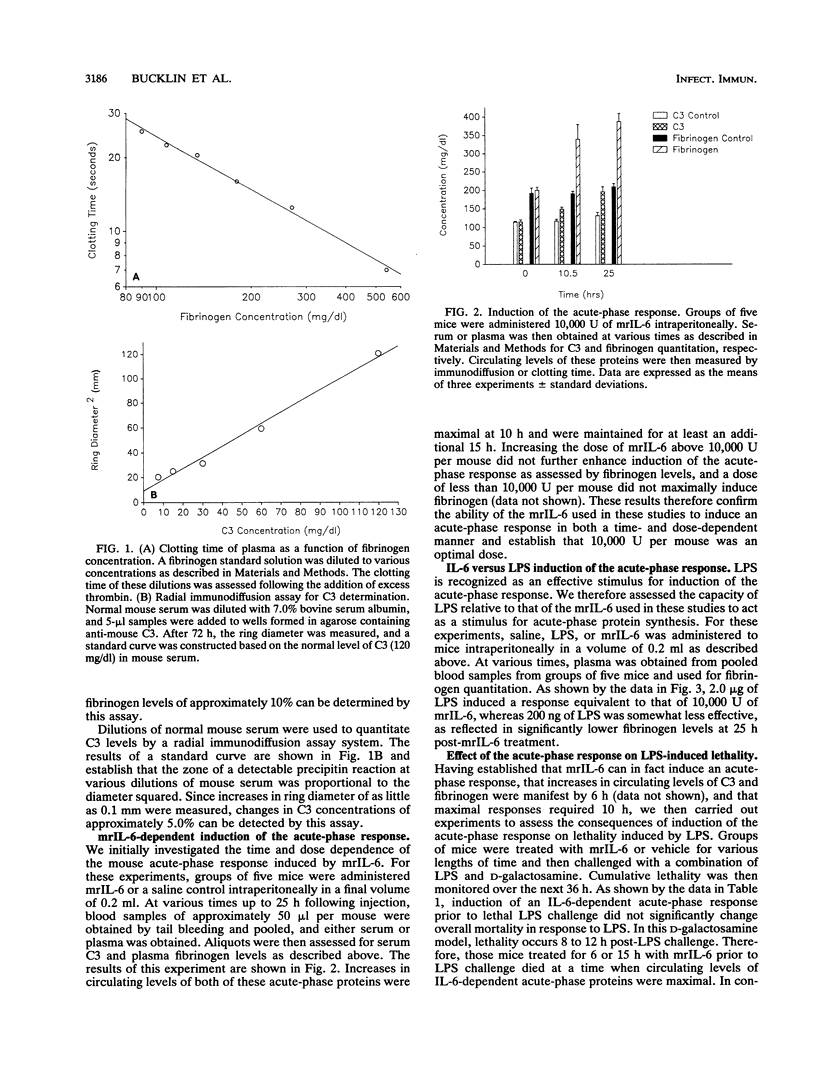
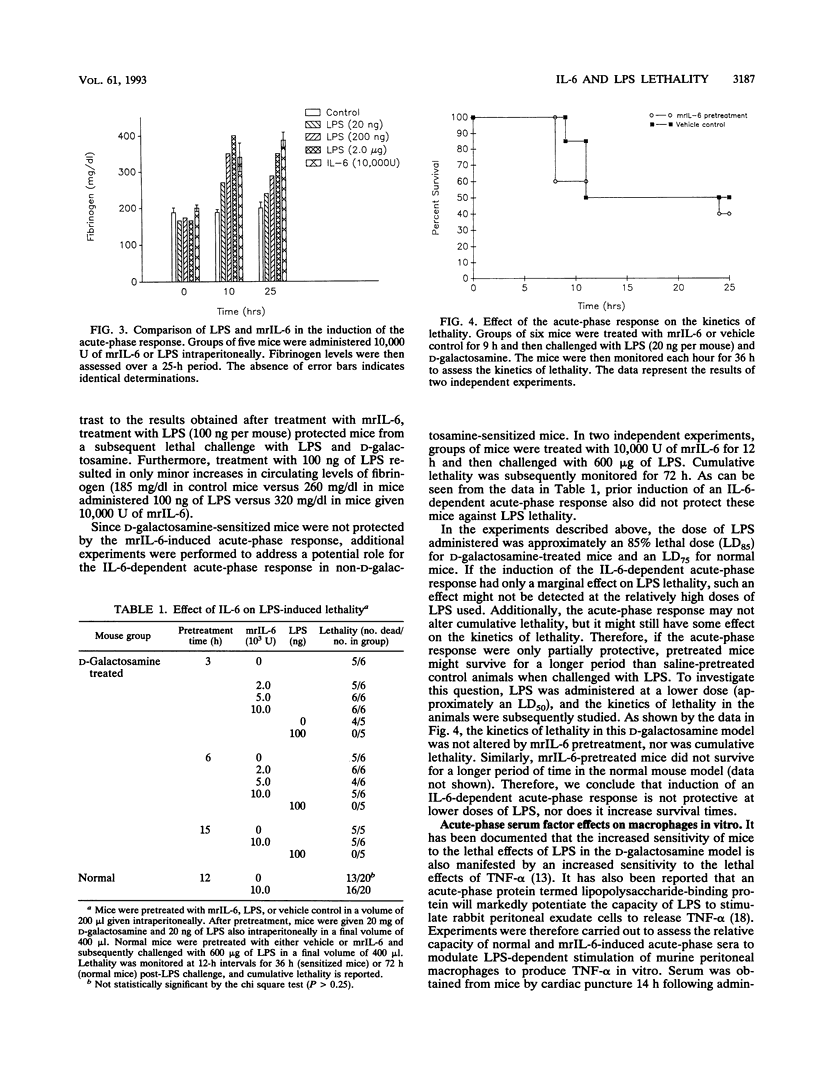
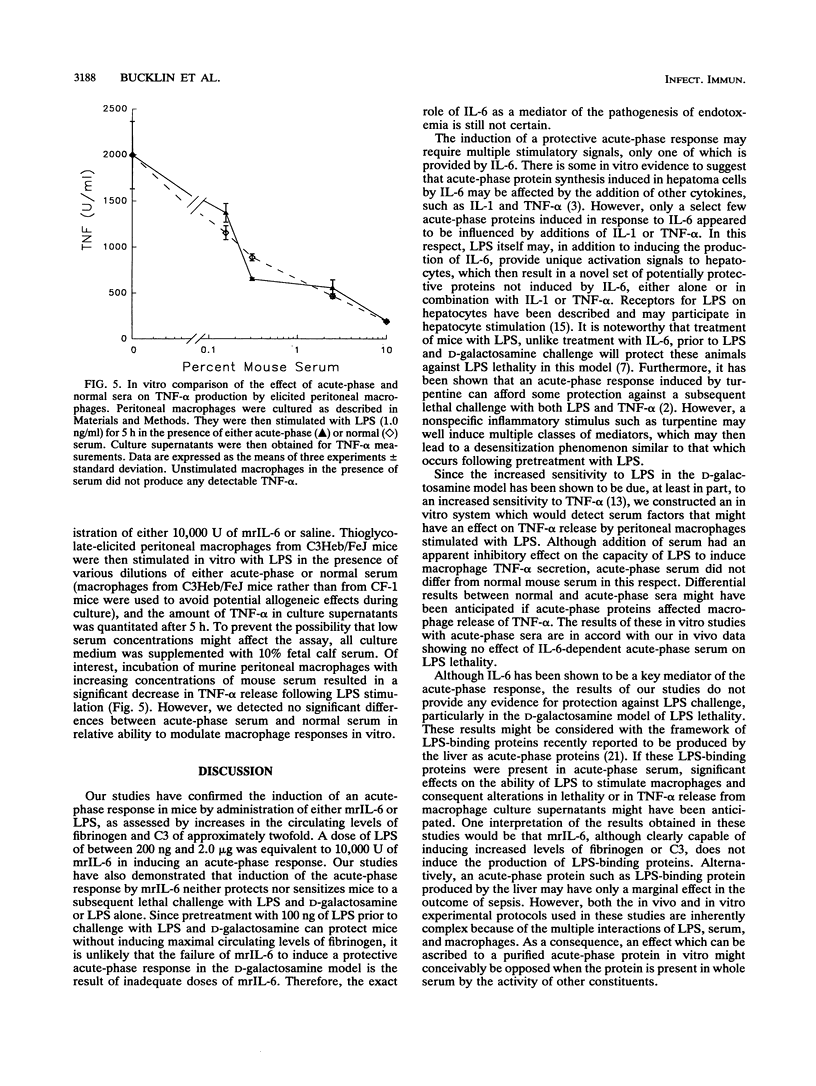
Abstract
Lipopolysaccharide (LPS), a component of gram-negative bacterial outer cell walls, can stimulate lymphoreticular cells to produce cytokines such as tumor necrosis factor alpha (TNF-alpha), interleukin-1 (IL-1), and IL-6. One of these proinflammatory cytokines, IL-6, induces hepatic synthesis of a class of proteins termed acute-phase proteins. D-Galactosamine inhibits acute-phase protein synthesis and concurrently sensitizes mice to a lethal dose of LPS approximately 10,000-fold. From these observations, we hypothesized that the acute-phase response may serve as a defense mechanism for protection of the host against the deleterious effects of LPS. To test this hypothesis, murine recombinant IL-6 (mrIL-6) was used to induce an acute-phase response prior to a lethal LPS challenge in both D-galactosamine-treated and normal mice. Induction of the acute-phase response by mrIL-6 was quantitated by measuring the concentrations of fibrinogen and complement component C3, two well-characterized acute-phase proteins, in the circulation. The effect of acute-phase and normal serum on TNF-alpha release by peritoneal macrophages stimulated with LPS in vitro was also examined. The results of these studies confirmed the induction of the acute-phase response by mrIL-6, as reflected in an approximate doubling in circulating levels of fibrinogen and C3. However, when either D-galactosamine-sensitized or normal mice were challenged with a lethal dose of LPS at various times after mrIL-6 administration, the acute-phase response induced by mrIL-6 did not alter either cumulative lethality or the kinetics of lethality. Additionally, compared with normal serum, acute-phase serum did not affect TNF-alpha release by peritoneal macrophages following LPS-mediated stimulation in vitro. Collectively, these studies would not support a dominant role for an IL-6-mediated acute-phase response as contributing to the resistance of normal mice compared with D-galactosamine-sensitized mice in LPS-induced lethal toxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Alcorn J. M., Fierer J., Chojkier M. The acute-phase response protects mice from D-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology. 1992 Jan;15(1):122–129. doi: 10.1002/hep.1840150121. [DOI] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Kishimoto T., Tran-Thi T. A., Decker K., Heinrich P. C. Regulation of synthesis and secretion of major rat acute-phase proteins by recombinant human interleukin-6 (BSF-2/IL-6) in hepatocyte primary cultures. Eur J Biochem. 1988 Apr 15;173(2):287–293. doi: 10.1111/j.1432-1033.1988.tb13997.x. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Keppler D. O., Pausch J., Decker K. Selective uridine triphosphate deficiency induced by D-galactosamine in liver and reversed by pyrimidine nucleotide precursors. Effect on ribonucleic acid synthesis. J Biol Chem. 1974 Jan 10;249(1):211–216. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Parent J. B. Membrane receptors on rat hepatocytes for the inner core region of bacterial lipopolysaccharides. J Biol Chem. 1990 Feb 25;265(6):3455–3461. [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Fielder A. H., Mirjah D. D. Isolation and study of murine C3. Immunology. 1977 Oct;33(4):491–499. [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Silverstein R., Turley B. R., Christoffersen C. A., Johnson D. C., Morrison D. C. Hydrazine sulfate protects D-galactosamine-sensitized mice against endotoxin and tumor necrosis factor/cachectin lethality: evidence of a role for the pituitary. J Exp Med. 1991 Feb 1;173(2):357–365. doi: 10.1084/jem.173.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Chedid L. A. Strategies for the treatment of endotoxemia: significance of the acute-phase response. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S630–S638. doi: 10.1093/clinids/9.supplement_5.s630. [DOI] [PubMed] [Google Scholar]
- van Vugt H., van Gool J., de Ridder L. Alpha 2 macroglobulin of the rat, an acute phase protein, mitigates the early course of endotoxin shock. Br J Exp Pathol. 1986 Jun;67(3):313–319. [PMC free article] [PubMed] [Google Scholar]


