Abstract
OBJECTIVE
Cytokines contribute to pancreatic β-cell death in type 1 diabetes. This effect is mediated by complex gene networks that remain to be characterized. We presently utilized array analysis to define the global expression pattern of genes, including spliced variants, modified by the cytokines interleukin (IL)-1β + interferon (IFN)-γ and tumor necrosis factor (TNF)-α + IFN-γ in primary rat β-cells.
RESEARCH DESIGN AND METHODS
Fluorescence-activated cell sorter–purified rat β-cells were exposed to IL-1β + IFN-γ or TNF-α + IFN-γ for 6 or 24 h, and global gene expression was analyzed by microarray. Key results were confirmed by RT-PCR, and small-interfering RNAs were used to investigate the mechanistic role of novel and relevant transcription factors identified by pathway analysis.
RESULTS
Nearly 16,000 transcripts were detected as present in β-cells, with temporal differences in the number of genes modulated by IL-1β + IFNγ or TNF-α + IFN-γ. These cytokine combinations induced differential expression of inflammatory response genes, which is related to differential induction of IFN regulatory factor-7. Both treatments decreased the expression of genes involved in the maintenance of β-cell phenotype and growth/regeneration. Cytokines induced hypoxia-inducible factor-α, which in this context has a proapoptotic role. Cytokines also modified the expression of >20 genes involved in RNA splicing, and exon array analysis showed cytokine-induced changes in alternative splicing of >50% of the cytokine-modified genes.
CONCLUSIONS
The present study doubles the number of known genes expressed in primary β-cells, modified or not by cytokines, and indicates the biological role for several novel cytokine-modified pathways in β-cells. It also shows that cytokines modify alternative splicing in β-cells, opening a new avenue of research for the field.
Type 1 diabetes is an autoimmune disease characterized by a progressive and selective destruction of the pancreatic β-cells. During insulitis, activated macrophages and T-cells release cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ in the vicinity of the β-cells, contributing for β-cell dysfunction and apoptosis (1,2). Expression of TNF-α and IL-1β was observed in pancreas of patients with recent type 1 diabetes onset and in animal models of the disease (1–3), prompting clinical trials based on the use of blockers of TNF-α (4) or IL-1β (5) to prevent type 1 diabetes.
In vitro exposure of rodent or human β-cells to IL-1β + IFN-γ or TNF-α + IFN-γ, but not to any of these cytokines alone, triggers β-cell apoptosis (1,6). IL-1β + IFN-γ affects the expression of several gene networks in β-cells, modulating pro- and antiapoptotic pathways, expression of cytokines and chemokines, and decreasing expression of genes involved in β-cell function (2,6–10). Less is known about the genes induced by TNF-α; both cytokines induce the key transcription factor nuclear factor (NF)-κB (11), but they affect kinase cascade pathways differently, such as IκB kinase, with the potential to trigger a differential gene expression outcome (11,12). We have previously addressed this issue by using a target microarray, the Apochip (13), to compare IL-1β– and TNF-α–induced genes. The findings obtained indicated some differences between these cytokines, mostly related to intensity of gene expression (12). These observations, however, were biased by the choice and limited number of probes included in the Apochip. Moreover, neither the Apochip nor usually utilized cDNA arrays (7–9) have the ability to identify splice variants of genes. This is a significant limitation, since recent data suggest that regulation of alternative splicing is of major importance for regulation of proteomic diversity and for cell physiology/pathology (14–16).
Cytokine composition and its respective concentrations may vary during insulitis, depending on the timing, degree of islet infiltration, immune cells present, and the pancreatic β-cell responses to the immune assault (10). This may explain why blocking TNF-α or IL-1β at different stages of the pre-diabetic period may be more or less effective in preventing diabetes in rodent models (1,17), suggesting that the contribution of the different cytokines and their downstream signaling pathways may also vary between individual type 1 diabetic patients. This reinforces the need for understanding separately and in detail the gene networks downstream of IL-1β + IFN-γ and TNF-α + IFN-γ, with the ultimate goal of devising targeted and individualized therapies to preserve β-cells in early type 1 diabetes. We have presently addressed this question by using primary rat β-cells treated for 6 or 24 h with combinations of IL-1β + IFN-γ and TNF-α + IFN-γ and performing array analysis using first the latest Affymetrix microarray, covering >28,000 genes, and then the Affymetrix exon-array, covering ∼850,000 exons and having the potential to identify most splice variants present in a cell. This was followed by global analysis of gene expression using Ingenuity Pathway Analysis (IPA) software, which indicated networks of special interest for subsequent studies. The data obtained doubles the number of known genes expressed in primary rat β-cells, modified or not by cytokines, and identifies several novel cytokine-modified pathways in β-cells, including cytokines/chemokines, Krebs cycle genes, hormone receptors, and hypoxia-inducible factor (HIF)-1α–regulated genes. It also indicates that cytokines modify alternative splicing in β-cells, opening a new avenue of research in the field.
RESEARCH DESIGN AND METHODS
Cell culture and cytokine exposure; viability and Western blot assay; nitric oxide and chemokine (CC-motif) ligand (CCL) 5 measurement; sample preparation for array analysis; real-time RT-PCR and normal PCR; immunofluorescence; promoter in silico analysis and promoter reporter assay are available at the online appendix Supplementary Methods at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1159/DC1.
Gene expression array data analysis.
The GeneChip Rat Genome 230 2.0 arrays (Affymetrix), containing 31,099 probesets representing >28,000 rat genes was used in the study. The GC-Robust MultiChip Average (GCRMA) (18) was used, as part of the GCRMA package in the Bioconductor site (http://www.bioconductor.org), to preprocess the raw data (CEL files). For the analysis, see Supplementary Methods. Pathway analysis was done by IPA 5.5 software.
Exon-array data analysis.
The CEL files corresponding to the GeneChip Rat Exon 1.0 ST Arrays (Affymetrix) were imported and analyzed by the ArrayAssist Exon software (Stratagene Software Solutions), as described in Supplementary Methods.
RNA interference.
Small-interfering RNA (siRNA) against activating factor (ATF) 4, HIF-1α, and IFN regulatory factor (IRF)-7 (supplementary Table 2), were used to knock down expression of the respective target genes. Allstars Negative Control siRNA (Qiagen, Venlo, Netherlands) was used as a negative control. Transfection using DharmaFECT1 (Thermo Scientific, Chicago, IL) was performed as previously described and validated (19).
Statistical analysis.
Comparisons between groups were carried out either by paired t test or by ANOVA followed by t tests with Bonferroni correction as required. A P ≤ 0.05 was considered as statistically significant. Array statistical analysis is described in Supplementary Methods.
RESULTS
Effect of IL-1β + IFN-γ or TNF-α + IFN-γ on the viability, nitric oxide production, and gene expression of rat β-cells.
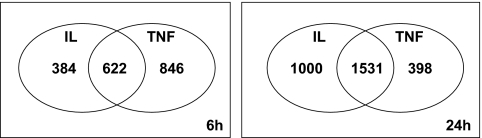
β-Cells were exposed to IL-1β + IFN-γ or TNF-α + IFN-γ and collected at 6 and 24 h for array analysis. Viability was not affected by the cytokine treatment after 24 h (supplementary Fig. 1A), but there was a twofold increase in apoptosis after 72 h (supplementary Fig. 1A) without significant changes in the percentage of necrotic cells (data not shown). Both cytokine combinations increased nitric oxide (NO) production after 24 h of exposure (supplementary Fig. 1B), with higher induction by IL-1β + IFN-γ as compared with TNF-α + IFN-γ. These results are similar to our previous observations (12), confirming biological activity of the cytokines. In the array analysis, nearly 16,000 probe sets, corresponding to 7,991 genes, were detected as present in control and/or cytokine-treated β-cells (supplementary Table 3). TNF-α + IFN-γ modified the expression of a higher number of genes compared with IL-1β + IFN-γ at 6 h, while this was inverted at 24 h, with higher number of IL-1β + IFN-γ–modified genes (Fig. 1). At 6 and 24 h, 67 and 48%, respectively, of the total number of cytokine-modified genes was differentially induced by IL-1β + IFN-γ or TNF-α + IFN-γ. Supplementart Tables 4–7 list all transcripts considered as modified by the different cytokine combinations at 6 and 24 h and classified by IPA. In Table 1, selected genes with a putative role in β-cell function/dysfunction and death were classified by one of the investigators (D.L.E.), using an adaptation of a previously described in-house classification (7,9).
FIG. 1.
Effects of cytokine exposure on gene expression in FACS-purified rat β-cells. Ven diagram showing the number of β-cell genes with the expression modified by cytokines after exposure to IL-1β + IFN-γ (IL) or TNF-α + IFN-γ (TNF) for 6 and 24 h. The diagram shows genes modified by IL-1β + IFN-γ alone (left part of the figure), TNF-α + IFN-γ alone (right) or both (center). Results of three independent array experiments were analyzed. mRNA expression was considered as modified by cytokines when P < 0.02 and fold change ≥1.5 compared with control condition.
TABLE 1.
Selected genes modulated by cytokine treatment detected by array analysis
| Probe | GenBank | Gene name/functional group | Symbol | 6 h |
24 h |
||
|---|---|---|---|---|---|---|---|
| IL + IFN | TNF + IFN | IL + IFN | TNF + IFN | ||||
| Arginine metabolism and NO formation | |||||||
| 1368266_at | NM_017134 | arg1 | Arg1 | 0.65 ± 0.06 | 0.76 ± 0.16 | 0.15 ± 0.03 | 0.28 ± 0.18 |
| 1370964_at | BF283456 | ASS | Ass | 5.93 ± 1.30 | 0.75 ± 2.55 | 14.21 ± 4.66 | 1.73 ± 1.35 |
| 1387667_at | L12562 | iNOS2 | Nos2 | 375.0 ± 59.78 | 109.3 ± 214.5 | 113.8 ± 16.03 | 57.80 ± 21.89 |
| Glucose metabolism | |||||||
| 1386916_at | NM_017321 | Aconitase 1 (Krebs) | Aco1 | 0.23 ± 0.12 | 0.38 ± 0.04 | 0.41 ± 0.04 | 0.56 ± 0.11 |
| 1367589_at | NM_024398 | Aconitase 2, mitochondrial (Krebs) | Aco2 | 0.45 ± 0.08 | 0.53 ± 0.07 | 0.61 ± 0.03 | 0.57 ± 0.06 |
| 1375295_at | AI009657 | Citrate synthase (Krebs) | Cs | 0.73 ± 0.18 | 0.60 ± 0.04 | 1.42 ± 0.15 | 1.31 ± 0.05 |
| 1367670_at | NM_017005 | Fumarase/fumarate hydratase 1 (Krebs) | Fh1 | 0.34 ± 0.03 | 0.58 ± 0.04 | 0.64 ± 0.03 | 0.77 ± 0.04 |
| 1370865_at | BI277627 | Isocitrate dehydrogenase 3 (NAD), γ (Krebs) | Idh3g | 0.61 ± 0.03 | 0.61 ± 0.09 | 0.56 ± 0.05 | 0.66 ± 0.02 |
| 1388160_a_at | AI171793 | Isocitrate dehydrogenase 3 (NAD+) β (Krebs) | Idh3B | 0.67 ± 0.23 | 0.74 ± 0.10 | 1.30 ± 0.18 | 1.39 ± 0.05 |
| 1372790_at | BG671530 | Malate dehydrogenase 1, NAD (soluble) (Krebs) | Mdh1 | 0.47 ± 0.06 | 0.53 ± 0.09 | 0.20 ± 0.02 | 0.29 ± 0.02 |
| 1372790_at | BG671530 | Malate dehydrogenase 1, NAD (soluble) (Krebs) | Mdh1 | 0.47 ± 0.06 | 0.53 ± 0.09 | 0.20 ± 0.02 | 0.29 ± 0.02 |
| 1367653_a_at | NM_033235 | Malate dehydrogenase 1, NAD (soluble) (Krebs) | Mdh1 | 0.67 ± 0.12 | 0.66 ± 0.03 | 0.52 ± 0.02 | 0.52 ± 0.05 |
| 1367653_a_at | NM_033235 | Malate dehydrogenase 1, NAD (soluble) (Krebs) | Mdh1 | 0.67 ± 0.12 | 0.66 ± 0.03 | 0.52 ± 0.02 | 0.52 ± 0.05 |
| 1369927_at | NM_031151 | Malate dehydrogenase 2, NAD (mitochondrial) (Krebs) | Mdh2 | 0.53 ± 0.16 | 0.48 ± 0.05 | 0.86 ± 0.03 | 0.79 ± 0.09 |
| 1390020_at | BI277513 | α-Ketoglutarate dehydrogenase (Krebs) | αkdgh | 0.69 ± 0.14 | 0.75 ± 0.09 | 0.44 ± 0.01 | 0.55 ± 0.06 |
| 1380813_at | AA891239 | Succinate dehydrogenase complex (Krebs) | Sdhb_predicted | 0.97 ± 0.23 | 1.00 ± 0.08 | 0.66 ± 0.10 | 0.89 ± 0.09 |
| 1372123_at | AI172320 | Succinate dehydrogenase complex (Krebs) | Sdhb_predicted | 0.84 ± 0.25 | 0.59 ± 0.11 | 1.43 ± 0.05 | 1.32 ± 0.07 |
| 1373017_at | AI237518 | Succinyl-CoA synthetase, β-subunit (Krebs) | Suclg2 | 0.84 ± 0.24 | 0.84 ± 0.06 | 0.65 ± 0.08 | 0.89 ± 0.02 |
| 1367617_at | NM_012495 | Aldolase A (glycolysis) | Aldoa | 0.77 ± 0.08 | 0.66 ± 0.01 | 1.35 ± 0.13 | 1.27 ± 0.04 |
| 1387312_a_at | NM_012565 | Glucokinase (glycolysis) | Gck | 0.20 ± 0.07 | 0.32 ± 0.14 | 0.22 ± 0.05 | 0.24 ± 0.10 |
| 1383519_at | BI294137 | Hexokinase 2 (glycolysis) | Hk2 | 4.99 ± 2.62 | 17.22 ± 5.44 | 16.77 ± 8.13 | 20.43 ± 10.53 |
| 1367575_at | NM_012554 | Enolase 1, alpha (glycolysis) | Eno1 | 0.70 ± 0.07 | 0.62 ± 0.04 | 1.71 ± 0.08 | 1.62 ± 0.19 |
| 1388318_at | BI279760 | Phosphoglycerate kinase 1 (glycolysis) | Pgk1 | 0.81 ± 0.10 | 0.70 ± 0.10 | 2.08 ± 0.16 | 1.61 ± 0.20 |
| 1386864_at | NM_053290 | Phosphoglycerate mutase 1 (glycolysis) | Pgam1 | 0.71 ± 0.16 | 0.70 ± 0.07 | 1.53 ± 0.10 | 1.52 ± 0.06 |
| 1378382_at | AI230014 | Phosphoglycerate mutase family member 5 (glycolysis) | Pgam5 | 1.05 ± 0.05 | 0.91 ± 0.05 | 1.64 ± 0.04 | 1.61 ± 0.10 |
| 1391577_at | BI293450 | Phosphoglycerate mutase family member 5 (glycolysis) | Pgam5 | 1.12 ± 0.07 | 0.90 ± 0.05 | 1.56 ± 0.04 | 1.52 ± 0.24 |
| 1367864_at | NM_031715 | Phosphofructokinase, muscle (glycolysis) | Pfkm | 0.46 ± 0.11 | 0.43 ± 0.08 | 0.21 ± 0.04 | 0.23 ± 0.12 |
| 1372182_at | BM389769 | Phosphofructokinase, platelet (glycolysis) | Pfkp | 4.98 ± 0.72 | 4.95 ± 0.34 | 7.76 ± 0.71 | 6.09 ± 0.13 |
| 1387263_at | NM_012624 | Pyruvate kinase, liver and red blood cell (glycolysis) | Pklr | 0.51 ± 0.16 | 0.32 ± 0.22 | 0.15 ± 0.01 | 0.27 ± 0.06 |
| 1368651_at | M17685 | Pyruvate kinase, liver and red blood cell (glycolysis) | Pklr | 0.51 ± 0.06 | 0.52 ± 0.07 | 0.20 ± 0.02 | 0.22 ± 0.15 |
| 1370200_at | BI284411 | Glutamate dehydrogenase 1 | Glud1 | 0.32 ± 0.06 | 0.24 ± 0.08 | 0.26 ± 0.01 | 0.31 ± 0.09 |
| 1387878_at | AW916644 | Glutamate dehydrogenase 1 | Glud1 | 0.38 ± 0.06 | 0.26 ± 0.02 | 0.45 ± 0.04 | 0.43 ± 0.03 |
| 1370870_at | M30596 | Malic enzyme 1 | Me1 | 0.51 ± 0.18 | 0.57 ± 0.09 | 0.54 ± 0.02 | 0.67 ± 0.08 |
| 1370067_at | NM_012600 | Malic enzyme 1 | Me1 | 0.56 ± 0.13 | 0.63 ± 0.09 | 0.56 ± 0.03 | 0.84 ± 0.07 |
| 1386917_at | NM_012744 | Pyruvate carboxylase | Pc | 0.38 ± 0.15 | 0.75 ± 0.10 | 0.47 ± 0.09 | 0.61 ± 0.10 |
| 1371388_at | BM389223 | Pyruvate dehydrogenase (lipoamide) β | Pdhb | 0.58 ± 0.10 | 0.52 ± 0.08 | 0.74 ± 0.05 | 0.73 ± 0.07 |
| 1372229_at | AI179119 | Pyruvate dehydrogenase kinase, isoenzyme 3 (mapped) | Pdk3 | 0.21 ± 0.01 | 0.54 ± 0.12 | 0.09 ± 0.03 | 0.49 ± 0.04 |
| 1370848_at | BI284218 | Solute carrier family 2 (facilitated glucose transporter), member 1 | Slc2a1 | 4.25 ± 0.37 | 2.22 ± 0.28 | 12.80 ± 5.29 | 9.07 ± 2.86 |
| 1387228_at | NM_012879 | Solute carrier family 2 (facilitated glucose transporter), member 2 | Slc2a2 | 0.37 ± 0.03 | 0.38 ± 0.04 | 0.27 ± 0.04 | 0.37 ± 0.08 |
| Lipid metabolism | |||||||
| 1367763_at | D13921 | Acetyl-coenzyme A acetyltransferase 1 | Acat1 | 0.47 ± 0.00 | 0.59 ± 0.06 | 0.30 ± 0.05 | 0.23 ± 0.12 |
| 1383416_at | AA899304 | Acetyl-coenzyme A acetyltransferase 1 | Acat1 | 0.32 ± 0.08 | 0.39 ± 0.12 | 0.36 ± 0.05 | 0.34 ± 0.05 |
| 1370939_at | D90109 | Acyl-CoA synthetase long-chain family member 1 | Acsl1 | 0.78 ± 0.22 | 1.45 ± 0.11 | 0.61 ± 0.09 | 1.22 ± 0.05 |
| 1368177_at | NM_057107 | Acyl-CoA synthetase long-chain family member 3 | Acsl3 | 0.78 ± 0.16 | 0.51 ± 0.10 | 1.92 ± 0.39 | 1.34 ± 0.33 |
| 1386926_at | NM_053607 | Acyl-CoA synthetase long-chain family member 5 | Acsl5 | 1.67 ± 0.11 | 1.41 ± 0.15 | 2.35 ± 0.13 | 2.17 ± 0.15 |
| 1367854_at | NM_016987 | ATP citrate lyase | Acly | 0.61 ± 0.06 | 0.66 ± 0.02 | 0.61 ± 0.01 | 0.71 ± 0.06 |
| 1398716_at | BG670822 | Carnitine palmitoyltransferase 1a, liver | Cpt1a | 0.96 ± 0.41 | 0.79 ± 0.23 | 0.47 ± 0.04 | 0.43 ± 0.12 |
| 1382882_x_at | AA963228 | Carnitine palmitoyltransferase 1a, liver | Cpt1a | 0.78 ± 0.04 | 0.78 ± 0.06 | 0.51 ± 0.07 | 0.44 ± 0.15 |
| 1392166_at | BE099838 | Carnitine palmitoyltransferase 1a, liver | Cpt1a | 0.77 ± 0.19 | 0.83 ± 0.13 | 0.52 ± 0.03 | 0.48 ± 0.15 |
| 1397700_x_at | BG670822 | Carnitine palmitoyltransferase 1a, liver | Cpt1a | 0.83 ± 0.47 | 0.91 ± 0.18 | 0.55 ± 0.05 | 0.40 ± 0.13 |
| 1386927_at | NM_012930 | Carnitine palmitoyltransferase 2 | Cpt2 | 0.29 ± 0.08 | 0.31 ± 0.03 | 0.17 ± 0.04 | 0.21 ± 0.09 |
| 1367740_at | M14400 | Ceatine kinase, brain | Ckb | 0.19 ± 0.07 | 0.15 ± 0.06 | 0.12 ± 0.03 | 0.09 ± 0.09 |
| 1390566_a_at | BI301453 | Creatine kinase, mitochondrial 1, ubiquitous | Ckmt1 | 6.60 ± 0.94 | 5.46 ± 1.91 | 6.07 ± 1.56 | 3.82 ± 0.76 |
| 1391534_at | BG666735 | Elongation of very-long-chain fatty acids (FEN1/Elo2, SUR4/Elo3, yeast)-like 2 (predicted) | Elovl2_predicted | 0.38 ± 0.28 | 0.36 ± 0.27 | 0.34 ± 0.04 | 0.39 ± 0.09 |
| 1388108_at | BE116152 | ELOVL family member 6, elongation of long-chain fatty acids (yeast) | Elovl6 | 0.43 ± 0.11 | 0.38 ± 0.08 | 0.36 ± 0.02 | 0.35 ± 0.06 |
| 1367857_at | NM_053445 | Fatty acid desaturase 1 | Fads1 | 0.21 ± 0.07 | 0.25 ± 0.07 | 0.28 ± 0.03 | 0.27 ± 0.14 |
| 1367707_at | NM_017332 | Fatty acid synthase | Fasn | 0.33 ± 0.09 | 0.12 ± 0.08 | 0.50 ± 0.02 | 0.50 ± 0.14 |
| 1371979_at | AI170663 | Sterol regulatory element– binding factor 2 (predicted) | Srebf2_predicted | 0.87 ± 0.16 | 0.87 ± 0.04 | 0.45 ± 0.06 | 0.43 ± 0.08 |
| 1389611_at | AA849857 | VLDL receptor | Vldlr | 0.75 ± 0.13 | 0.34 ± 0.12 | 2.39 ± 0.20 | 2.28 ± 0.07 |
| 1387455_a_at | NM_013155 | VLDL lipoprotein receptor | Vldlr | 0.63 ± 0.15 | 0.50 ± 0.09 | 2.79 ± 0.25 | 2.35 ± 0.09 |
| Chemokines/cytokines/adhesion molecules | |||||||
| 1367973_at | NM_031530 | Chemokine (C-C motif) ligand 2 | Ccl2 | 962.7 ± 378.5 | 987.1 ± 713.1 | 511.9 ± 362.3 | 124.1 ± 163.7 |
| 1369814_at | AF053312 | Chemokine (C-C motif) ligand 20/ | Ccl20 | 168.9 ± 15.58 | 62.2 ± 119.2 | 139.7 ± 15.95 | 14.86 ± 5.29 |
| 1369983_at | NM_031116 | Chemokine (C-C motif) ligand 5 | Ccl5 | 0.60 ± 1.55 | 77.43 ± 32.63 | 4.42 ± 2.94 | 182.1 ± 121.9 |
| 1379935_at | BF419899 | Chemokine (C-C motif) ligand 7 | Ccl7 | 131.3 ± 45.08 | 128.0 ± 74.85 | 59.30 ± 6.36 | 7.41 ± 2.48 |
| 1387316_at | NM_030845 | Chemokine (C-X-C motif) ligand 1 (GRO-alpha) | Cxcl1 | 392.4 ± 101.8 | 36.4 ± 155.4 | 189.8 ± 84.53 | 6.64 ± 3.37 |
| 1372064_at | BI296385 | Similar to chemokine (C-X-C motif) ligand 16 | Cxcl16 | 15.71 ± 2.78 | 19.87 ± 3.52 | 17.06 ± 1.37 | 24.66 ± 2.03 |
| 1368760_at | NM_031530 | Chemokine (C-X-C motif) ligand 2/ | Cxcl2 | 282.1 ± 61.17 | 15.91 ± 203.9 | 47.3 ± 21.72 | 1.54 ± 0.40 |
| 1373544_at | AI170387 | Chemokine (C-X-C motif) ligand 9 | Cxcl9 | 238.2 ± 232.7 | 588.0 ± 339.3 | 199.8 ± 88.6 | 288.0 ± 161.2 |
| 1382454_at | AI044222 | Chemokine (C-X-C motif) ligand 9 | Cxcl9 | 290.5 ± 172.6 | 913.8 ± 269.6 | 197.2 ± 216.5 | 334.7 ± 398.9 |
| 1387202_at | NM_012967 | Intercellular adhesion molecule 1 | Icam1 | 192.9 ± 29.93 | 541.5 ± 110.6 | 93.2 ± 29.84 | 152.8 ± 32.92 |
| 1368375_a_at | AF015718 | IL-15 | Il15 | 10.36 ± 0.45 | 32.08 ± 1.36 | 6.84 ± 1.37 | 20.42 ± 7.31 |
| 1368474_at | NM_012889 | Vascular cell adhesion molecule 1 | Vcam1 | 3.13 ± 0.95 | 34.83 ± 5.74 | 2.50 ± 0.86 | 28.37 ± 12.45 |
| IFN-γ signaling | |||||||
| 1369956_at | NM_053783 | IFN-γ receptor 1 | Ifngr | 1.47 ± 0.34 | 1.46 ± 0.28 | 1.81 ± 0.26 | 1.74 ± 0.15 |
| 1368073_at | NM_012591 | IRF-1 | Irf1 | 66.39 ± 15.09 | 105.83 ± 19.34 | 19.85 ± 3.56 | 34.76 ± 2.17 |
| 1371560_at | AA893384 | IRF-3 | Irf3 | 0.81 ± 0.09 | 0.87 ± 0.23 | 0.70 ± 0.05 | 0.58 ± 0.06 |
| 1383564_at | BF411036 | IRF- 7 | Irf7 | 90.31 ± 27.87 | 427.28 ± 111.88 | 22.64 ± 11.73 | 104.71 ± 53.34 |
| 1372097_at | BF284262 | IRF-8 | Irf8 | 81.26 ± 2.01 | 57.63 ± 16.59 | 12.28 ± 2.73 | 3.57 ± 2.74 |
| 1375796_at | BI300770 | IFN-γ–induced GTPase | Igtp | 32.18 ± 11.58 | 45.65 ± 10.40 | 7.96 ± 4.76 | 11.24 ± 9.04 |
| 1373992_at | AI408440 | Similar to IFN-inducible GTPase | MGC108823 | 140.5 ± 42.77 | 482.2 ± 131.4 | 144.4 ± 18.54 | 370.8 ± 95.71 |
| 1377950_at | AA955213 | Similar to IFN-inducible GTPase | RGD1309362 | 63.4 ± 13.8 | 235.0 ± 73.5 | 29.1 ± 11.5 | 142.2 ± 43.5 |
| 1368835_at | AW434718 | STAT1 | Stat1 | 14.50 ± 1.75 | 19.51 ± 1.89 | 5.50 ± 0.69 | 8.67 ± 1.42 |
| 1372757_at | BM386875 | STAT1 | Stat1 | 7.38 ± 0.85 | 8.27 ± 0.61 | 4.74 ± 0.49 | 7.28 ± 1.02 |
| 1387354_at | NM_032612 | STAT1 | Stat1 | 28.13 ± 9.16 | 23.80 ± 8.68 | 9.90 ± 1.91 | 13.39 ± 4.09 |
| 1389571_at | BG666368 | STAT2 | Stat2 | 17.98 ± 5.43 | 25.50 ± 2.68 | 32.83 ± 16.23 | 49.37 ± 12.41 |
| 1370224_at | BE113920 | STAT3 | Stat3 | 2.66 ± 0.43 | 2.86 ± 0.25 | 2.92 ± 0.66 | 1.91 ± 0.73 |
| 1371781_at | BI285863 | STAT3 | Stat3 | 2.02 ± 0.15 | 2.96 ± 0.34 | 1.62 ± 0.22 | 1.66 ± 0.28 |
| 1387876_at | AI177626 | STAT5B | Stat5b | 1.33 ± 0.39 | 1.23 ± 0.11 | 3.24 ± 1.02 | 1.41 ± 0.31 |
| 1383478_at | BG671504 | Janus kinase 1 | Jak1 | 1.06 ± 0.16 | 0.98 ± 0.09 | 2.71 ± 0.11 | 2.74 ± 0.45 |
| 1384060_at | BG663208 | Janus kinase 1 | Jak1 | 1.31 ± 0.04 | 0.97 ± 0.23 | 3.71 ± 0.38 | 3.38 ± 0.45 |
| 1368856_at | NM_031514 | Janus kinase 2 | Jak2 | 9.74 ± 5.58 | 17.88 ± 3.18 | 4.23 ± 1.48 | 5.79 ± 1.98 |
| 1380110_at | AI229643 | Janus kinase 2 | Jak2 | 12.28 ± 1.16 | 14.90 ± 2.81 | 7.27 ± 0.75 | 9.56 ± 1.54 |
| 1368251_at | NM_012855 | Janus kinase 3 | Jak3 | 1.46 ± 0.12 | 3.24 ± 0.85 | 0.99 ± 0.24 | 1.53 ± 0.20 |
| 1376666_at | AI170864 | Suppressor of cytokine signaling 6 (predicted) | Socs6_predicted | 0.90 ± 0.17 | 1.28 ± 0.25 | 1.51 ± 0.08 | 1.37 ± 0.09 |
| 1391484_at | BF284786 | Suppressor of cytokine signaling 7 (predicted) | Socs7_predicted | 1.32 ± 0.06 | 1.53 ± 0.13 | 1.25 ± 0.03 | 1.41 ± 0.18 |
| NF-κB regulation | |||||||
| 1383474_at | BI274988 | IL-1 receptor–associated kinase 2 | Irak2 | 3.55 ± 0.66 | 3.57 ± 1.51 | 3.01 ± 0.20 | 1.34 ± 0.23 |
| 1370968_at | AA858801 | NFκ light-chain gene enhancer in B-cells 1, p105 | Nfkb1 | 19.06 ± 3.30 | 17.76 ± 0.97 | 7.67 ± 1.59 | 7.48 ± 1.72 |
| 1389538_at | AW672589 | NFκ light-chain gene enhancer in B-cells inhibitor, α | Nfkbia | 55.16 ± 26.23 | 82.84 ± 18.93 | 25.93 ± 8.42 | 20.09 ± 5.05 |
| 1367943_at | NM_030867 | NFκ light-chain gene enhancer in B-cells inhibitor, β | Nfkbib | 4.40 ± 1.97 | 5.78 ± 0.95 | 7.26 ± 2.31 | 5.67 ± 1.34 |
| 1375989_a_at | AI170362 | NFκ light polypeptide gene enhancer in B-cells 2, p49/p100 | Nfkb2 | 12.32 ± 2.84 | 18.22 ± 5.02 | 10.63 ± 6.55 | 11.33 ± 5.34 |
| 1376835_at | BI293600 | NFκ light polypeptide gene enhancer in B-cells inhibitor, έ | Nfkbie | 11.21 ± 3.38 | 41.27 ± 4.76 | 2.04 ± 1.40 | 7.89 ± 1.86 |
| 1378032_at | AI176265 | NFκ light polypeptide gene enhancer in B-cells inhibitor, ζ (predicted) | Nfkbiz_predicted | 12.91 ± 2.15 | 9.86 ± 1.36 | 13.48 ± 2.26 | 10.71 ± 1.32 |
| Others transcription factors | |||||||
| 1379368_at | AI237606 | B-cell leukemia/lymphoma 6 (predicted) | Bcl6_predicted | 1.90 ± 0.90 | 2.23 ± 0.86 | 9.63 ± 1.51 | 10.86 ± 2.22 |
| 1385592_at | BI289386 | Bcl6 interacting corepressor (predicted) | Bcor_predicted | 1.34 ± 0.04 | 1.53 ± 0.11 | 2.48 ± 0.52 | 1.76 ± 0.07 |
| 1391632_at | AA964568 | CCAAT/enhancer binding protein (C/EBP), δ | Cebpd | 0.74 ± 0.05 | 0.60 ± 0.03 | 2.21 ± 0.63 | 1.74 ± 0.09 |
| 1387343_at | NM_013154 | CCAAT/enhancer binding protein (C/EBP), δ | Cebpd | 2.34 ± 0.36 | 1.11 ± 0.10 | 10.82 ± 3.47 | 5.42 ± 0.99 |
| 1375043_at | BF415939 | FBJ murine osteosarcoma viral oncogene homolog | Fos | 0.33 ± 0.08 | 0.70 ± 0.18 | 0.33 ± 0.07 | 0.29 ± 0.28 |
| 1388761_at | AI180339 | Histone deacetylase 1 (predicted) | Hdac1_predicted | 0.59 ± 0.04 | 0.72 ± 0.09 | 0.34 ± 0.05 | 0.56 ± 0.02 |
| 1396820_at | AW530195 | Histone deacetylase 1 (predicted) | Hdac1_predicted | 0.97 ± 0.16 | 0.99 ± 0.24 | 0.54 ± 0.02 | 0.56 ± 0.03 |
| 1370908_at | AA892297 | Histone deacetylase 2 | Hdac2 | 0.61 ± 0.04 | 0.97 ± 0.16 | 0.73 ± 0.05 | 0.82 ± 0.02 |
| 1387076_at | NM_024359 | HIF-1, α-subunit | Hif1a | 2.02 ± 0.17 | 1.58 ± 0.31 | 2.07 ± 0.20 | 2.16 ± 0.19 |
| 1369681_at | NM_017339 | ISL1 transcription factor, LIM/homeodomain 1 | Isl1 | 0.53 ± 0.08 | 0.14 ± 0.05 | 0.31 ± 0.23 | 0.29 ± 0.06 |
| 1393138_at | BE329377 | Jun D proto-oncogene | Jund | 1.31 ± 0.10 | 1.43 ± 0.05 | 2.25 ± 0.23 | 1.83 ± 0.23 |
| 1369516_at | NM_022852 | Pancreatic and duodenal homeobox gene 1 | Pdx1 | 1.01 ± 0.23 | 0.21 ± 0.03 | 0.49 ± 0.06 | 0.25 ± 0.21 |
| 1369242_at | NM_013001 | Paired box gene 6 | Pax6 | 0.50 ± 0.01 | 0.52 ± 0.08 | 0.35 ± 0.04 | 0.53 ± 0.05 |
| 1374404_at | BI288619 | Proto-oncogene c-jun | Jun | 2.17 ± 1.36 | 2.44 ± 1.09 | 4.95 ± 0.94 | 2.76 ± 0.36 |
| 1369788_s_at | NM_021835 | Proto-oncogene c-jun | Jun | 4.71 ± 1.35 | 6.30 ± 1.77 | 7.39 ± 0.13 | 6.65 ± 1.20 |
| 1389528_s_at | BI288619 | Proto-oncogene c-jun | Jun | 4.00 ± 0.93 | 3.05 ± 0.68 | 9.12 ± 1.52 | 7.04 ± 2.01 |
| Hormones | |||||||
| 1387235_at | NM_021655 | Chromogranin A | Chga | 0.64 ± 0.16 | 0.65 ± 0.01 | 0.30 ± 0.02 | 0.27 ± 0.05 |
| 1368034_at | NM_012526 | Chromogranin B | Chgb | 0.79 ± 0.13 | 0.66 ± 0.02 | 0.30 ± 0.02 | 0.35 ± 0.04 |
| 1369888_at | NM_012707 | Glucagon | Gcg | 0.71 ± 0.04 | 0.77 ± 0.06 | 0.10 ± 0.01 | 0.16 ± 0.06 |
| 1387815_at | NM_019129 | Insulin 1 | Ins1 | 0.94 ± 0.09 | 1.04 ± 0.12 | 0.75 ± 0.06 | 0.79 ± 0.02 |
| 1370077_at | NM_019130 | Insulin 2 | Ins2 | 0.86 ± 0.07 | 0.99 ± 0.09 | 0.68 ± 0.03 | 0.75 ± 0.01 |
| 1387660_at | M25390 | Islet amyloid polypeptide | Iapp | 0.86 ± 0.10 | 0.85 ± 0.02 | 0.48 ± 0.03 | 0.60 ± 0.06 |
| 1368559_at | NM_017091 | Proprotein convertase subtilisin/kexin type 1 | Pcsk1 | 0.48 ± 0.18 | 0.48 ± 0.07 | 0.18 ± 0.03 | 0.27 ± 0.05 |
| 1387247_at | M83745 | Proprotein convertase subtilisin/kexin type 1 | Pcsk1 | 0.41 ± 0.14 | 0.56 ± 0.06 | 0.17 ± 0.02 | 0.23 ± 0.14 |
| 1387155_at | NM_012746 | Proprotein convertase subtilisin/kexin type 2 | Pcsk2 | 0.78 ± 0.08 | 0.76 ± 0.08 | 0.45 ± 0.02 | 0.58 ± 0.04 |
| 1397662_at | BF395791 | Proprotein convertase subtilisin/kexin type 2 | Pcsk2 | 0.92 ± 0.17 | 1.05 ± 0.08 | 0.26 ± 0.04 | 0.55 ± 0.14 |
| 1367778_at | NM_019331 | Proprotein convertase subtilisin/kexin type 3 | Pcsk3 | 0.80 ± 0.06 | 0.51 ± 0.01 | 0.67 ± 0.05 | 0.57 ± 0.03 |
| 1367762_at | NM_012659 | Somatostatin | Sst | 0.48 ± 0.06 | 0.48 ± 0.04 | 0.08 ± 0.01 | 0.08 ± 0.08 |
| Hormone receptors | |||||||
| 1369787_at | NM_012688 | Cholecystokinin A receptor | Cckar | 0.23 ± 0.04 | 0.25 ± 0.04 | 0.06 ± 0.01 | 0.11 ± 0.12 |
| 1368481_at | NM_012714 | Gastric inhibitory polypeptide receptor | Gipr | 0.55 ± 0.05 | 0.64 ± 0.16 | 0.14 ± 0.02 | 0.12 ± 0.08 |
| 1369699_at | NM_012728 | GLP-1 receptor | Glp1r | 0.47 ± 0.34 | 0.96 ± 0.26 | 0.32 ± 0.04 | 0.39 ± 0.08 |
| 1368924_at | NM_017094 | Growth hormone receptor | Ghr | 0.41 ± 0.07 | 0.49 ± 0.17 | 0.31 ± 0.06 | 0.44 ± 0.07 |
| 1370384_a_at | M57668 | Prolactin receptor | Prlr | 0.36 ± 0.12 | 0.38 ± 0.18 | 0.17 ± 0.06 | 0.23 ± 0.20 |
| 1370789_a_at | L48060 | Prolactin receptor | Prlr | 0.15 ± 0.08 | 0.38 ± 0.14 | 0.21 ± 0.01 | 0.21 ± 0.05 |
| 1376944_at | AI407163 | Prolactin receptor | Prlr | 0.41 ± 0.03 | 0.49 ± 0.03 | 0.17 ± 0.04 | 0.35 ± 0.11 |
| 1392612_at | AW142962 | Prolactin receptor | Prlr | 0.37 ± 0.11 | 0.50 ± 0.12 | 0.13 ± 0.03 | 0.14 ± 0.10 |
| 1387177_at | NM_017238 | Vasoactive intestinal peptide receptor 2 | Vipr2 | 0.35 ± 0.07 | 0.36 ± 0.06 | 0.13 ± 0.02 | 0.11 ± 0.03 |
| Free radical scavanger/DNA damage | |||||||
| 1367995_at | NM_012520 | Catalase | Cat | 0.79 ± 0.09 | 0.72 ± 0.09 | 1.96 ± 0.14 | 2.12 ± 0.29 |
| 1367774_at | NM_031509 | Glutathione S-transferase A3 | Gsta3 | 0.58 ± 0.19 | 0.45 ± 0.16 | 6.34 ± 1.77 | 1.78 ± 0.13 |
| 1389832_at | BE113459 | Glutathione S-transferase, ω 1 | Gsto1 | 0.62 ± 0.12 | 0.66 ± 0.04 | 1.54 ± 0.15 | 1.59 ± 0.05 |
| 1387023_at | NM_031154 | Glutathione S-transferase, μ type 3 | Gstm3 | 0.38 ± 0.06 | 0.38 ± 0.04 | 0.14 ± 0.01 | 0.12 ± 0.05 |
| 1388122_at | X02904 | Glutathione S-transferase, π 2 | Gstp2 | 0.83 ± 0.32 | 0.52 ± 0.14 | 4.61 ± 1.84 | 6.30 ± 2.10 |
| 1372016_at | BI287978 | Growth arrest and DNA damage–inducible 45 β | Gadd45b | 44.52 ± 6.02 | 21.01 ± 34.64 | 131.03 ± 53.38 | 38.81 ± 10.31 |
| 1388792_at | AI599423 | Growth arrest and DNA damage–inducible 45 γ | Gadd45g | 3.85 ± 1.06 | 2.43 ± 0.88 | 3.53 ± 0.48 | 1.89 ± 0.54 |
| 1388267_a_at | M24327 | Metallothionein 1a | Mt1a | 2.41 ± 1.74 | 3.61 ± 1.23 | 26.36 ± 4.49 | 30.52 ± 4.33 |
| 1371237_a_at | AF411318 | Metallothionein 1a | Mt1a | 3.02 ± 1.19 | 4.03 ± 1.07 | 36.70 ± 11.37 | 40.64 ± 9.73 |
| 1374911_at | AW251534 | Oxidative stress responsive gene | RGD1303142 | 1.01 ± 1.07 | 1.00 ± 0.34 | 8.43 ± 2.51 | 6.51 ± 0.66 |
| 1372941_at | BI273897 | p53 and DNA damage regulated 1 | Pdrg1 | 2.01 ± 0.50 | 1.30 ± 0.03 | 2.61 ± 0.18 | 2.07 ± 0.07 |
| 1380071_at | BI285978 | Poly (ADP-ribose) polymerase family, member 12 (predicted) | Parp12_predicted | 7.04 ± 1.25 | 8.87 ± 0.94 | 2.89 ± 0.13 | 4.96 ± 0.65 |
| 1383251_at | AW524533 | Poly (ADP-ribose) polymerase family, member 2 (predicted) | Parp2_predicted | 0.99 ± 0.18 | 0.77 ± 0.20 | 1.22 ± 0.09 | 1.40 ± 0.13 |
| 1376144_at | AA819679 | Poly (ADP-ribose) polymerase family, member 9 (predicted) | Parp9_predicted | 27.68 ± 9.82 | 48.25 ± 13.19 | 15.17 ± 1.94 | 22.40 ± 2.13 |
| 1370173_at | BG671549 | Superoxide dismutase 2, mitochondrial | Sod2 | 6.74 ± 0.41 | 7.02 ± 0.19 | 7.51 ± 1.27 | 5.53 ± 0.35 |
| 1370172_at | AA892254 | Superoxide dismutase 2, mitochondrial | Sod2 | 8.69 ± 0.50 | 9.23 ± 0.82 | 13.07 ± 0.94 | 9.18 ± 0.63 |
| Endoplasmic reticulum stress/apoptosis related | |||||||
| 1369268_at | NM_012912 | ATF3 | Atf3 | 15.73 ± 3.69 | 23.27 ± 4.00 | 53.89 ± 24.87 | 26.59 ± 27.09 |
| 1367624_at | NM_024403 | ATF4 | Atf4 | 1.33 ± 0.08 | 1.07 ± 0.05 | 2.32 ± 0.27 | 2.16 ± 0.12 |
| 1368066_at | NM_053812 | BCL2-antagonist/killer 1 | Bak1 | 6.45 ± 3.37 | 5.77 ± 0.93 | 3.83 ± 0.76 | 6.81 ± 2.79 |
| 1369122_at | AF235993 | Bcl2-associated X protein | Bax | 1.17 ± 0.06 | 1.24 ± 0.06 | 2.34 ± 0.22 | 2.32 ± 0.22 |
| 1377759_at | BG666928 | BH3-interacting domain death agonist | Bid | 6.29 ± 1.66 | 3.48 ± 1.36 | 9.39 ± 1.30 | 4.30 ± 0.71 |
| 1370283_at | M14050 | Bip | Hspa5 | 0.89 ± 0.10 | 0.91 ± 0.05 | 1.46 ± 0.03 | 1.35 ± 0.06 |
| 1370283_at | M14050 | Bip | Hspa5 | 0.89 ± 0.10 | 0.91 ± 0.05 | 1.46 ± 0.03 | 1.35 ± 0.06 |
| 1381173_at | BG375010 | Caspase 4, apoptosis-related cysteine peptidase | Casp4 | 17.97 ± 7.54 | 15.89 ± 5.15 | 5.70 ± 0.89 | 17.31 ± 4.98 |
| 1387818_at | NM_053736 | Caspase 4, apoptosis-related cysteine peptidase | Casp4 | 18.89 ± 7.24 | 26.13 ± 13.11 | 41.24 ± 27.83 | 65.61 ± 19.29 |
| 1389170_at | BF283754 | Caspase 7 | Casp7 | 0.40 ± 0.12 | 0.31 ± 0.18 | 0.57 ± 0.09 | 0.72 ± 0.08 |
| 1367529_at | BE113989 | Derlin1 | RGD1311835 | 0.81 ± 0.06 | 0.78 ± 0.08 | 1.52 ± 0.20 | 1.44 ± 0.08 |
| 1374581_at | BM384392 | Derlin1 | RGD1311835 | 0.91 ± 0.08 | 0.80 ± 0.09 | 1.73 ± 0.18 | 1.43 ± 0.13 |
| 1389615_at | BI284801 | Derlin1 | RGD1311835 | 0.58 ± 0.19 | 0.55 ± 0.02 | 2.34 ± 0.25 | 2.07 ± 0.17 |
| 1369590_a_at | NM_024134 | Chop | Ddit3 | 1.66 ± 0.27 | 1.19 ± 0.33 | 7.86 ± 1.11 | 7.10 ± 0.91 |
| 1383011_at | AI501182 | Eukaryotic translation initiation factor 2A | Eif2a | 1.62 ± 0.09 | 1.62 ± 0.25 | 1.86 ± 0.20 | 1.70 ± 0.05 |
| 1388898_at | AI236601 | Heat shock 105 kDa/110 kDa protein 1 | Hsph1 | 0.71 ± 0.08 | 0.74 ± 0.15 | 2.01 ± 0.66 | 1.50 ± 0.20 |
| 1385620_at | BF525282 | Heat shock 105 kDa/110 kDa protein 1 | Hsph1 | 0.54 ± 0.07 | 0.31 ± 0.15 | 3.56 ± 0.85 | 1.35 ± 0.20 |
| 1388721_at | BG380282 | Heat shock 22 kDa protein 8 | Hspb8 | 35.81 ± 13.54 | 12.67 ± 5.18 | 9.02 ± 2.86 | 5.92 ± 2.36 |
| 1368247_at | NM_031971 | Heat shock 70 kD protein 1A | Hspa1a | 1.21 ± 0.16 | 1.25 ± 0.23 | 5.57 ± 1.02 | 1.52 ± 1.14 |
| 1370912_at | BI278231 | Heat shock 70 kD protein 1B (mapped) | Hspa1b | 1.19 ± 0.26 | 1.17 ± 0.35 | 6.15 ± 1.09 | 2.03 ± 1.48 |
| 1388851_at | BI282281 | Heat shock 70 kDa protein 9A (predicted) | Hspa9a_predicted | 0.80 ± 0.07 | 0.69 ± 0.03 | 1.97 ± 0.10 | 1.91 ± 0.12 |
| 1386894_at | NM_022229 | Heat shock protein 1 (chaperonin) | Hspd1 | 0.52 ± 0.01 | 0.49 ± 0.01 | 1.37 ± 0.11 | 1.24 ± 0.05 |
| 1372701_at | AI237597 | Heat shock protein 1, α | Hspca | 0.76 ± 0.12 | 0.76 ± 0.18 | 2.79 ± 0.20 | 1.63 ± 0.26 |
| 1388850_at | BG671521 | Heat shock protein 1, α | Hspca | 0.67 ± 0.06 | 0.79 ± 0.09 | 3.06 ± 0.68 | 1.66 ± 0.27 |
| 1398240_at | NM_024351 | Heat shock protein 8 | Hspa8 | 0.66 ± 0.06 | 0.68 ± 0.08 | 1.51 ± 0.10 | 1.21 ± 0.05 |
| 1368195_at | NM_134419 | Hspb-associated protein 1 | Hspbap1 | 0.81 ± 0.77 | 1.00 ± 0.25 | 3.89 ± 0.30 | 2.22 ± 0.34 |
| 1370174_at | BI284349 | Myeloid differentiation primary response gene 116 | Myd116 | 6.11 ± 0.81 | 6.55 ± 4.61 | 21.46 ± 2.39 | 9.67 ± 6.56 |
| 1382615_at | BI284366 | Sec61 α1 subunit (S. cerevisiae) | Sec61a1 | 0.76 ± 0.18 | 0.97 ± 0.09 | 0.75 ± 0.29 | 0.54 ± 0.04 |
| 1375659_at | BG381529 | Sec61, α-subunit 2 (S. cerevisiae) (predicted) | Sec61a2_predicted | 0.77 ± 0.06 | 0.84 ± 0.03 | 0.71 ± 0.04 | 0.81 ± 0.01 |
| 1372533_at | AI175790 | Similar to mKIAA0212 protein (predicted) | edem | 1.35 ± 0.12 | 2.06 ± 0.14 | 1.44 ± 0.05 | 1.51 ± 0.16 |
| 1370695_s_at | AB020967 | Tribbles homolog 3 (Drosophila) | Trib3 | 1.88 ± 0.76 | 0.88 ± 0.13 | 8.15 ± 3.61 | 7.74 ± 0.85 |
| 1370694_at | AB020967 | Tribbles homolog 3 (Drosophila) | Trib3 | 1.94 ± 1.04 | 1.16 ± 0.23 | 9.76 ± 1.28 | 7.81 ± 0.47 |
| 1386321_s_at | H31287 | Tribbles homolog 3 (Drosophila) | Trib3 | 2.12 ± 0.61 | 1.30 ± 0.16 | 14.66 ± 2.32 | 10.47 ± 1.31 |
| 1369065_a_at | NM_017290 | Serca2 | Atp2a2 | 0.40 ± 0.08 | 0.45 ± 0.07 | 0.21 ± 0.02 | 0.25 ± 0.07 |
| 1370426_a_at | AI175492 | Serca2 | Atp2a2 | 0.51 ± 0.06 | 0.52 ± 0.03 | 0.63 ± 0.06 | 0.65 ± 0.03 |
| Cell cycle | |||||||
| 1390343_at | AA998893 | Cyclin C | Ccnc | 0.61 ± 0.15 | 0.59 ± 0.03 | 0.48 ± 0.01 | 0.68 ± 0.04 |
| 1389101_at | BE120340 | Cyclin C | Ccnc | 0.90 ± 0.14 | 0.81 ± 0.21 | 1.33 ± 0.21 | 1.62 ± 0.04 |
| 1371643_at | AW143798 | Cyclin D1 | Ccnd1 | 0.48 ± 0.12 | 0.69 ± 0.14 | 0.43 ± 0.12 | 0.55 ± 0.17 |
| 1369935_at | NM_012766 | Cyclin D3 | Ccnd3 | 0.38 ± 0.11 | 0.52 ± 0.02 | 0.62 ± 0.12 | 0.62 ± 0.10 |
| 1371953_at | AI408309 | Cyclin G2 (predicted) | Ccng2_predicted | 4.59 ± 0.72 | 4.51 ± 1.85 | 2.45 ± 0.33 | 2.54 ± 0.37 |
| 1388370_at | AA945706 | Cyclin I (predicted) | Ccni_predicted | 0.93 ± 0.06 | 1.05 ± 0.06 | 0.74 ± 0.02 | 0.88 ± 0.04 |
| 1368050_at | NM_053662 | Cyclin L1 | Ccnl1 | 1.61 ± 0.35 | 1.96 ± 0.55 | 2.76 ± 0.47 | 1.80 ± 0.76 |
| 1390815_at | BF282870 | Cyclin M1 (predicted) | Cnnm1_predicted | 1.08 ± 0.11 | 0.76 ± 0.17 | 0.49 ± 0.04 | 0.38 ± 0.05 |
| 1391270_at | BE112177 | Cyclin M3 (predicted) | Cnnm3_predicted | 0.37 ± 0.05 | 0.33 ± 0.05 | 0.19 ± 0.04 | 0.37 ± 0.07 |
| 1384214_a_at | AI045459 | Cyclin T2 (predicted) | Ccnt2_predicted | 0.50 ± 0.23 | 0.75 ± 0.30 | 0.27 ± 0.06 | 0.42 ± 0.09 |
| 1390470_at | BE107044 | Cyclin T2 (predicted) | Ccnt2_predicted | 1.73 ± 0.29 | 1.21 ± 0.12 | 0.53 ± 0.09 | 0.71 ± 0.16 |
| Splicing machinery | |||||||
| Serine-rich and serine-rich–related protein | |||||||
| 1376252_at | AI145784 | Splicing factor, arginine/serine-rich 3 (SRp20) (predicted) | Sfrs3_predicted | 1.03 + 0.08 | 0.76 + 0.27 | 0.39 + 0.02 | 0.50 + 0.12 |
| 1379010_at | AA956727 | Splicing factor, arginine/serine-rich 3 (SRp20) (predicted) | Sfrs3_predicted | 2.11 + 0.43 | 1.18 + 0.28 | 3.98 + 0.87 | 2.05 + 0.67 |
| 1376594_at | AW524517 | Similar to splicing factor, arginine/serine-rich 1 (ASF/SF2) | Vezf1_predicted | 1.73 + 0.60 | 1.45 + 0.23 | 3.16 + 0.36 | 3.44 + 0.51 |
| 1383537_at | BF522715 | Similar to splicing factor, arginine/serine-rich 1 (ASF/SF2) | Vezf1_predicted | 1.84 + 0.46 | 1.79 + 0.30 | 1.67 + 0.29 | 1.80 + 0.11 |
| 1371838_at | AI411155 | Similar to splicing factor, arginine/serine-rich 2 | Sfrs2 | 1.11 + 0.07 | 1.13 + 0.12 | 1.43 + 0.04 | 1.33 + 0.04 |
| 1371839_at | AA819369 | Similar to splicing factor, arginine/serine-rich 2 | Sfrs2 | 0.63 + 0.13 | 0.66 + 0.07 | 1.59 + 0.10 | 1.33 + 0.17 |
| 1368992_a_at | AI104005 | Splicing factor, arginine/serine-rich 5 | Sfrs5 | 0.56 + 0.09 | 0.77 + 0.17 | 0.53 + 0.03 | 0.57 + 0.06 |
| 1371999_at | BI303641 | Splicing factor arginine/serine-rich 6 (SRP55-2) (isoform 2) | Sfrs6 | 0.37 + 0.13 | 0.71 + 0.06 | 0.19 + 0.04 | 0.20 + 0.09 |
| 1381623_at | BF391476 | Ssimilar to Sfrs4 protein (predicted) | Sfrs4_predicted | 1.23 + 0.37 | 1.59 + 0.14 | 2.08 + 0.22 | 1.89 + 0.17 |
| 1370188_at | AW252670 | Splicing factor, arginine/serine-rich 10 (transformer 2 homolog, Drosophila) | Sfrs10 | 1.02 + 0.16 | 1.04 + 0.06 | 2.18 + 0.29 | 1.41 + 0.13 |
| 1371425_at | BF396399 | Serine/arginine repetitive matrix 1 (predicted) | Srrm1_predicted | 1.29 + 0.21 | 1.20 + 0.13 | 1.40 + 0.09 | 1.37 + 0.06 |
| 1383410_at | BI290777 | Signal recognition particle 54 | Srp54 | 0.91 + 0.09 | 0.88 + 0.06 | 1.50 + 0.13 | 1.21 + 0.03 |
| 1371596_at | AI008971 | Ribonucleic acid binding protein S1 | Rnps1 | 1.01 + 0.34 | 0.90 + 0.12 | 1.84 + 0.08 | 1.54 + 0.06 |
| Heterogeneous nuclear ribonucleoprotein family | |||||||
| 1398883_at | BI296284 | Heterogeneous nuclear ribonucleoprotein A2/B1 (predicted) | Hnrpa2b1_predicted | 0.77 + 0.10 | 0.85 + 0.05 | 0.45 + 0.04 | 0.53 + 0.06 |
| 1371505_at | BG381750 | Heterogeneous nuclear ribonucleoprotein C | Hnrpc | 1.15 + 0.03 | 1.15 + 0.06 | 2.25 + 0.28 | 2.05 + 0.18 |
| 1367931_a_at | X60790 | Polypyrimidine tract binding protein 1 | Ptbp1 | 0.67 + 0.12 | 0.60 + 0.02 | 0.83 + 0.14 | 0.73 + 0.10 |
| 1370919_at | AI103467 | Heterogeneous nuclear ribonucleoprotein M | Hnrpm | 0.85 + 0.09 | 0.81 + 0.03 | 0.79 + 0.07 | 0.78 + 0.07 |
| Other splicing factors | |||||||
| 1389975_at | BE116949 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 4 (Hu antigen D) | HuD | 0.63 + 0.12 | 0.59 + 0.11 | 0.32 + 0.02 | 0.33 + 0.06 |
| 1394546_at | AI556229 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 4 (Hu antigen D) | HuD | 0.82 + 0.37 | 0.59 + 0.03 | 0.10 + 0.01 | 0.21 + 0.09 |
| 1395083_at | AA926313 | Neuro-oncological ventral antigen 1 | Nova1 | 0.34 + 0.18 | 0.68 + 0.18 | 0.22 + 0.03 | 0.37 + 0.11 |
| 1388476_at | AI101391 | Tial1 cytotoxic granule–associated RNA binding protein–like 1 (mapped) | Tial1 | 1.15 + 0.21 | 1.03 + 0.21 | 1.39 + 0.02 | 1.32 + 0.05 |
| 1374463_at | AI172068 | Quaking homolog, KH domain RNA binding (mouse) | Qki | 1.33 + 0.04 | 1.07 + 0.22 | 1.87 + 0.08 | 1.57 + 0.16 |
| 1370899_at | AI599699 | Splicing factor proline/glutamine rich (polypyrimidine tract binding protein associated) | Sfpq | 0.42 + 0.05 | 0.48 + 0.02 | 0.40 + 0.08 | 0.30 + 0.13 |
| 1386896_at | AF393783 | KH domain containing, RNA binding, signal transduction–associated 1 | Khdrbs1 | 0.88 + 0.12 | 0.82 + 0.04 | 2.15 + 0.19 | 1.66 + 0.17 |
| 1398773_at | NM_130405 | KH domain containing, RNA binding, signal transduction–associated 1 | Khdrbs1 | 0.96 + 0.18 | 0.96 + 0.04 | 1.31 + 0.06 | 1.39 + 0.05 |
| 1372496_at | BG371538 | Ribonucleoprotein PTB-binding 1 (protein raver-1) | Raver1h | 0.55 + 0.24 | 0.68 + 0.07 | 3.26 + 0.38 | 1.85 + 0.29 |
| 1371367_at | BE107459 | TAR DNA-binding protein 43 (TDP-43) | Tardbp | 0.93 + 0.07 | 0.83 + 0.10 | 0.40 + 0.03 | 0.51 + 0.05 |
Data are means ± SE of three independent experiments and are expressed as fold change versus control cells, studied at the same time points. Gene expression was considered modified by cytokines when P ≤ 0.02 (paired t test) and expression level ≥ 1.5-fold higher or lower as compared with control conditions. Primary rat β-cells were left untreated or exposed to IL1β + IFN-γ (IL + IFN) or TNF-α + IFN-γ (TNF + IFN) for 6 and 24 h.
Analysis of gene networks and pathways regulated by IL-1β + IFN-γ or TNF-α + IFN-γ in rat β-cells.
IPA analysis identified 50 and 100 IL-1β + IFN-γ–modified and 50 and 86 TNF-α + IFN-γ–modified networks containing >12 focus genes and representing key transcription factors and their interactions with target genes after 6 and 24 h, respectively (data not shown). The networks regulated by the transcription factors NF-κB (supplementary Fig. 2A) and Myc (supplementary Fig. 2B) were among the top scores for both cytokines. Depending on the cytokines tested, however, these networks often contained different groups of genes regulated by the same transcription factor. Different temporal patterns of transcription factor activation may lead to a differential induction of downstream genes (11). IL-1β induced an earlier and more sustained NF-κB activation, represented by nuclear p65, as compared with TNF-α (supplementary Fig. 2C).
The canonical pathways regulated by IL-1β + IFN-γ or TNF-α + IFN-γ after 24 h were identified by IPA, and the top 32 pathways are shown in supplementary Fig. 3. Among these, many were related to local inflammatory responses, such as IFN signaling, antigen presentation, antiviral responses, and production of cytokines or chemokines. Several of the pathways were involved in the intracellular signaling induced by cytokines (such as those mediated by Janus kinase/signal transducers and activators of transcription, HIF-1α and NF-κB), apoptosis, cell cycle regulation, cell metabolism (e.g., Krebs [citrate] cycle), or in endoplasmic reticulum stress. Based on the identification of these pathways, we focused on novel pathways of particular relevance for insulitis/β-cell apoptosis, aiming to identify regulatory transcription factors by use of siRNA strategy (see below).
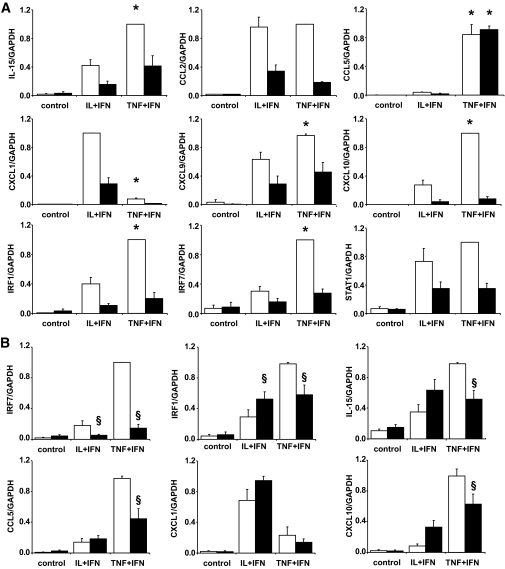
Differential inflammatory signature of IL-1β and TNF-α.
Cytokines regulate expression of many genes involved in the inflammatory response, such as “chemokines/cytokines/adhesion molecules” and “IFN-γ signaling” (Table 1 and supplementary Fig. 3). For some of these genes and pathways, there was a different regulation by IL-1β and TNF-α, with TNF-α + IFN-γ preferentially inducing IL-15, chemokine (CXC-motif) ligand (CXCL) 9 (or Mig), CXCL10 (or IP-10), CCL5 (or RANTES), IRF-1, IRF-7, and signal transducer and activator of transcription-1 (STAT1)-α, while IL-1β preferentially upregulated CCL2 (or MCP-1) and CXCL1 (or Groα) (Table 1). These differences were to a large extent confirmed by real-time RT-PCR (Fig. 2A). TNF-α–induced higher CCL5 and IRF-7 expression was also confirmed at the protein level (supplementary Fig. 4A–C). TNF-α + IFN-γ leads also to higher expression of IFN-β, a downstream gene of IRF-7, than IL-1β + IFN-γ (supplementary Fig. 4E). TNF-α–induced IRF-7 expression upregulates expression of IRF-1 and proinflammatory chemokines in other tissues (20,21). Use of a specific siRNA against IRF-7 induced a 90% knock down of IRF-7, which partially prevented TNF-α + IFN-γ–induced, but not IL-1β + IFN-γ–induced, IRF-1, CCL5 (confirmed at protein level) (supplementary Fig. 4C), IL-15, and CXCL10 expression (Fig. 2B). The role of IRF-7 is apparently specific for genes preferentially induced by TNF-α + IFN-γ, since CXCL1 expression, which is higher after IL-1β + IFN-γ exposure (Fig. 2), was not significantly decreased by IRF-7 knock down (Fig. 2B). These observations were confirmed by the use of a second siRNA against IRF-7 (data not shown).
FIG. 2.
TNF-α and IL-1β differentially modulate expression of β-cell genes involved in the inflammatory response. Gene expression was analyzed by real-time RT-PCR. A: FACS-purified rat β-cells were exposed or not (control) to IL-1β + IFN-γ (IL+IFN) or to TNF-α + IFN-γ (TNF+IFN) for 6 h (□) or 24 h (■). B: FACS-purified rat β-cells were transfected with siRNA control (□) or siRNA against IRF-7 (■) and exposed or not (control) to IL-1β + IFN-γ (IL+IFN) or to TNF-α + IFN-γ (TNF+IFN) for 24 h. Results are means ± SE of three to six independent experiments. *P < 0.05 vs. IL+IFN at the same time point; §P < 0.05 vs. siControl at the same time point and treatment.
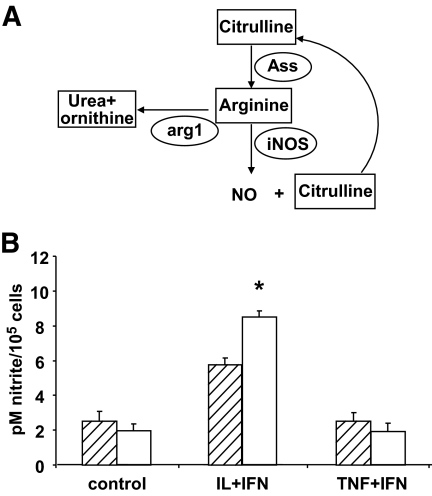
Differential modulation of the citrulline-NO cycle by IL-1β and TNF-α.
IL-1β + IFN-γ treatment in β-cells led to higher expression of inducible NO synthase (iNOS) (Table 1) and NO accumulation in the medium (supplementary Fig. 1B) than TNF-α + IFN-γ. iNOS utilizes arginine as the substrate for NO formation, generating citrulline as a by-product. Citrulline can be used to regenerate arginine by the citrulline-NO cycle (Fig. 3A) (22), which is regulated by argininosuccinate synthetase (ASS) expression (22). The array analysis indicated that ASS is strongly induced by IL-1β + IFN-γ but not by TNF-α + IFN-γ (Table 1). In addition, IL-1β + IFN-γ inhibited the expression of arginase-1 (arg1) more efficiently than TNF-α + IFN-γ (Table 1), preserving arginine for NO formation (Fig. 3A). In line with the mRNA data, IL-1β + IFN-γ, but not TNF-α + IFN-γ, induced NO formation in the absence of arginine but presence of citrulline (Fig. 3B).
FIG. 3.
Differential usage of the NO synthesis pathway by IL-1β and TNF-α. A: Schematic view of the NO synthesis pathway. Elliptical shapes represent enzymes. B: Synthesis of NO by rat primary β-cells cultured in arginine-citrulline–free medium (▨) or in medium containing 1 mmol/l citrulline (□) and exposed to IL-1β + IFN-γ (IL+IFN) or TNF-α + IFN-γ (TNF+IFN) for 48 h. Results are mean of five independent experiments. *P < 0.05 vs. arginine-citrulline–free medium.
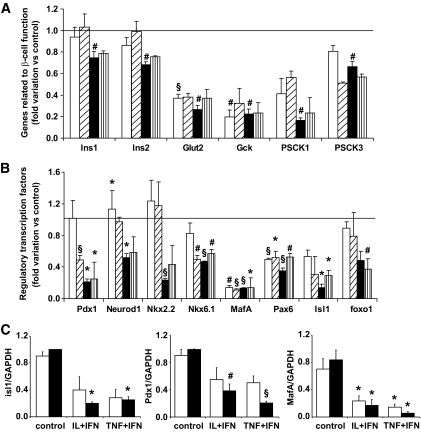
Cytokines decrease the expression of genes involved in maintenance of a differentiated β-cell phenotype.
We next examined the expression of a group of 14 genes (Fig. 4) previously shown to be of particular relevance for the induction and maintenance of the differentiated phenotype in β-cells (23). These genes are either directly related to β-cell–differentiated functions (Fig. 4A) or function as master regulatory transcription factors (Fig. 4B). They were all inhibited by IL-1β + IFN-γ or TNF-α + IFN-γ, a finding confirmed by real-time RT-PCR for selected genes (Fig. 4C). For many of these genes, inhibition was already present after 6 h of cytokine exposure (Fig. 4), suggesting an early and specific effect.
FIG. 4.
Cytokines decrease the expression of genes involved in the maintenance of a differentiated β-cell phenotype. Expression of genes related to β-cell function (A) or regulatory transcription factors (B) were analyzed by microarray (n = 3) in FACS-purified rat β-cells exposed to IL-1β + IFN-γ for 6 h (□) or 24 h (■) or to TNF-α + IFN-γ for 6 h (▨) or 24 h (vertical striped bars). Results are shown as fold change compared with control (no cytokine added), considered as one (line). C: confirmation by real-time RT-PCR of cytokine effects on the expression of PDX-1, MafA, and Isl1; □, 6 h; ■, 24 h. Results are means ± SE of three to four independent experiments. *P < 0.05; #P < 0.01; §P < 0.001 vs. control.
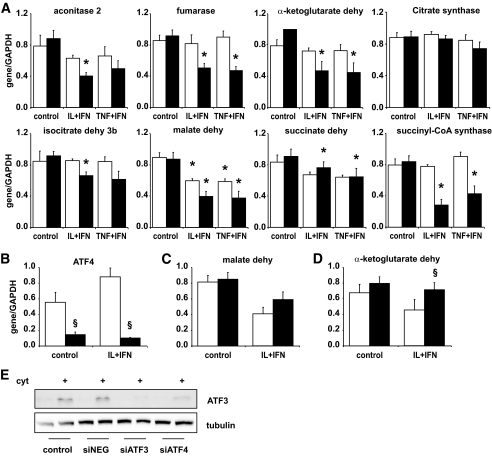
Cytokines decrease the expression of genes encoding enzymes of the Krebs cycle.
Exposure of β-cells to IL-1β + IFN-γ or TNF-α + IFN-γ decreased to a similar extent expression of genes encoding enzymes of the Krebs cycle (Table 1, glucose metabolism). This was confirmed by real-time RT-PCR for seven of eight genes present in the Krebs cycle, with a more important inhibitory effect at 24 h (Fig. 5A). The promoter region of these genes was analyzed by an in silico approach, and a binding site for (ATF4 identified as overrepresentative in this set of genes. ATF4 is induced by cytokines (Table 1 and Fig. 5B) and has an important role in the unfolded protein response (UPR) in β-cells (24,25). Against this background, we analyzed the role of ATF4 knock down on cytokine-induced changes in Krebs cycle–regulating genes. Since both cytokine combinations have similar effects on this group of genes, we used only IL-1β + IFNγ. siRNA targeting ATF4 inhibited cytokine-induced ATF4 expression by >80% (Fig. 5B). Expression of ATF3, an ATF4-regulated gene, was significantly decreased confirming functional consequences of ATF4 knock down (Fig. 5E). Inhibition of ATF4 expression partially prevented the inhibitory effects of cytokines on the expression of the two Krebs cycles genes analyzed, namely malate dehydrogenase and α-ketoglutarate dehydrogenase (Fig. 5C). ATF4 knock down was confirmed at the functional level by Western blot for ATF3, a downstream gene of ATF4. Cytokine-induced expression of ATF3 was prevented by ATF4 knock down, at a similar level of the inhibition observed when a siATF3 was used (Fig. 5E).
FIG. 5.
Cytokines inhibit expression of genes encoding Krebs cycle enzymes, which is partially dependent of ATF4 activation. A: Confirmation by real-time RT-PCR of microarray analysis data in rat purified β-cells untreated (control) or exposed to a combination of IL-1β + IFN-γ (IL+IFN) or TNF-α + IFN-γ (TNF+IFN) for 6 h (□) or 24 h (■). Results are means ± SE of four to five independent experiments. *P ≥ 0.05 vs. control. B and C: β-Cells were transfected with siControl (□) or siATF4 (■) and then left untreated or treated with IL-1β + IFN-γ (IL+IFN) for 24 h. B: Confirmation of ATF4 knockdown (KD) by real-time RT-PCR. C: Effects of ATF4 KD on the expression of malate dehydrogenase and α-ketoglutarase dehydrogenase. Results are mean of six independent experiments. §P < 0.05 cytokines + siATF4 vs. cytokines + siControl. Dehy = dehydrogenase. E: Western blot for ATF3 protein in cells transfected with siATF4 or siATF3. The figure is representative of four independent experiments.
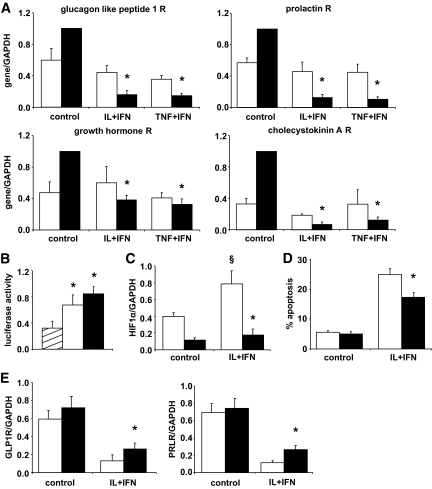
Cytokines decrease the expression of incretin and hormone receptors at least in part via activation of HIF-1α.
IL-1β + IFNγ and TNF-α + IFNγ inhibited the expression of key hormone receptors in rat β-cells (Table 1). This was confirmed by real-time RT-PCR for the receptors of glucagon-like peptide (GLP)-1, prolactin (PRL), growth hormone (GH), and cholecystokinin A (CCKA) (Fig. 6A). Cytokine-induced inhibition was in the range of 50–90% and more marked after 24 h (Fig. 6A). Analysis of the promoter region of these genes found a common binding site for the transcription factor HIF-1 (26,27). Cytokines upregulated transcriptional activity (Fig. 6B) and mRNA expression (Table 1 and Fig. 6C) of HIF-1α, the regulatory HIF-1 subunit (26). This could in part be explained by cytokine activation of AKT (supplementray Fig. 5C). HIF-1α knock down inhibited by 75% cytokine-induced HIF-1α expression (Fig. 6D) and by 60% HIF transcriptional activity (supplementary Fig. 5A and B). Knock down of HIF-1α partially prevented cytokine-induced apoptosis in β-cells (Fig. 6D) and inhibition of two receptors analyzed, GLP-1 receptor (R) and PRLR (Fig. 6E). This partial effect of HIF-1α knock down in GLP-1R and PRLR expression suggest that other transcription factors may be involved in this process, as supported by the in silico identification of other relevant candidate transcription factors (supplementary Table 8).
FIG. 6.
Cytokines decrease expression of key hormone receptor genes partially via HIF-1α induction. Rat purified β-cells were left untreated (control) or exposed to a combination of IL-1β + IFN-γ (IL+IFN) or TNF-α + IFN-γ (TNF+IFN). A: Real-time RT-PCR to confirm microarray analysis of hormone receptors (R) expression after cytokine exposure for 6 h (□) or 24 h (■). Results are means ± SE of three to four experiments. *P ≤ 0.05 vs. control. B: Luciferase reporter assay of HIF-1α activation by cytokines. Cells were cotransfected with an HRE luciferase reporter gene and the internal control pRL-CMV, then left untreated (▨) or exposed to IL+IFN (□) or the positive control Cobalt chloride (CoCl2, ■) for 12 h. Results are normalized for Renilla luciferase activity and are means ± SE of five experiments. *P < 0.05 vs. untreated cells. C–E: HIF-1α knockdown by siRNA. Cells were transfected with siControl (□) or siHIF-1α (■) and then left untreated or treated with IL+IFN for 24 h. Results are means ± SE of four to six experiments. C: HIF-1α knockdown analyzed by real-time RT-PCR. *P ≤ 0.05 vs. siControl under the same treatment and §P ≤ 0.05 vs. control (not cytokine treated). D: Viability of cells after HIF-1α knockdown and 48 h cytokine exposure. *P ≤ 0.05 vs. siControl + cytokines. E: Expression of PRLR and GLP-1R after HIF-1α knockdown and 24-h cytokine exposure, measured by real-time RT-PCR. *P ≤ 0.05 vs. siControl + cytokines.
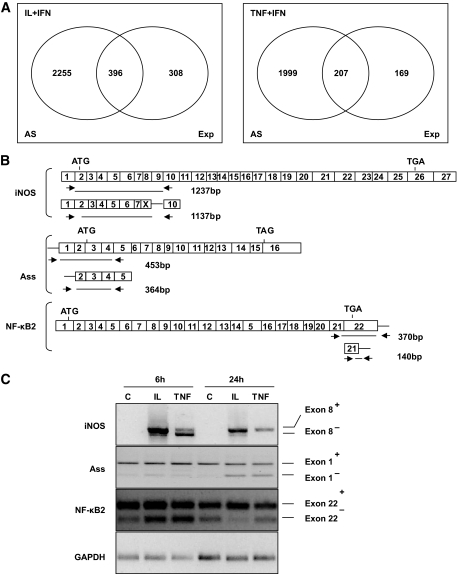
Cytokines regulate the splicing machinery and alternative splicing in primary β-cells.
A large number of cytokine-modified genes are involved in alternative splicing (Table 1, splicing machinery). To determine whether this triggers modifications in the splice variants present in β-cells, 24-h cytokine-treated samples from the three β-cell–independent preparation/experiments used in the initial array analysis (Fig. 1 and Table 1) were pooled as previously described (7,9) and analyzed for the presence of splice variants using the rat exon-array from Affymetrix. Cytokine treatment led to important changes in alternative splicing, with IL-1β + IFNγ potentially modulating differential splicing of 2,651 genes (21% of the total number of the expressed genes) (supplementary Table 9, Fig. 7A). From these, only 396 were also modified at the expression level. These findings suggest that >50% of IL-1β + IFN-γ–modulated genes undergo alternative splicing. For TNF-α + IFN-γ, there was induction of alternative splicing in 2,206 genes (19%), with only 207 of these being also modified at expression level (supplementary Table 10, Fig. 7A). The spliced genes were classified according with their putative molecular function as shown in supplementary Tables 11 and 12. Alternative splicing was confirmed for three genes analyzed by RT-PCR (Fig. 7), namely iNOS and ASS, which participate in the citrulline-NO cycle (Fig. 3A) and the NF-κB subunit p100/p52 (NF-κB2). iNOS was not detected in control cells, but it was induced by cytokines, and there was a difference in the size of the amplified region after 6 and 24 h of cytokine treatment (Fig. 7C). At 6 h, there was amplification of two bands of 1,237 and 1,137 bp, the second one corresponding to iNOS lacking exon 8 or 9 (by sequencing analysis of the PCR product we confirmed that exon 8 is missing (data not shown), while at 24 h the majority of the amplified bands contained exon 8 (Fig. 7C). This confirms that posttranscriptional processing of iNOS is differentially modified by cytokines at different time points. Using the same approach, we observed that cytokines decreased utilization of exon 1 from ASS while it increased utilization of exon 22 from NF-κB2 (Fig. 7C).
FIG. 7.
Cytokines induce alternative splicing in rat pancreatic β-cells. A: Ven diagram representing the number of β-cell genes that undergo alternative splicing (alternative splicing) and/or expression (Exp) changes after 24 h of cytokine treatment compared with control condition, as identified by exon array analysis (GeneChip Rat exon 1.0 ST Array). B: Schematic diagram of inducible iNOS, ASS, and NFκB subunit p100/p52 exon structures and of the PCR primers presently used to identify spliced forms. Start (ATG) and stop (TGA or TAG) codons are indicated in the figure. The arrows show the positions of the PCR primers, while the lines below indicate the size of the amplified region in the presence or absence of the respective exon analyzed. C: RT-PCR of rat primary β-cells exposed to control condition (C), IL-1β + IFN-γ (IL), or TNF-α + IFN-γ (TNF) for 6 or 24 h to amplify the regions of iNOS, ASS, and NF-κB indicated in B. GAPDH was amplified in parallel to control for the amount of cDNA loaded in each reaction. The figure is representative of three to five experiments.
DISCUSSION
We have presently used state-of-the-art array analysis of fluorescence-activated cell sorter–purified β-cells to unveil the global pattern of genes modified by the inflammatory cytokines IL-1β + IFN-γ and TNF-α + IL-1β. The use of primary and pure cell preparations (>90% β-cells) is of special relevance, since it enabled us to obtain a broad picture of β-cell responses to proapoptotic inflammatory mediators without the confounding signals generated by other endocrine and nonendocrine islet cells. We cannot, however, discard that interactions between β-cells and others cells in the islets, and with infiltrating mononuclear cells during insulitis, will lead to changes in β-cell gene expression that are not detected by the present model. The array data were evaluated by both nonbiased pathway analysis (IPA) and investigator-based analysis. Selected pathways were chosen for additional studies, with special emphasis on the role of novel transcription factors. Prompted by the observation of cytokine-induced changes in a large number of genes involved in alternative splicing, an exon-array analysis was performed to evaluate the presence of splicing variants in β-cells. The following are main novel observations of the study. 1) Nearly 8,000 genes were detected as present in β-cell, with 96% confirmation of selected cytokine-modified genes by real-time RT-PCR. This more than doubles the known β-cell expressed genes. 2) There are temporal, qualitative, and quantitative differences in the genes induced by TNF-α and IL-1β regarding inflammation and NO production. This is probably secondary to the differential expression and usage of transcription factors such as NF-κB and IRF-7. 3) Key gene networks related to β-cell–differentiated phenotype and the Krebs cycle are similarly inhibited by TNF-α + IFN-γ and IL-1β + IFN-γ. 4) Cytokines induce major changes in alternative splicing of genes, indicating a novel level of functional regulation in β-cells.
IL-1β + IFN-γ induces a higher expression of iNOS and ASS and a more marked inhibition of arg1 as compared with TNF-α + IFN-γ, leading to higher NO production from either arginine or citrulline (supplementary Fig. 1B and Fig. 3). This enables continuous NO production in inflammation sites where arginine is usually depleted. NO formation induced by proinflammatory cytokines contributes for β-cell death in some rodent models of diabetes (1). Furthermore, 46% of cytokine-modulated genes are NO dependent in INS-1E cells (8), suggesting that differences in NO production may explain why IL-1β + IFN-γ modulates a higher number of genes compared with TNF-α + IFN-γ at 24 h (Fig. 1).
Exposure of β-cells to proinflammatory cytokines during insulitis induce release of chemokines and cytokines, which may contribute to recruit and activate immune cells and thus amplify local inflammation and the autoimmune assault (2,10). The present data suggest differential roles for IL-1β and TNF-α in this “dialogue” between the β-cells and the immune system. Thus, while TNF-α + IFN-γ induces higher expression of IL-15, CCL5, CXCL9, and CXCL10, IL-1β + IFN-γ preferentially induces CCL2 and CXCL1. These inflammatory mediators contribute for insulitis and destruction of β-cells by the immune system (1,2,10), and the present observations suggest that the balance between TNF-α and IL-1β expression during insulitis can lead to different outcomes. These differences may reflect differential usage of two key transcription factors, namely NF-κB and IRF-7. Thus, higher and earlier activation of NF-κB by IL-1β + IFN-γ, as presently shown in primary β-cells, probably explains the higher expression of NF-κB target genes such as CCL2 (28). On the other hand, TNF-α preferentially triggers IRF-7 and IRF-1 activation (present data). In other cell types, TNF-α–induced IFN-β expression synergistically activates the IRF-7/1–STAT-1 pathway, leading to sustained expression of cytokines and chemokines (21). TNF-α + IFN-γ leads to higher induction of IFN-β expression in β-cells than IL-1β + IFN-γ, which may explain the differences in the expression of chemokines/cytokines induced by IL-1β or TNF-α. The role of STAT-1 in this process was previously shown in islets from STAT-1 knockout mice (29), and we presently show that IRF-7 knock down partially prevents TNF-α + IFN-β–induced expression of IRF-1, IL-15, CCL5, and CXCL1.
Loss of differentiated β-cell functions is another important consequence of exposure to cytokines (30). We presently describe three gene networks whose inhibition may contribute to this outcome, namely key transcription factors for the maintenance of β-cell phenotype, mRNAs encoding receptors for growth factors and incretins, and mRNAs encoding enzymes of the Kreb's cycle. Zhou et al. (23) reported that inducing expression of the transcription factors neurogenin 3 (Ngn3), pancreatic and duodenal homeobox-1 (Pdx-1), and mammalian homologue of avian MafA/L-Maf (MafA) reprograms pancreatic mouse exocrine cells into cells that closely resemble β-cells. Reprogramming of pancreatic exocrine cells to β-cells should benefit patients with type 1 diabetes, an autoimmune disease characterized by local inflammation (2,10). Insulin epitopes are targets of the immune assault in type 1 diabetes (31), and new insulin-producing cells will be recognized and attacked by the immune system (32). The present data suggest that immune mediators of insulitis, such as cytokines, will push back newly developed β-cells into a dedifferentiated state, preceding actual β-cell death.
The hormones GLP-1, CCKA, PRL, and GH are involved in mitotic and functional activation of rodent β-cells (33,34). Due to these characteristics, GLP-1 analogs are being presently tested as an adjuvant therapy in early type 1 diabetes (35). Of concern, cytokines induce an early and profound inhibition of mRNAs encoding for the receptors of GLP-1, CCKA, PRL, and GH, which may prevent the restorative effects of these hormones. These mRNAs are inhibited in parallel, suggesting the role for a common inhibitory transcription factor downstream of cytokines. In silico analysis and siRNA experiments suggest that HIF-1α is at least in part involved in this inhibitory effect of cytokines. HIF-1 is a key regulator of adaptive cellular responses to hypoxia, and it is active when its regulatory subunit HIF-1α is stabilized during hypoxia (26). HIF-1α stabilization/activation has important roles in other cellular responses, such as glucose metabolism, cell growth/apoptosis, and the inflammatory response (26,36,37). We now show that cytokines induce both HIF-1α mRNA expression and transcriptional activity in β-cells and that HIF-1α knock down partially prevents cytokine-induced inhibition of key hormone receptors and β-cell apoptosis. This suggests a novel role for HIF-1α in β-cells, as one of the mediators of cytokine-induced β-cell dysfunction and death. Of note, prolonged β-cell exposure to high glucose triggers HIF-1α expression (38), while constitutive HIF-1α expression in β-cells impairs glucose-stimulated insulin release (39).
We have previously shown that cytokine-induced NO formation in β-cells inhibits mitochondrial glucose oxidation via functional impairment of the enzyme aconitase (40). We presently show that cytokines also inhibit expression of several mRNAs encoding enzymes of the Krebs cycle. This is mediated, at least in part, via ATF4 activation, as suggested by both in silico analysis and siRNA. The transcription factor ATF4 is part of the UPR response in cytokine-treated β-cells (24,25). Endoplasmic reticulum stress may contribute to HIF-1α activation (41), potentially linking three cytokine-induced effects in β-cells, namely endoplasmic reticulum stress, HIF-1α activation, and inhibition of the Krebs cycle. Additional experiments are now required to further investigate this possibility and to clarify how gene networks regulating mitochondria and endoplasmic reticulum function may provide the signaling for β-cell apoptosis.
Cytokines modulate expression of several genes related to the alternative splicing machinery (present data), which is in line with recent proteomic data (42). Alternative splicing is an important determinant of cellular function. More than 85% of the human genes may undergo alternative splicing (14,43), and many of these spliced forms are tissue specific, contributing for the generation of proteomic diversity (43). The complex interactions required for correct splicing can be disturbed by changes in the expression of splicing factors and cellular energy stores (15,44). By exon-array analysis, we presently observed that cytokines modulate the expression of splicing variants in β-cells, with potentially 20% of the detected genes showing alternative splicing. This findings must be interpreted with caution, since they represent a pool of three experiments that precludes adequate statistical analysis. In addition, this methodology can lead to false positive detection (45). Here, for at least three of the modified genes (iNOS ASS, and NF-κB2) there was independent confirmation by RT-PCR. Cytokine-induced iNOS splicing variants may provide another level of regulation of iNOS activity in a tissue-specific way (46). Many of the presently identified genes are modified only at the splicing level, without changes in expression. This indicates a new level of complexity in the effects of cytokines (and potentially of other modulators of β-cell function and survival) that must be taken into account in future studies. The functional impact of these diverse splice variants in β-cells remain to be investigated, but data available from other tissues indicate that it is huge, increasing the number of molecule species that are involved in normal regulation of cell or disease susceptibility (14–16,43,47,48). Interestingly, splicing may also have a role in the augmentation of autoimunity in type 1 diabetes (49).
In conclusion, the present study doubles the number of known genes modified by cytokines in primary rat β-cells and suggests temporal, qualitative, and quantitative differences between the effects of TNF-α + IFN-γ and IL-1β + IFN-γ. Cytokines decrease the expression of genes related to β-cell function and growth/regeneration, indicating that immune mediators of insulitis can push back newly formed β-cells into a dedifferentiated state. Interestingly, cytokines modify alternative splicing in β-cells, indicating a new level of complexity in the β-cell responses to immune-mediated damage.
Supplementary Material
ACKNOWLEDGMENTS
This work has been supported by grants from the European Union (Projects Savebeta and Naimit in the Framework Programs 6 and 7 of the European Community); the FNRS (Fonds National de la Recherche Scientifique) and ARC (Actions de Recherche Concerteé de la Communauté Française), Belgium; the Belgium Program on Interuniversity Poles of Attraction initiated by the Belgian state (IUAP P5/17 and P6/40); and the Expert Center Grant 2008.40.001 from the Dutch Diabetes Research Foundation. M.L.C. is the recipient of a scholarship from CAPES (Brazilian Coordination for the Improvement of Higher Education Personnel). F.M. is the recipient of a Postdoctoral Fellowship from FNRS, Belgium. No potential conflicts of interest relevant to this article were reported.
We thank the personnel from Laboratory of Experimental Medicine–Université Libre de Bruxelles, M.A. Neef, G. Vandenbroeck, M. Urbain, J. Schoonheydt, R. Leeman, S. Mertens, R. Makhnas, and A.E. Musaya for excellent technical support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 335.
REFERENCES
- 1.Eizirik DL, Mandrup-Poulsen T: A choice of death-the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 2001;44:2115–2133 [DOI] [PubMed] [Google Scholar]
- 2.Kaminitz A, Stein J, Yaniv I, Askenasy N: The vicious cycle of apoptotic β-cell death in type 1 diabetes. Immunol Cell Biol 2007;85:582–589 [DOI] [PubMed] [Google Scholar]
- 3.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I: Macrophages and dendritic cells infiltrating islets with or without β-cells produce tumour necrosis factor-α in patients with recent-onset type 1 diabetes. Diabetologia 2007;50:596–601 [DOI] [PubMed] [Google Scholar]
- 4.Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T: Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 2009;32:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickersgill LM, Mandrup-Poulsen TR: The anti-interleukin-1 in type 1 diabetes action trial-background and rationale. Diabetes Metab Res Rev 2009;25:321–324 [DOI] [PubMed] [Google Scholar]
- 6.Eizirik DL, Moore F, Flamez D, Ortis F: Use of a systems biology approach to understand pancreatic β-cell death in type 1 diabetes. Biochem Soc Trans 2008;36:321–327 [DOI] [PubMed] [Google Scholar]
- 7.Cardozo AK, Kruhoffer M, Leeman R, Orntoft T, Eizirik DL: Identification of novel cytokine-induced genes in pancreatic β-cells by high-density oligonucleotide arrays. Diabetes 2001;50:909–920 [DOI] [PubMed] [Google Scholar]
- 8.Kutlu B, Cardozo AK, Darville MI, Kruhoffer M, Magnusson N, Orntoft T, Eizirik DL: Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 2003;52:2701–2719 [DOI] [PubMed] [Google Scholar]
- 9.Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhoffer M, Orntoft T, Eizirik DL: A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic β-cells. J Biol Chem 2001;276:48879–48886 [DOI] [PubMed] [Google Scholar]
- 10.Eizirik DL, Colli ML, Ortis F: The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 11.Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL: Cytokine-induced pro-apoptotic gene expression in insulin-producing cells is related to rapid, sustained and non-oscillatory NF-κB activation. Mol Endocrinol 2006;20:1867–1879 [DOI] [PubMed] [Google Scholar]
- 12.Ortis F, Pirot P, Naamane N, Kreins AY, Rasschaert J, Moore F, Théatre E, Verhaeghe C, Magnusson N, Chariot A, Orntoft T, Eizirik DL: TNF-α and IL-1β induction of NF-κB and its downstream genes has a pro-apoptotic role in pancreatic β-cells. Diabetologia 2008;51:1213–1225 [DOI] [PubMed] [Google Scholar]
- 13.Magnusson NE, Cardozo AK, Kruhoffer M, Eizirik DL, Orntoft TF, Jensen JL: Construction and validation of the APOCHIP, a spotted oligo-microarray for the study of β-cell apoptosis. BMC Bioinformatics 2005;6:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ: Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 2008; 40: 1413– 1415 [DOI] [PubMed] [Google Scholar]
- 15.Blencowe BJ: Alternative splicing: new insights from global analyses. Cell 2006;126:37–47 [DOI] [PubMed] [Google Scholar]
- 16.Colobran R, Pujol-Borrell R, Armengol MP, Juan M: The chemokine network: II. on how polymorphisms and alternative splicing increase the number of molecular species and configure intricate patterns of disease susceptibility. Clin Exp Immunol 2007;150:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW: β-Cell apoptosis in diabetes. Apoptosis 2009;14:1389–1404 [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F: A model based background adjustment for oligonucleotide expression array. J Am Stat Assoc 2004;99:909–917 [Google Scholar]
- 19.Moore F, Colli ML, Cnop M, Esteve MI, Cardozo AK, Cunha DA, Bugliani M, Marchetti P, Eizirik DL: PTPN2, a candidate gene for type 1 diabetes, modulates interferon-γ–induced pancreatic β-cell apoptosis. Diabetes 2009;58:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sgarbanti M, Marsili G, Remoli AL, Orsatti R, Battistini A: IRF-7: new role in the regulation of genes involved in adaptive immunity. Ann N Y Acad Sci 2007;1095:325–333 [DOI] [PubMed] [Google Scholar]
- 21.Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB: TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol 2008;9:378–387 [DOI] [PubMed] [Google Scholar]
- 22.Flodstrom M, Niemann A, Bedoya FJ, Morris SM, Jr, Eizirik DL: Expression of the citrulline-nitric oxide cycle in rodent and human pancreatic β-cells: induction of argininosuccinate synthetase by cytokines. Endocrinology 1995;136:3200–3206 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA: In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008; 1476–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eizirik DL, Cardozo AK, Cnop M: The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008; 29: 42– 61 [DOI] [PubMed] [Google Scholar]
- 25.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL: Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 2005;54:452–461 [DOI] [PubMed] [Google Scholar]
- 26.Ke Q, Costa M: Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 2006;70:1469–1480 [DOI] [PubMed] [Google Scholar]
- 27.O'Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB: PGC-1α is coupled to HIF-1α-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A 2009;106:2188–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutlu B, Darville MI, Cardozo AK, Eizirik DL: Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic β-cells. Diabetes 2003;52:348–355 [DOI] [PubMed] [Google Scholar]
- 29.Gysemans CA, Ladriere L, Callewaert H, Rasschaert J, Flamez D, Levy DE, Matthys P, Eizirik DL, Mathieu C: Disruption of the γ-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of β-cells. Diabetes 2005;54:2396–2403 [DOI] [PubMed] [Google Scholar]
- 30.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL: Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54(Suppl. 2):S97– S107 [DOI] [PubMed] [Google Scholar]
- 31.Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA: Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature 2005;435:224–228 [DOI] [PubMed] [Google Scholar]
- 32.Sibley RK, Sutherland DE, Goetz F, Michael AF: Recurrent diabetes mellitus in the pancreas iso- and allograft: a light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 1985;53:132–144 [PubMed] [Google Scholar]
- 33.Nielsen JH, Svensson C, Galsgaard ED, Moldrup A, Billestrup N: β-Cell proliferation and growth factors. J Mol Med 1999;77:62–66 [DOI] [PubMed] [Google Scholar]
- 34.Ahrén B: Potentiators and inhibitors of insulin secretion. In Advances in Molecular Cell Biology Vol. 29.Bittar EE, Howel SL. Eds.Greenwich, CT, Jay Press, 1999, p. 191– 202 [Google Scholar]
- 35.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A: Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008;57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehne N, Brune B: HIF-1 in the inflammatory microenvironment. Exp Cell Res 2009;315:1791–1797 [DOI] [PubMed] [Google Scholar]
- 37.Piret JP, Mottet D, Raes M, Michiels C: Is HIF-1α a pro- or an anti-apoptotic protein? Biochem Pharmacol 2002;64:889–892 [DOI] [PubMed] [Google Scholar]
- 38.Bensellam M, Jonas J-C: High glucose activates hypoxia inducible factors 1 and 2 in cultured rat islets and INS-1E cell monolayers. In Proceedings of the 44th Annual Meeting of the European Association for the Study of Diabetes, Rome, Italy,7–11 September 2008 [Google Scholar]
- 39.Gribble FM: Intolerant of glucose and gasping for oxygen. J Clin Invest 2009; 15: 247– 249 [DOI] [PubMed] [Google Scholar]
- 40.Welsh N, Eizirik DL, Bendtzen K, Sandler S: Interleukin-1 β-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology 1991;129:3167–3173 [DOI] [PubMed] [Google Scholar]
- 41.Werno C, Zhou J, Brune B: A23187, ionomycin and thapsigargin upregulate mRNA of HIF-1α via endoplasmic reticulum stress rather than a rise in intracellular calcium. J Cell Physiol 2008;215:708–714 [DOI] [PubMed] [Google Scholar]
- 42.D'Hertog W, Overbergh L, Lage K, Ferreira GB, Maris M, Gysemans C, Flamez D, Cardozo AK, Van den Bergh G, Schoofs L, Arckens L, Moreau Y, Hansen DA, Eizirik DL, Waelkens E, Mathieu C: Proteomics analysis of cytokine-induced dysfunction and death in insulin-producing INS-1E cells: new insights into the pathways involved. Mol Cell Proteomics 2007;6:2180–2199 [DOI] [PubMed] [Google Scholar]
- 43.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB: Alternative isoform regulation in human tissue transcriptomes. Nature 2008;456:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long JC, Caceres JF: The SR protein family of splicing factors: master regulators of gene expression. Biochem J 2009;417:15–27 [DOI] [PubMed] [Google Scholar]
- 45.Gaidatzis D, Jacobeit K, Oakeley EJ, Stadler MB: Overestimation of alternative splicing caused by variable probe characteristics in exon arrays. Nucleic Acid Res 2009; 37:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eissa NT, Strauss AJ, Haggerty CM, Choo EK, Chu SC, Moss J: Alternative splicing of human inducible nitric-oxide synthase mRNA. tissue-specific regulation and induction by cytokines. J Biol Chem 1996;271:27184–27187 [DOI] [PubMed] [Google Scholar]
- 47.Gardina PJ, Clark TA, Shimada B, Staples MK, Yang Q, Veitch J, Schweitzer A, Awad T, Sugnet C, Dee S, Davies C, Williams A, Turpaz Y: Alternative splicing and differential gene expression in colon cancer detected by a whole genome exon array. BMC Genomics 2006;7:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Duan S, Bleibel WK, Wisel SA, Huang RS, Wu X, He L, Clark TA, Chen TX, Schweitzer AC, Blume JE, Dolan ME, Cox NJ: Identification of common genetic variants that account for transcript isoform variation between human populations. Hum Genet 2009; 125: 81– 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diez J, Park Y, Zeller M, Brown D, Garza D, Ricordi C, Hutton J, Eisenbarth GS, Pugliese A: Differential splicing of the IA-2 mRNA in pancreas and lymphoid organs as a permissive genetic mechanism for autoimmunity against the IA-2 type 1 diabetes autoantigen. Diabetes 2001;50:895–900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.