DS, equivalent to murine I-A subregion molecules. J Exp Med. 1982 Aug 1;156(2):550–566. doi: 10.1084/jem.156.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Krüger C., Schütt C., Obertacke U., Joka T., Müller F. E., Knöller J., Köller M., König W., Schönfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991 Aug;85(2):297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laug W. E., Tokes Z. A., Benedict W. F., Sorgente N. Anchorage independent growth and plasminogen activator production by bovine endothelial cells. J Cell Biol. 1980 Feb;84(2):281–293. doi: 10.1083/jcb.84.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Kato K., Tobias P. S., Kirkland T. N., Ulevitch R. J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992 Jun 1;175(6):1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Lynn W. A., Raetz C. R., Qureshi N., Golenbock D. T. Lipopolysaccharide-induced stimulation of CD11b/CD18 expression on neutrophils. Evidence of specific receptor-based response and inhibition by lipid A-based antagonists. J Immunol. 1991 Nov 1;147(9):3072–3079. [PubMed] [Google Scholar]
- Maliszewski C. R., Ball E. D., Graziano R. F., Fanger M. W. Isolation and characterization of My23, a myeloid cell-derived antigen reactive with the monoclonal antibody AML-2-23. J Immunol. 1985 Sep;135(3):1929–1936. [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J., Tobias P., Wolfson E., Ulevitch R. Regulatory mechanisms of host responsiveness to endotoxin (lipopolysaccharide). Pathobiology. 1991;59(3):185–188. doi: 10.1159/000163641. [DOI] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Patrick D., Betts J., Frey E. A., Prameya R., Dorovini-Zis K., Finlay B. B. Haemophilus influenzae lipopolysaccharide disrupts confluent monolayers of bovine brain endothelial cells via a serum-dependent cytotoxic pathway. J Infect Dis. 1992 May;165(5):865–872. doi: 10.1093/infdis/165.5.865. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Quagliarello V., Scheld W. M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992 Sep 17;327(12):864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Schütt C., Schilling T., Grunwald U., Schönfeld W., Krüger C. Endotoxin-neutralizing capacity of soluble CD14. Res Immunol. 1992 Jan;143(1):71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Espevik T. Cytokine regulation of interleukin 6 production by human endothelial cells. Cell Immunol. 1989 Jul;121(2):372–382. doi: 10.1016/0008-8749(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., Vukajlovich S. W., Morrison D. C. Endotoxin interactions with serum proteins relationship to biological activity. Prog Clin Biol Res. 1988;272:47–62. [PubMed] [Google Scholar]
- Tobias P. S., Mathison J. C., Ulevitch R. J. A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J Biol Chem. 1988 Sep 25;263(27):13479–13481. [PubMed] [Google Scholar]
- Tobias P. S., Mathison J., Mintz D., Lee J. D., Kravchenko V., Kato K., Pugin J., Ulevitch R. J. Participation of lipopolysaccharide-binding protein in lipopolysaccharide-dependent macrophage activation. Am J Respir Cell Mol Biol. 1992 Sep;7(3):239–245. doi: 10.1165/ajrcmb/7.3.239. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Interactions of bacterial lipopolysaccharides with serum proteins. Prog Clin Biol Res. 1988;272:309–318. [PubMed] [Google Scholar]
- Wispelwey B., Hansen E. J., Scheld W. M. Haemophilus influenzae outer membrane vesicle-induced blood-brain barrier permeability during experimental meningitis. Infect Immun. 1989 Aug;57(8):2559–2562. doi: 10.1128/iai.57.8.2559-2562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Tobias P. S., Ulevitch R. J., Ramos R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989 Oct 1;170(4):1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J. Protective antibody to endotoxin core: the emperor's new clothes? J Infect Dis. 1988 Aug;158(2):286–290. doi: 10.1093/infdis/158.2.286. [DOI] [PubMed] [Google Scholar]


