Abstract
Meiotic recombination ensures accurate chromosome segregation during the first meiotic division and provides a mechanism to increase genetic heterogeneity among the meiotic products. Unlike homologous recombination in somatic (vegetative) cells, where sister chromatid interactions prevail and crossover formation is avoided, meiotic recombination is targeted to involve homologs, resulting in crossovers to connect the homologs before anaphase of the first meiotic division. The mechanisms responsible for homolog choice and crossover control are poorly understood, but likely involve meiosis-specific recombination proteins, as well as meiosis-specific chromosome organization and architecture. Much progress has been made to identify and biochemically characterize many of the proteins acting during meiotic recombination. This review will focus on the proteins that generate and process heteroduplex DNA, as well as those that process DNA junctions during meiotic recombination, with particular attention to how recombination activities promote crossover resolution between homologs.
1 Introduction
HR plays a critical role during meiosis to ensure that the paternal and maternal homologs segregate from one another during the first meiotic division. This is achieved by the physical connections between homologs, called chiasmata, that are formed as a consequence of crossovers (COs) generated during meiotic recombination (Fig. 1, part A). Genetic analysis of tetrads and octads resulting from fungal meiosis led to the Holliday model (Holliday 1964) and its revisions to the present version shown in Fig. 2, the DSBR-SDSA model (see chapter by J.E. Haber, this BOOK, on the evolution of recombination models). The central tenets of the Holliday model, heteroduplex DNA and four-way cross-stranded junctions, or Holliday junctions (HJs), still represent the critical intermediates during meiotic recombination. Here, we focus on the biochemistry of meiotic recombination as it pertains to the formation and processing of the heteroduplex DNA and DNA junction intermediates (see Table 1 for a list of proteins that are discussed). Additional important aspects of meiotic DNA transactions are discussed in more depth in other chapters, including the initiation of meiotic recombination by DSB formation (S. Keeney, this SERIES) and the mechanism of homology search by RecA-like proteins (C. Prévost, this BOOK). This review will concentrate on results with the budding yeast Saccharomyces cerevisiae (Table 1), with references to other organisms where the proteins or mechanisms appear to differ from budding yeast. The chapters specifically dedicated to organisms, including fission yeast Schizosaccharomyces pombe (G. Cromie and G.R. Smith, this BOOK) and Arabidopsis thaliana (G.H. Jones and F.C.H. Franklin, this SERIES), will offer more detail on these systems. The reader is also referred to excellent previous reviews on meiotic recombination (Cromie and Smith 2007; Gerton and Hawley 2005; Hunter 2007; Krogh and Symington 2004; Orr-Weaver and Szostak 1985; Paques and Haber 1999; Roeder 1997; Zickler and Kleckner 1999).
Fig. 1.
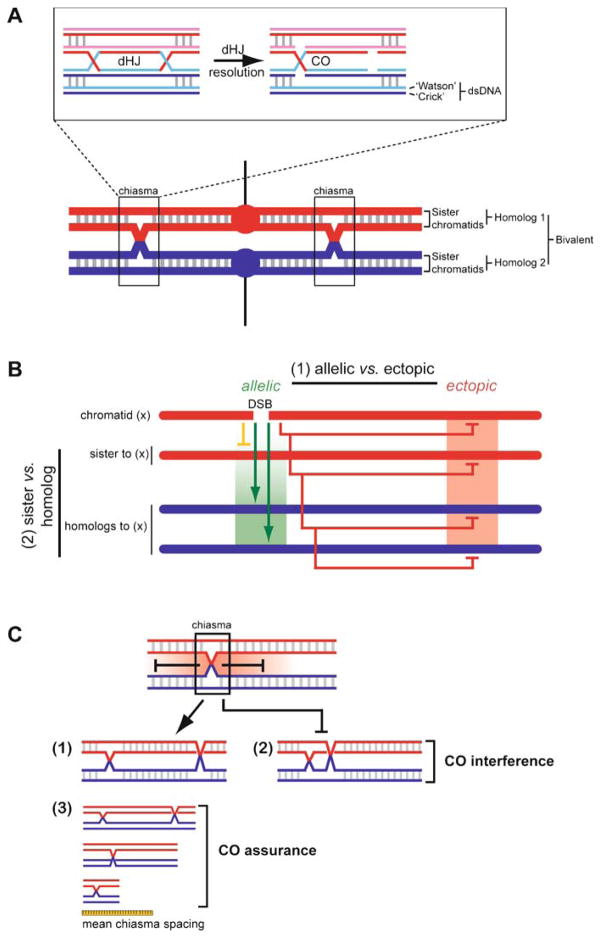
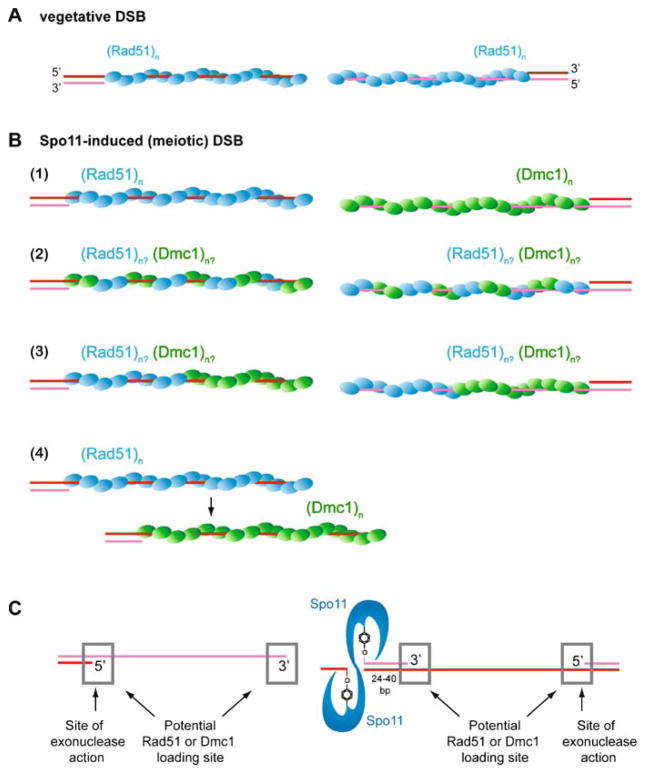
Meiotic crossovers establish physical connection between homologs. A The two homologs each consist of two sister chromatids (red and blue lines each depicting a dsDNA molecule) held together by cohesion (represented by grey lines between the sisters). Upon bipolar attachment of the kinetochores (red/blue circles) to the meiosis I spindle (black lines), the CO points between homologs (indicated as chiasmata) provide a counterforce to the spindle force acting on the kinetochores, signaling correct bipolar attachment of the paired homologs (bivalent) and ensuring high-fidelity chromosome segregation during meiosis I division. Resolution of a double-Holliday junction (dHJ) by alternate incision, as shown in the box, represents a mechanism to generate a chiasma. The individual DNA strands involved are shown in the box. B Meiotic recombination entails objectives unique from those in vegetative cells. (1) As in vegetative cells, recombination is minimized between ectopic sequences but is instead directed toward allelic sites. Mechanisms responsible for the biochemical differentiation between ectopic (homeologous) and allelic (homologous) sites are poorly known but probably involve mismatch repair factors and regulation at the level of heteroduplex quality during preliminary DNA strand exchange events. (2) Meiotic recombination promotes a regulated level of DSB repair directed to the homolog, at the exclusion of the sister chromatid. The biochemical basis of sister vs. homolog discrimination is also poorly understood and remains an outstanding question for recombination applications specific to meiosis. C Crossovers are an essential outcome of the meiotic recombination agenda, but only under strict limitations of number (incidence) and distribution. Where one CO occurs in a bivalent, the probability of a second CO nearby is far below what would be expected by random distribution. This suggests that the number and spacing of COs is regulated; a phenomenon known as CO (or chiasma) interference. It is unclear at what level (pre-DSB, DSB, SEI, dHJ) interference is imposed. Not all organisms display interference (e.g., Schizosaccharomyces pombe does not) (Munz 1994), and not all CO pathways are associated with interference (see Fig. 12). Although the underlying mechanism(s) for CO interference remain to be explained, interference results in the non-random spacing of chiasmata on chromosomes that undertake multiple CO events (1) and (2). Interference may also play a role in CO assurance (3), the observation that all bivalents earn at least one chiasma, even on chromosomes that are smaller than the mean chiasma spacing
Fig. 2.
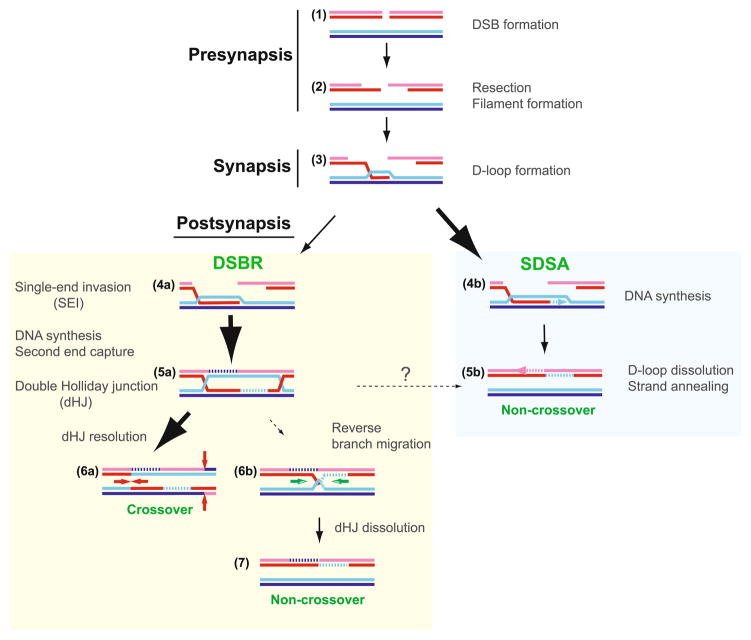
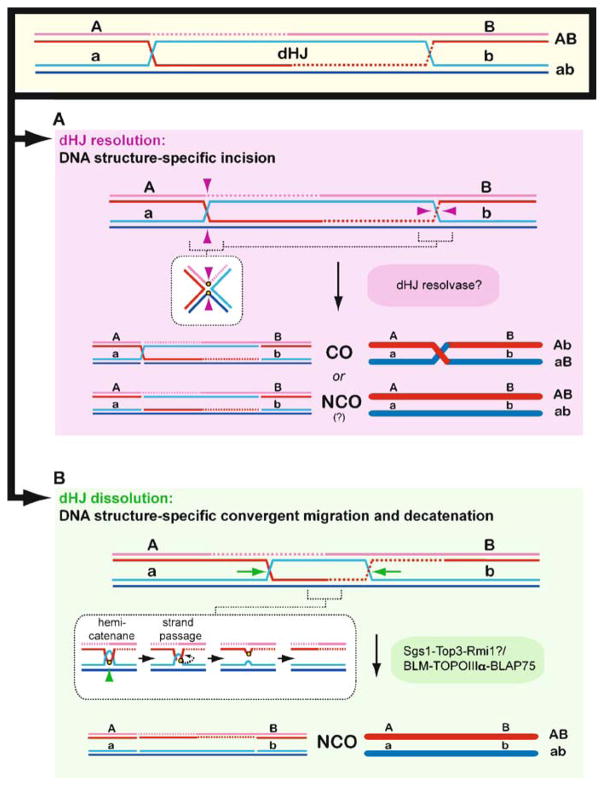
Mechanistic stages of homologous recombination. Meiotic recombination is initiated by a Spo11-mediated double-stranded DNA break (DSB) (1). During presynapsis, the initial break is resected to form 3′-OH ending single-stranded DNA tails to allow formation of filaments by the DNA strand exchange proteins, Rad51 and Dmc1 (2). During synapsis, a joint molecule is formed between the broken DNA and an unbroken template from the other homolog, positioning the 3′-OH end for DNA synthesis (3). During postsynapsis, meiotic recombination bifurcates into at least two primary pathways that repair the DSBs: most breaks are repaired to NCO products by SDSA, but a fraction of breaks are repaired to CO products by DSBR. SDSA (4b, 5b) dissolves the initial D-loop to reanneal the extended invading strand to the second end of the break site, resulting in NCO products (Nassif et al. 1994; Resnick 1976). Second end capture and dHJ formation (DSBR, 5a, 6a,b, 7) (Szostak et al. 1983) account for the main CO pathway in budding yeast, nematodes, and mammals (termed CO pathway 1). Possible scenarios for CO pathway 2 (predominant in fission yeast) and 3 (predominant in Drosophila) are shown in Fig. 12. The joint molecule physically identified as the SEI intermediate (4a) appears to be a stabilized D-loop and is a CO-specific intermediate in meiosis (Hunter and Kleckner 2001). The dHJ intermediate (5a) is critical for CO formation, possibly through resolution by structure-specific endonucleases resembling the bacterial RuvC enzyme (6a). Resolution of dHJs might be biased to CO products, such that there is no NCO outcome (“?” in 5a to 5b transition). Alternatively, a minor fraction of dHJs may be dissolved into NCO products by a RecQ-family helicase, a topoisomerase III, and a junction specificity factor, involving reverse-branch migration that confines heteroduplex DNA to the recipient chromosome (6b-7) (Wu and Hickson 2003) (see Fig. 11)
Table 1.
Saccharomyces cerevisiae proteins involved in meiotic recombination
| Protein | Function |
|---|---|
| DSB formation/processing | |
| Spo11a | DSB formation |
| Ski8/Rec103 | Required for DSB formation; direct interaction with Spo11 |
| Mei4a | Required for DSB formation; subcomplex with Mer2/Rec107, Rec114 |
| Mer2/Rec107a | Required for DSB formation; subcomplex with Mei4, Rec114 |
| Rec114a | Required for DSB formation; subcomplex with Mei4, Mer2/Rec107; interaction with Rec102 |
| Rec102a | Required for DSB formation; subcomplex with Rec104 that interacts with Spo11 |
| Rec104a | Required for DSB formation; subcomplex with Rec102 that interacts with Spo11 |
| Mre11-Rad50-Xrs2 | Complex required for DSB formation and processing with DNA unwinding, DNA endonuclease and 3′-5′ exonuclease, and DNA tethering activities (Xrs2 is NBS1 in mammals) |
| Sae2/Com1 | ssDNA endonuclease, working in conjunction with MRX/N complex, required for DSB processing (mammalian homolog CtIP) |
| Exo1 | 5′-3′ Exonuclease; possible role in resection of meiotic DSBs (see also postsynapsis) |
| Srs2 | 3′-5′ DNA helicase with anti-recombination function; Rad51-ssDNA nucleoprotein filament disruption (similar function for mammalian BLM and RECQL5) |
| Rad51/Dmc1 filament formation | |
| RPA | Heterotrimeric single-stranded DNA binding protein, binds resected tails and likely displaced strand in D-loop, function in MMR |
| Rad51 | Homology search and DNA strand exchange |
| Rad52 | Mediator of Rad51, reannealing during second end capture and SDSA |
| Rad59 | Reannealing during second end capture and SDSA? |
| Rad55-Rad57 | Rad51 paralog complex, mediator of Rad51 (five mammalian Rad51 paralogs RAD51B, RAD51C, RAD51D, XRCC2, XRCC3) |
| Hed1a | Meiosis-specific inhibitor of Rad51 |
| Dmc1a | Homology search and DNA strand exchange |
| Mei5-Sae3a | Cofactor complex of Dmc1 (and possibly Rad51 in some organisms) |
| Mnd1-Hop2a | Cofactor complex of Dmc1 (and possibly Rad51 in some organisms) |
| Rad54 | Stabilization of Rad51 filament; enhances synapsis in Rad51-mediated in vitro recombination reactions |
| Rdh54/Tid1 | Stabilization of Dmc1 filament; enhances synapsis in Rad51 (Dmc1?)-mediated in vitro recombination reactions |
| Postsynapsis | |
| Rad54 | Turnover of Rad51-dsDNA product complexes; branch migration? |
| Rdh54/Tid1 | Turnover of Rad51/Dmc1-dsDNA product complexes; branch migration? |
| DNA polymerases and cofactors | Polδ and possibly Polλ are involved in DNA synthesis from invading 3′ end; Polδ is PCNA/RFC-dependent and the involvement of these cofactors is inferred; Polδ/PCNA/RFC also function in MMR |
| Mer3a | Helicase/DNA translocase with functions in heteroduplex DNA extension |
| Msh2 | MutS homolog with functions in MMR and possibly heteroduplex rejection, in complexes with Msh3 and Msh6 |
| Msh3 | MutS homolog with functions in MMR and possibly heteroduplex rejection, in complexes with Msh2 |
| Msh6 | MutS homolog with functions in MMR and possibly heteroduplex rejection, in complexes with Msh2 |
| Mlh1 | MutL homolog with functions in MMR (as complexes with Mlh2, Mlh3, and Pms1) and CO promotion in the Msh4-Msh5 pathway (as complex with Mlh3) |
| Mlh2 | MutL homolog with function in MMR in complex with Mlh1 |
| Mlh3 | MutL homolog with function in CO promotion in the Msh4-Msh5 pathway in complex with Mlh1 |
| Pms1 | MutL homolog with function in MMR and possibly heteroduplex rejection (note that mammalian homolog is called Pms2) |
| Rad1-Rad10 | 3′ Flap endonuclease with function in repair of large insertion/deletion loops; possible function in removing 3′ flaps resulting from excess DNA synthesis (XPF-ERCC1 in mammals) |
| Exo1 | 5′-3′ Exonuclease, function in MMR and CO formation (see also presynapsis) |
| Msh4a-Msh5a | Complex with function in CO pathway 1 |
| Mus81-Mms4 | Complex with function in CO pathway 2 partially distinct from Msh4-Msh5 (Mms4 is Eme1 is fission yeast and mammals) |
| Sgs1-Top3-Rmi1 | Complex with 3′-5′ DNA helicase and type 1 topoisomerase activity, functions to dissolve joint molecules (analogous to BLM-TOPOIIIα-BLAP75/RMI1 in mammals) |
| Srs2 | 3′-5′ DNA helicase with anti-recombination function |
The proteins are specifically expressed during meiosis in S. cerevisiae but not all are meiosis-specific in other organisms. Mer2/Rec107 protein is produced by meiosis-specific splicing involving the meiosis-specific splicing factors Mer1 and Mre2 (Engebrecht et al. 1991; Nakagawa and Ogawa 1997).
2 Biochemistry of Meiotic Recombination
The RAD52 epistasis group (RAD50, RAD51, RAD52, RAD54, RDH54/TID1, RAD55, RAD57, RAD59, MRE11, XRS2) forms the core of the recombination pathway in somatic and meiotic cells, aided by context-specific factors (Table 1). Meiotic recombination differs from recombination in somatic (vegetative) cells in significant aspects. First, recombination is strongly induced in meiosis (100- to 10000-fold), as we now know by DSBs introduced by the Spo11 protein (Figs. 1, part B, 2, 3). A similar increase in recombination (up to 4000-fold) in vegetative cells is induced by DSBs during gene targeting in budding yeast (Orr-Weaver et al. 1981). Second, meiotic recombination is designed to favor homologs over sisters, which are the preferred template for DSB repair in somatic cells (Fig. 1, part B). A third unique aspect of meiotic recombination in most eukaryotes concerns meiotic CO control and interference (Fig. 1, part C). Interference, precisely chiasma interference, defines the observation that a CO affects the probability of a second CO in its vicinity. The earliest studies on meiotic recombination in Drosophila established the existence of positive interference, showing that exchange (CO) in one interval decreased the probability of exchange (CO) in a nearby interval (Muller 1916; Sturtevant 1915). The mechanistic bases for the homolog bias and CO outcome of meiotic recombination are possibly related, as they both serve to establish the physical connection between homologs in the bivalent (Fig. 1, part A) that ensure proper chromosome segregation during the first meiotic division. These mechanisms likely involve meiosis-specific chromosome structures including the synaptonemal complex (Zickler and Kleckner 1999), meiosis-specific proteins, including Dmc1 and its cofactors (Table 1), meiosis-specific aspects of the S-phase preceding the first meiotic division (Cha et al. 2000; Watanabe et al. 2001), and meiosis-specific DNA checkpoint controls (Hochwagen and Amon 2006; Lydall et al. 1996). While many of the core recombination factors and meiosis-specific recombination proteins have been identified, our understanding of the mechanisms by which the recombination machinery is altered to accommodate the specific biological challenges of meiosis is still rather rudimentary. This review focuses on the biochemical properties of meiotic recombination proteins and is structured according to the mechanistic progression of meiotic recombination (Fig. 2).
2.1 DSB Formation: Spo11 and its Control
The induction of meiotic recombination occurs largely through DSBs catalyzed by the Spo11 nuclease, a meiosis-specific protein with homology to the Top6A subunit of archaeal type IIB topoisomerases that is conserved in all eukaryotes (Bergerat et al. 1997) (see also S. Keeney, this SERIES, for a more detailed discussion). Spo11 functions similar to type II topoisomerases in that it forms a covalent intermediate between the active-site tyrosine and the 5′-end of the DSB, as deduced from in vivo studies (Fig. 3) (Keeney et al. 1997). Mutational analysis (Spo11-Y135F in S. cerevisiae) demonstrated that the active site is essential for in vivo function (Bergerat et al. 1997). Unfortunately, the biochemistry of this initiation step is still lacking, due to the difficulty in purifying Spo11 protein and likely due to the complex control of Spo11 by at least nine other factors (see Table 1 and below) and the possible requirement for meiotic chromatin or chromosome structure.
Fig. 3.
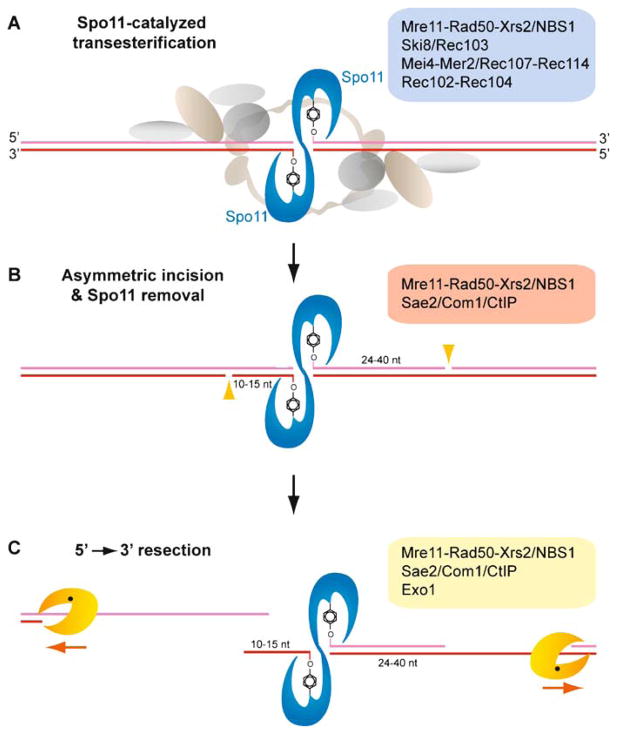
Spo11-catalyzed DSBs and asymmetric end processing by 5′ → 3′ resection. A Spo11 is a type II-related topoisomerase that catalyzes DSB formation at “hotspots” by a transesterification mechanism involving a covalent intermediate between a tyrosine residue of a Spo11 subunit and each 5′ end. A legion of factors (see blue box) is implicated in Spo11 DSB initiation, and their biochemical contributions to Spo11 activity need explanation. B Spo11 remains covalently bound to its product DNA break ends and can be isolated in two populations, bound to oligonucleotides of asymmetric lengths (Neale et al. 2005). The enzymes involved in the endonucleotlytic incision are shown in the red box. One population is associated with short oligonucleotides, 10–15 nt, while a second population is recovered in association with oligonucleotides 24–40 nt in length. This result provides a possible mechanism to establish asymmetry of break ends at or near the timing of Spo11-catalyzed DSBs, although the underlying basis of the asymmetry is unknown. The two populations of oligonucleotide-Spo11 complexes imply that 5′ → 3′ resection initiates at nicks positioned asymmetrically to the Spo11 cleavage complex. C A number of factors are implicated in DNA end resection (see yellow box), but the primary exonuclease or endonuclease activities remain uncertain. Resection is processive up to ~ 500 nt on each break end and is possibly coupled to Rad51 and Dmc1 loading. The short oligonucleotide-Spo11 complex (10–15nt) is suggested to separate readily from its complementary strand, generating a free 3′ end (Neale et al. 2005). The longer oligonucleotide-Spo11 complex (24–40nt) may remain paired to its complementary strand and therefore resection may generate a gapped region instead of a free end. These asymmetries may imply differential assembly of Rad51 and Dmc1 filaments (see Fig. 5). Alternatively, the oligonucleotides associated with Spo11 may remain base-paired to their complements, but the larger duplex extent on one side may present a binding site or interaction surface for a mediator protein specific for Dmc1 or Rad51 (see Fig. 4)
Meiotic DSB formation by Spo11 depends in vivo on five meiosis-specific proteins (Mei4, Mer2/Rec107, Rec102, Rec104, Rec114) and Ski8/Rec103 protein, which exerts a dual function in RNA metabolism and meiotic recombination (Fig. 3) (Arora et al. 2004). Extensive analyses have elucidated the physical and genetic interactions between these proteins (see Table 1) (Arora et al. 2004; Kee et al. 2004; Li et al. 2006). Ski8/Rec103 has the classical seven-bladed propeller structure of WD repeat proteins (Madrona and Wilson 2004; Seet et al. 2006). The WD repeat is a widely employed protein interaction motif, and Ski8/Rec103 appears to serve as a scaffold for the assembly of the Spo11 cleavage complex with direct interactions to Spo11 (Arora et al. 2004). Unfortunately, little is known about the biochemical activities of these proteins and therefore how they regulate or promote Spo11 DSB activity.
Meiotic DSB formation by Spo11 also depends on the Mre11-Rad50-Xrs2 (MRX) complex (Table 1), as no meiosis-specific DSBs are formed in deletion mutants of these three genes in budding yeast (NBS1 is the mammalian Xrs2 ortholog and the inclusive complex is referred to as MRX/N). The MRX/N complex exerts numerous functions in DNA damage checkpoints, mitotic DSB repair, and meiotic recombination (D’Amours and Jackson 2002; Keeney 2001). The strict dependency of Spo11 cleavage on the MRX complex is not conserved in Arabidopsis (Puizina et al. 2004) or in the fission yeast S. pombe, where meiotic DSBs are formed in the rad50 and rad32 (mre11) mutants with the proper timing, albeit at a reduced level compared to wild-type cells (Young et al. 2004). Since the MRX complex’s conserved function in meiotic recombination appears to be DSB resection, the biochemical properties and cellular functions of this complex are discussed below (Sect. 2.2).
The biochemistry of the initiation of meiotic recombination and its control is shrouded in mystery. There is no mechanistic understanding to explain how Spo11 cleavage is targeted to a particular site. Moreover, it appears that Spo11 cleavage is restricted to a single sister chromatid in the bivalent (Fig. 1, part B), as the artificial HIS4::LEU2 hotspot is cleaved with 25% efficiency, which is best explained by cleavage of a single sister chromatid in the bivalent in the absence of evidence for multiple cleavages (Hunter and Kleckner 2001). Lastly, it is unclear how Spo11 activity is restrained to make only a single cleavage per site during meiotic prophase. In analogy to DNA replication, where origin firing is limited to once per cell cycle, Spo11 cleavage may have a similar licensing requirement to limit its activity to once per meiosis at a given site (Blow and Laskey 1988).
2.2 Resection
Once generated by Spo11 and its associated factors, resection of the DSB proceeds in an apparent 5′-3′ direction, resulting in the 3′-OH ending ssDNA tail needed for Rad51/Dmc1 filament formation and DNA strand invasion (Figs. 2, 3). Mechanistically, resection could be achieved by a 5′-3′ dsDNA exonuclease, a 5′-3′ ssDNA exonuclease in combination with a DNA helicase, or by an ssDNA endonuclease in combination with a DNA helicase. In vivo data using separation of function, non-null mutations in RAD50 and MRE11 implicated the MRX/N complex in DSB resection. Such rad50-s mutations map near to the Walker A box ATP binding consensus sequence (Alani et al. 1990). Mutants in the phosphoesterase motif of MRE11 that eliminated all its nuclease activities in vitro displayed the same phenotype as rad50s mutants by accumulating unresected meiotic DSBs with 5′ ends covalently attached to the Spo11 protein (Keeney et al. 1997; Moreau et al. 1999; Nairz and Klein 1997; Tsubouchi and Ogawa 1998; Usui et al. 1998). This key result not only demonstrated that Spo11 delivers the meiotic DSB, but it also identified a post-DSB role for the MRX/N complex in meiotic recombination. How does the MRX/N complex function during DSB resection and what other enzymes are involved in this process?
2.2.1 The Multifunctional MRX/N Complex
The MRX/N complex is a multiprotein assembly with several functions in DNA damage signaling, NHEJ, and recombination (Hopfner and Tainer 2003; Krogh and Symington 2004). MRX/N is one of the first protein complexes found at DSBs and a primary sensor for the activation of the Mec1/Tel1 signaling pathways in S. cerevisiae. However, this signaling role and the effector pathways controlled by it, as well as its role in NHEJ, will not be further discussed here (for reviews see D’Amours and Jackson 2002; Stracker et al. 2004).
Mre11 has an N-terminal phosphoesterase motif that provides the single catalytic center for all its nuclease activities (see below). This nuclease function is essential for Mre11 in meiosis, but in vegetative (somatic) cells, the phenotypes of nuclease-deficient Mre11 mutants are far less severe than those of null mutants (Moreau et al. 1999). In addition to its nuclease domain required for DSB processing in meiosis, Mre11 also contains two DNA binding domains in the C-terminal half of the protein, which are required for meiotic DSB formation (Furuse et al. 1998). Rad50 is an SMC-type (structural maintenance of chromosomes) protein with an N-terminal Walker A and a C-terminal Walker B box that compose an intramolecular ATPase domain and a central extended intramolecular coiled-coil domain (de Jager et al. 2001b). The Rad50 ATPase activity is essential for all functions of the MRX complex in vivo (Alani et al. 1990). Binding of ATP or non-hydrolyzable ATP analogs stimulates Rad50 DNA binding by inducing dimerization (Hopfner et al. 2000), suggesting that DNA binding is regulated by the nucleotide cofactor cycle. The ATPase domain of Rad50 is related to the ABC transporter ATPases (Hopfner and Tainer 2003), which have been shown to share structural similarity with adenylate kinases. In fact, human and yeast Mre11-Rad50 complexes display adenylate kinase activity (ATP + AMP ↔ ADP + ADP) (Bhaskara et al. 2007), but it is unclear how this activity impacts the biochemical and cellular functions of the MRX/N complex. Two Mre11 subunits interact with the ATPase heads of a Rad50 dimer, forming a hetero-tetramer. The Xrs2/NBS1 subunit lacks apparent enzymatic activity and associates with the Mre11-Rad50 assembly by binding to Mre11 through a C-terminal binding site (Shima et al. 2005). Key features of Xrs2/NBS1 are an FHA and a tandem BRCT domain, which are known phospho-specific protein interaction modules (Seet et al. 2006). These domains probably confer regulated protein interactions on the MRX/N complex, but the target proteins during meiotic recombination have not been identified yet.
The biochemical properties of Mre11-Rad50 and/or MRX/N complexes are expansive and include nuclease, DNA unwinding, DNA annealing, and DNA tethering activities in vitro (for review see D’Amours and Jackson 2002; Krogh and Symington 2004). Mre11 exhibits Mn2+-dependent 3′-5′ dsDNA exonuclease activity, as well as ssDNA and dsDNA endonuclease activities, which are enhanced by the presence of Rad50 and Xrs2/NBS1 (Furuse et al. 1998; Paull and Gellert 1998; Trujillo et al. 1998; Usui et al. 1998). The ssDNA endonuclease activity appears responsible for the processing of a covalent protein-DNA intermediate defined by Spo11 bound to the 5′-end of the DSB; Mre11 ssDNA endonuclease activity releases Spo11 as an oligonucleotide-bound form (Neale et al. 2005) (Fig. 3). Spo11 removal may in fact be the essential function of Mre11 in meiosis. This might explain why Mre11 nuclease-deficient mutants display a much more severe phenotype in meiotic cells than in vegetative cells, if further break end processing requires nucleolytic removal of the protein that catalyzed the DSB. Furthermore, an unexpected asymmetry in the physical properties of the break ends on either side of the Spo11 cleavage may lead to a distinction between the two ends, and mechanistic implications are discussed later (see Fig. 3). The nucleolytic release of Spo11 by the MRX/N endonuclease activity furthermore requires an associated unwinding activity, which may also be supplied by the MRX/N complex, as the human MRN complex displays weak strand dissociation activity (Paull and Gellert 1999). The unwinding activity is stimulated by, but is not dependent on, ATP. This feature and the absence of a motor domain found in traditional DNA helicases makes it unlikely that MRX/N functions by translocating on ssDNA, and instead suggests a stoichiometric mechanism of binding to ssDNA akin to ssDNA binding proteins. Beyond a contribution to Spo11 release, the unwinding activity might also be involved in further processing of the DSB, although there is no direct evidence for this at present. It is also possible that a DNA helicase cooperates with the MRX/N complex in this function. Finally, human Mre11 was also found to reanneal complementary ssDNA in vitro, an activity that was abrogated when RPA was bound to ssDNA (de Jager et al. 2001a). The biological significance of this biochemical activity is unclear.
The DNA tethering activity of the MRX/N complex is of particular interest, and led to the elegant suggestion that the MRX/N complex functions like “molecular Velcro” to coordinate the two ends of a DSB or two DNA molecules (de Jager et al. 2001b). The coiled-coil domain of Rad50 folds into a 50 nm long stalk protruding from the ATPase head domain. The apex of the coiled-coil contains a zinc-hook, which can non-covalently link two Rad50 coiled-coil domains by shared binding of a zinc atom (Hopfner et al. 2002). The coiled-coil domains are rather flexible in solution, leading to the possibility of an intramolecular connection between the two Rad50 coiled-coils within a single MRX/N assembly. Such an intramolecular interaction would frustrate intermolecular interactions between MRX/N assemblies needed for DNA tethering. Direct observation by atomic force microscopy provided a solution to this conundrum, demonstrating that DNA binding by MRN stiffens the coiled-coil, which will prevent intramolecular interactions and favor the intermolecular associations needed for tethering (Moreno-Herrero et al. 2005). The Rad50 zinc-hook mutant in budding yeast is deficient in meiotic DSB formation (Wiltzius et al. 2005), suggesting a function of DNA tethering by MRX in this process, either by coordinating the recombining homologs or by putting in place a tether that bridges the future DSB.
In sum, biochemical analysis of the MRX/N complex and the genetic analysis of MRX mutants in yeast have provided evidence for multiple functions of MRX/N in meiosis. The DNA tethering function is important for Spo11-dependent DSB formation, but it is unclear at the moment whether MRX/N also coordinates the two DSB ends of the meiotic DSB. In addition, the MRX/N ssDNA endonuclease activity is critical for initial processing of the covalent Spo11-DNA intermediate, releasing a Spo11–oligonucleotide complex, and may also be involved in further resection of the DSB. An important but not essential role of the MRX complex in post-DSB events during meiotic recombination is indicated by results from inducing meiotic DSBs by meiosis-specific expression of the HO-endonuclease in a rad50 deletion strain that is deficient in Spo11-mediated DSB formation (Malkova et al. 1996). While recombination was induced by the meiotic HO-mediated DSBs, some DSBs were not repaired, suggesting that Rad50 and by implication the MRX complex are required downstream of Spo11 removal from meiotic DSBs.
2.2.2 Sae2/Com1
SAE2 (a.k.a. COM1) was identified in genetic screens for mutations with the meiotic phenotype of rad50-s mutants (McKee and Kleckner 1997a; Prinz et al. 1997). Like rad50-s (or mre11-s) mutations, null mutants of SAE2 accumulate unresected meiotic DSBs. Sae2 exhibits ssDNA endonuclease activity, and cooperatively cleaves hairpin structures in the presence of the MRX complex (Lengsfeld et al. 2007). Unlike MRX, Sae2 is not involved in DSB formation. Moreover, meiosis-specific expression of the VDE-endonuclease in a Spo13-deficient yeast strain demonstrated that DSBs are processed in the absence of Sae2 (Neale et al. 2002). These results suggest that Sae2 specifically collaborates with the MRX complex in the release of Spo11 from the break during meiosis. Sae2 is also a phosphorylation substrate for the Mec1/Tel1 kinases during meiosis (Cartagena-Lirola et al. 2006). Sae2 phosphorylation site mutants display a defect in meiotic DSB end-processing (Cartagena-Lirola et al. 2006) but normal catalytic activity (Lengsfeld et al. 2007), leaving open the in vivo function of Sae2 phosphorylation. Sae2 is conserved in eukaryotes and is called CtIP in mammals (Penkner et al. 2007; Sartori et al. 2007; Uanschou et al. 2007). The meiotic phenotypes of Sae2 mutants in Arabidopsis and C. elegans are consistent with a role in meiotic DSB end processing (Penkner et al. 2007; Uanschou et al. 2007). In sum, the processing of Spo11-mediated DSBs requires at least two nucleases, the MRX complex and Sae2. While it is clear that the Mre11 nuclease activity is essential for Spo11 removal, it needs to be established whether this also holds for the Sae2 nuclease activity or whether Sae2 acts as an MRX/N co-factor during this process. The biochemical details of how these proteins interact and cooperate can now be defined, having the proteins available, but will require the cognate substrate of Spo11 covalently attached to 5′ ends of a DSB.
2.2.3 Exo1
Exo1 is a 5′-3′ exonuclease of the Rad2 family, first identified by a biochemical approach in meiotic S. pombe cells. In S. pombe, Exo1 activity is induced in meiosis (Szankasi and Smith 1992). Exo1 has been implicated at multiple stages during meiotic recombination, including end-resection, MMR, and CO control (Tran et al. 2004). In budding yeast, exo1-Δ mutants exhibit spore viability slightly decreased from wild-type (79% relative to 98%) (Khazanehdari and Borts 2000), with a pattern of increased two- or zero-viable spore tetrads consistent with meiosis I non-disjunction. CO is reduced approximately twofold, and is associated with shorter gene conversion tract lengths (Khazanehdari and Borts 2000). In Exo1−/− mutant mice, homolog pairing and synaptonemal complex formation are normal, but COs are severely reduced and resemble levels in Mlh1−/− and Mlh3−/− mice (Wei et al. 2003) (see Sect. 2.9.3). Exo1 displays non-processive 5′-3′ exonuclease activity with a twofold preference for dsDNA over ssDNA (Fiorentini et al. 1997). This activity depends on a divalent cation and can use Mg2+ or Mn2+ with almost equal efficiency. The exonucleolytic properties of Exo1 are suited for continued resection at meiotic DSBs after the initial processing by MRX/N-Sae2. However, genetic analysis has shown that meiotic DSBs are resected in exo1 mutants; nevertheless, the hyper-resection seen in meiotic mutants with DNA pairing defects (for example dmc1, see below) is decreased in Exo1-deficient cells (Tsubouchi and Ogawa 2000). Hence, it is possible that Exo1 contributes to 5′-3′ processing after Spo11 turnover by MRX/N-Sae2 in wild-type cells, but other activities may compensate in its absence. It is unclear whether the effect of the exo1 mutants on CO is an indirect consequence of a resection defect or indicative of an additional subsequent role of the protein in CO formation (see Sect. 2.9).
2.3 Rad51/Dmc1 Filament Formation
2.3.1 The Homology Search and DNA Strand Exchange Protein Rad51
Rad51 is the evolutionarily conserved RecA homolog found in all eukaryotes and performs the central aspect of recombination: homology search and DNA strand invasion (Aboussekhra et al. 1992; Bianco et al. 1998; Shinohara et al. 1992; Sung 1994). Rad51 is essential for mitotic and meiotic recombination (Hunter 2007; Krogh and Symington 2004; Paques and Haber 1999). rad51-Δ mutants in budding yeast show nearly complete meiotic failure; the few spores formed (on the order of 1% relative to 80–90% in wild-type) are inviable. Meiotic cells accumulate hyper-resected DSBs and exhibit a reduced yield of physical recombinants (Shinohara et al. 1992). Most relevant to the role of RAD51 in promoting the meiotic agenda of regulated interhomolog exchange is the observation that the interhomolog bias is lost in the rad51-Δ mutant; the ratio of interhomolog to intersister joint molecules is reduced by 7.3-fold (from ~2.4 in wild-type to 0.33 in rad51-Δ) (Schwacha and Kleckner 1997). Any model for meiotic recombination must therefore account for the role of Rad51 in promoting interhomolog joint molecules.
S. cerevisiae Rad51 protein forms a right-handed filament with a helical pitch of 130 Å on dsDNA and ssDNA, as determined by crystallographic and electron microscopic studies (Conway et al. 2004; Ogawa et al. 1993; Yu et al. 2001). Binding of ATP induces a high-affinity DNA binding state in Rad51, and ATP hydrolysis lowers this affinity. This effectively links DNA binding with the nucleotide cofactor cycle, although there are organism-specific variations in how nucleotide-cofactor regulates Rad51 DNA binding (for a discussion see Heyer 2007). As for all filament-forming proteins, including RecA, nucleation of the Rad51 filament (binding of the first subunit(s) to DNA) is the rate-limiting step, suggesting the need for cofactors that are functionally equivalent to bacterial RecFOR and RecBCD that load RecA on ssDNA (Bianco et al. 1998). Unlike RecA, which displays a kinetic delay binding to dsDNA, effectively targeting the protein to ssDNA, Rad51 has little preference for ssDNA over dsDNA (Bianco et al. 1998; Zaitseva et al. 1999). This opens a question of how Rad51 is targeted to ssDNA to form the presynaptic filament, which is key for homology search and DNA strand invasion. Compared to RecA, Rad51 displays a significantly lower (over 100-fold reduced) ATPase activity, particularly on dsDNA (Bianco et al. 1998). This lower ATPase activity leads to reduced dynamics of the Rad51-dsDNA filament in turning over and releasing bound DNA. The biochemical differences between RecA and Rad51 suggest that Rad51 needs additional cofactors (mediators) for the assembly of a dynamic and functional Rad51 presynaptic filament and for the dissociation of Rad51-dsDNA complexes. This is true for Rad51 in both vegetative and meiotic cells, although genetic evidence indicates certain differences in mediator requirement between meiotic and mitotic recombination. Furthermore, meiotic recombination uses Rad51 in a highly coordinated series of events that feature its meiosis-specific paralog, Dmc1 (see Sect. 2.3.4). This invokes meiosis-specific regulation of Rad51.
2.3.2 Rad52, Rad55-Rad57, RPA: Mediators of Rad51 Filament Assembly on ssDNA
At least three distinct cofactors help in the assembly of Rad51 on ssDNA to form the presynaptic filament in mitotic and meiotic recombination: the heterotrimeric ssDNA binding protein RPA, the heterodimer of the Rad51 paralogs Rad55-Rad57, and the Rad52 protein (Fig. 4).
Fig. 4.
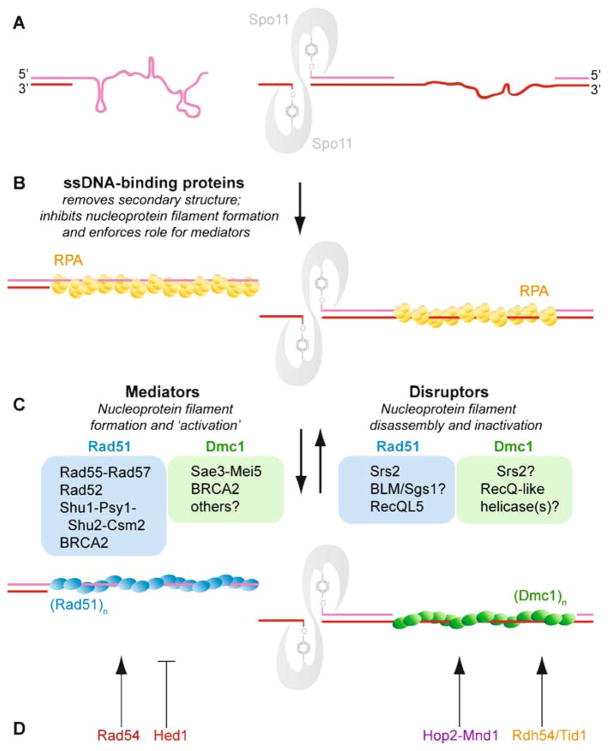
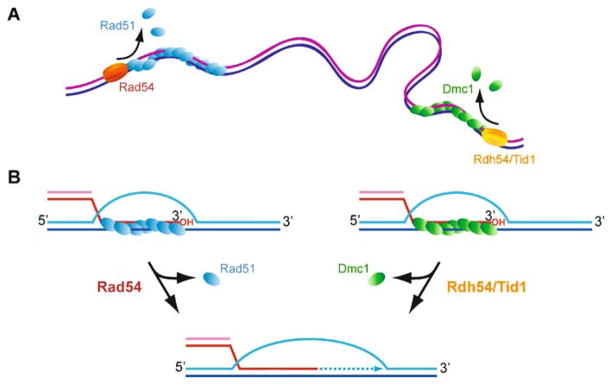
Regulation of Rad51 and Dmc1 nucleoprotein filament formation and function. A The ssDNA generated by 5′ → 3′ resection is likely to form secondary structures inhibitory to the formation of active Rad51 or Dmc1 nucleoprotein filaments. B RPA overcomes this challenge to Rad51 or Dmc1 by melting ssDNA secondary structure during binding, but RPA binds avidly to ssDNA and therefore inhibits nucleoprotein filament formation because RPA is poorly displaced by Rad51 or Dmc1 alone. C Aside from indirectly promoting nucleoprotein filament formation on ssDNA, RPA presents an opportunity for regulation of Rad51 or Dmc1 loading on ssDNA by enforcing a role for mediators. Mediators are a class of proteins that can best be characterized as factors that promote functional filaments (assayed by capacity for DNA strand exchange), although the mechanisms by which they promote a functional filament may be diverse and include: (1) regulation of RPA displacement and Rad51 nucleation on ssDNA, (2) regulation of Rad51 stability (turnover rates) on ssDNA, at an dsDNA-ssDNA junction, or on heteroduplex DNA, (3) filament nucleation and regulation of filament growth, or (4) a function with the free subunit pool. The asymmetry of Spo11 cleavage complexes may suggest a role for mediators specific to nucleation of Rad51 or Dmc1 at one or the other face of the cleavage complex. In some contexts, Srs2 may be considered a mediator of functional filaments if its displacement of Rad51 allows proper registry of a contiguous filament rather than small Rad51 patches on a ssDNA lattice that maybe out of register from one another. Biochemically, Srs2 removes Rad51 from ssDNA; Dmc1 remains to be tested. D Other factors further regulate the function of an assembled Rad51-ssDNA or Dmc1-ssDNA filament. Rad54 stabilizes the Rad51-ssDNA filament and promotes the DNA strand exchange activity of Rad51 filaments by mechanisms that may include topological remodeling of the dsDNA target. Rdh54/Tid1 also stimulates Rad51 DNA strand exchange (Petukhova et al. 2000), and by analogy, Rdh54/Tid1 likely interacts with the Dmc1 filament to promote its DNA strand exchange activity. Genetically, Hed1 appears to inhibit DNA strand exchange activity catalyzed by the Rad51 filament, but biochemical data is lacking. Hop2-Mnd1 promotes duplex capture by the Dmc1 filament
RPA has multiple functions during recombination and is generally expected to be the first protein to access ssDNA generated in vivo. First, in Rad51 filament assembly, RPA is critical to counteract secondary structure in ssDNA. Secondary structure in the ssDNA would interrupt the formation of functional Rad51 presynaptic filaments, because Rad51 also binds dsDNA. Secondly, RPA binds the displaced strand and consequently stabilizes this intermediate (Eggler et al. 2002). In vitro, Rad51 is strongly stimulated by substoichiometric RPA in reactions with ssDNA that has the potential to form secondary structure, but not with ssDNA devoid of secondary structure (Sugiyama et al. 1997; Sung 1994). However, because RPA displays much higher affinity to ssDNA than Rad51, Rad51 is extremely slow to form filaments on ssDNA precoated with RPA, leading to inhibition of Rad51-mediated DNA strand exchange when RPA-coated ssDNA is used. This challenge to Rad51 filament nucleation and propagation is managed by a class of proteins called mediators. The ability of the recombination mediator proteins Rad52 and Rad55-Rad57 to allow Rad51-mediated DNA strand exchange with RPA-coated ssDNA has been demonstrated in vitro (New et al. 1998; Shinohara and Ogawa 1998; Sung 1997a,b). Furthermore, in vivo Rad51 filament formation in meiosis depends on Rad52 and Rad55-Rad57, consistent with the biochemical data (Gasior et al. 1998). Rad51 filament formation is assessed by the formation of transient, Spo11-dependent immunostaining foci that form during meiotic prophase (Bishop 1994). These foci likely represent the Rad51 presynaptic filament and Rad51-mediated pairing intermediates, and their dependence on Rad52 and Rad55-Rad57 indicates that functional Rad51 filaments require mediator contributions. What are the biochemical properties of these mediators and the mechanisms involved in Rad51 nucleoprotein filament promotion?
Rad52 appears to be important to all applications of Rad51-mediated DNA strand exchange. In rad52-Δ mutants in budding yeast, Rad51 foci fail to appear and the few spores that form are inviable (< 1% viability) (Borts et al. 1986; Gasior et al. 1998; Resnick et al. 1986). The frequency of CO recombinants in surviving spores is reduced by 100- to 1000-fold relative to wild-type levels (Borts et al. 1986), and the true reduction is probably even greater than this value, because most of the recombinants scored in surviving spores may in fact represent half-crossovers (non-reciprocal events that yield apparent crossovers but are in fact the result of pathological events). Despite the failure to form Rad51 foci, Dmc1 filaments must be somewhat functional in rad52-Δ mutants; physical analysis of recombination intermediates in rad52-Δ cells shows that SEIs reach wild-type levels, but interhomolog dHJs are reduced eightfold (Lao et al. 2007). These events are very likely mediated by Dmc1, because of the mediator defect of rad52 mutants. Furthermore, recombination in rad52-Δ single mutants is indistinguishable from recombination in rad52-Δ dmc1-Δ, i.e., nearly completely abolished. The apparent epistatic relationship between RAD51 and RAD52 in meiotic recombination, as assayed by the null alleles, conceals a later role for Rad52. However, analysis of the rad52-327 allele that encodes a protein defective in physical interaction with Rad51, demonstrated a role of Rad52 in second-end capture during DSBR (Lao et al. 2007) (see Sect. 2.8).
Rad52 protein has a conserved N-terminal DNA binding domain and forms a multimeric ring-shaped structure that binds ssDNA on the outside face of the ring (Kagawa et al. 2002; Shinohara et al. 1998; Singleton et al. 2002; Stasiak et al. 2000). S. cerevisiae Rad52 binds to Rad51 through a C-terminal binding domain, and also interacts directly with RPA, based on genetic, cytological, and two-hybrid data (Firmenich et al. 1995; Gasior et al. 1998; Hays et al. 1998; Krejci et al. 2002). Rad52 is critical for the ejection of RPA upon Rad51 binding to ssDNA (Sugiyama and Kowalczykowski 2002). In analogy to the related T4 UvsY protein (Beernink and Morrical 1999), one could envision that Rad52 kinks the RPA-coated ssDNA template to allow nucleation of the Rad51 filament. In vegetative cells, the recombination defect of budding yeast rad52 mutants is much more extreme than that of rad55 (or rad57) mutants and also than that ofrad51mutants, becauseofthemultiplerolesofRad52inHR(Rad51 filament formation discussed here, strand annealing in DSBR and SDSA discussed below)as well as in SSA (Krogh andSymington2004; Paques and Haber 1999). However, a tight requirement for both Rad52 and Rad55-Rad57 is suggested for the meiotic setting (Gasior et al. 2001, 1998). In summary, Rad52 plays a significant role in Rad51 filament formation, likely by facilitating the nucleation of Rad51 filaments on RPA-coated ssDNA. How its mediator function intersects with Rad55-Rad57 mediator function to accomplish meiotic Rad51 filament assembly remains to be defined. It should be noted that Rad52 protein does not exert an equally important role in HR in vertebrates, as the respective mutants in mice display very mild phenotypes (Rijkers et al. 1998). It is unclear which other protein(s) have usurped Rad52 functions.
Rad55/57
Rad55 and Rad57 are Rad51 paralogs and share with Rad51 the RecA core, which comprises the ATPase domain (Krogh and Symington 2004; Paques and Haber 1999). They form a heterodimer that displays ATPase activity, but is unable to perform strand invasion reactions (Sung 1997b). While Rad55-Rad57 is not essential for Rad51 focus formation after ionizing radiation in mitotic cells (Lisby et al. 2004), it is absolutely required for meiotic Rad51 focus formation in vivo (Gasior et al. 1998). rad57-Δ mutants in budding yeast resemble rad52-Δ mutants for their meiotic phenotypes, but may yield slightly higher viable spores (< 10% viable) (Borts et al. 1986). In essence, rad51, rad57, and rad55 mutants are identical at the level of meiotic phenotype defined by joint molecule yields in the physical analysis of recombination intermediates. Like RAD51, RAD55 and RAD57 are required for the interhomolog bias observed in budding yeast meiotic recombination (Schwacha and Kleckner 1997), likely because of their Rad51 mediator role. Rad55-Rad57 interacts with Rad51 protein (Sung 1997b), but in contrast to Rad52, no interaction with RPA has been reported. Yet, Rad55-Rad57 is needed for Rad51 DNA strand exchange activity with RPA-coated ssDNA in vitro (Sung 1997b), although the mechanisms involved are unclear. Rad55-Rad57 might be a nucleating factor as proposed for Rad52. Alternatively, Rad55-Rad57 might also stabilize Rad51 filaments or short Rad51 patches that lead to formation of a longer filament. It is likely that Rad52 and Rad55-Rad57 play distinct, non-overlapping roles in Rad51 filament formation, since both are required for focus formation during meiosis in vivo (Gasior et al. 1998). Biochemical analysis of Rad51-mediated recombination reactions in the presence of RPA and both mediators (Rad52, Rad55-Rad57) should provide some insights. While there is no functional equivalent of the RecBCD complex in eukaryotes, the bacterial RecFOR complex that targets RecA filament formation to a ssDNA–dsDNA junction might serve as a useful paradigm (Morimatsu and Kowalczykowski 2003). Such a model would predict a competition between resection and filament nucleation, as both processes act on the same intermediate (see Fig. 5, part C).
Fig. 5.
Models for Dmc1 and Rad51-induced DSB ends: cofilaments or asymmetric filaments. A Rad51 is the sole RecA homolog employed during vegetative recombination in S. cerevisiae. It assembles as symmetric filaments on each DSB end, although each end may be differentially regulated (for DNA strand exchange or second-end capture in DSBR) and these details await biochemical explanation. B Dmc1 is a meiosis-specific Rad51 paralog that functions in collaboration with Rad51 for the purposes of homolog-directed DNA strand exchange with resolution to CO. There are at least four possibilities for the collaborative relationship of Rad1 and Dmc1 in filaments: 1 Rad51 and Dmc1 may assemble as separate filaments on each break end; 2 Rad51 and Dmc1 may assemble as mixed filaments on each break end; 3 Rad51 and Dmc1 may assemble as patchy cofilaments on each break end; or 4 Rad51 and Dmc1 may assemble consecutively on the same ssDNA regions during different stages of meiotic recombination. C The asymmetry suggested for Spo11-induced DSB processing (Neale et al. 2005) presents several opportunities to direct different loading of Rad51 and Dmc1 to one or the other break end. Furthermore, the break ends may remain associated but the different oligo lengths adjacent to the Spo11 homodimer may present binding sites for recruitment of Rad51- or Dmc1-specific mediators
The Shu1-Psy1-Shu2-Csm2 complex in budding yeast (Shor et al. 2005) might represent yet another Rad51 cofactor complex (reviewed in Heyer 2007). The similarity of Shu1 and Psy3 with distant Rad51 paralogs (fission yeast Rlp 1/mammalian Xrcc2 and fission yeast Rdl1/mammalian Rad51D, respectively) and the known function of the fission yeast and mammalian proteins in Rad51 focus formation in vivo (Haruta et al. 2006; Martin et al. 2006) provides motivation for further genetic and biochemical studies.
2.3.3 Hed1: A Meiosis-Specific Rad51 Inhibitor
In its fundamental properties, Rad51 filament assembly in meiotic cells may closely resemble filament assembly in vegetative cells, but meiotic recombination appears to invoke a unique mode for the temporal or physical control of subsequent Rad51 DNA strand exchange activity. Hed1 is an unusual meiosis-specific protein that appears to antagonize the function of Rad51 protein (Tsubouchi and Roeder 2006). Since Rad51 is required for meiotic recombination, it has been speculated that Hed1 coordinates Rad51 and Dmc1 function. Hed1 colocalizes with Rad51 at meiotic DSBs, and the dependence of this Hed1 localization on Rad51 (Tsubouchi and Roeder 2006) suggests that Hed1 functions after assembly of the Rad51 filament (Fig. 4, part D). Hed1 inhibition of Rad51 function can be overcome by increased levels of Rad51 or Rad54, at least in Dmc1-deficient cells, where overexpression of these proteins suppresses the meiotic arrest and DSB repair defects of dmc1 mutants (Shinohara et al. 2003; Tsubouchi and Roeder 2003). This suggests that Hed1 might affect the Rad51-Rad54 interaction. It will be of interest to determine the mechanism of Hed1 inhibition of Rad51 -mediated recombination. Since Rad51 is required for meiosis, the Hed1 inhibition of its function is likely to be transient.
2.3.4 Dmc1: The Meiosis-Specific RecA Homolog
Key to the fundamental outcome of meiotic recombination is the meiosis-specific expression of additional recombination factors that target DNA strand exchange to the homologs, at the relative exclusion of the sister chromatid. Meiotic homolog bias in most eukaryotes apparently cannot be achieved by modulation of Rad51 alone. The meiosis-specific recombination factors therefore include the Rad51 paralog, Dmc1, and its accessory factors, the Mei5-Sae3 and Hop2-Mnd1 complexes (Fig. 4). DMC1 was identified in a screen for meiosis-specific transcripts and is essential for interhomolog recombination during meiosis (Bishop et al. 1992; Hunter and Kleckner 2001; Schwacha and Kleckner 1997). The fundamental role for Dmc1 in directing meiotic recombination to homologs is inferred by the spore inviability of dmc1 mutants and the absence of junction intermediates (SEI, dHJ) (Schwacha and Kleckner 1997), leading to a dramatic decrease in CO formation (10–30% of wild-type levels) (Bishop et al. 1992; Rockmill and Roeder 1994; Rockmill et al. 1995). Like rad51-Δ mutants, dmc1-Δ mutants accumulate hyper-resected DSBs. Also like rad51-Δ mutants, dmc1-Δ mutants in budding yeast show low spore formation (1% relative to 79% in wild-type) and low spore viability (below 2.5% relative to 94% in wild-type) (Bishop et al. 1992). The poor spore formation is probably explained by arrest in meiotic prophase in certain strain backgrounds.
Dmc1 is a RecA homolog with unique N- and C-terminal extensions, and it forms the typical helical filament on DNA and performs DNA pairing reactions in vitro. The conditions for Dmc1 filament formation are more narrow than for Rad51 or RecA, which led to initial difficulties to develop robust assays for this protein (reviewed in Neale and Keeney 2006). The specific conditions that allow filament formation and more efficient recombination by Dmc1 (and also human Rad51) include high levels of Ca2+ or increased salt concentrations (Bugreev et al. 2005; Lee et al. 2005; Sauvageau et al. 2005; Sehorn et al. 2004). The free intracellular (cytosolic) Ca2+ concentration is less than 1 μM in mammals, i.e., substantially below the 100–400 μM concentration required for optimal in vitro stimulation (Bugreev et al. 2005). One could surmise that these experimental conditions substitute for cofactors found in vivo.
Similar to RecA and Rad51, nucleation of the Dmc1 filament is expected to be rate-limiting, in particular on RPA-coated ssDNA. The Sae3-Mei5 complex is a Dmc1-specific cofactor and may function in this mediator context (see below). Like Rad51, Dmc1 displays low ATPase activity compared to RecA and displays little preference for ssDNA over dsDNA (Hong et al. 2001; Li et al. 1997). These biochemical properties necessitate a factor that dissociates Dmc1-dsDNA complexes, and Rdh54/Tid1 appears to be specific for Dmc1 in this respect (Holzen et al. 2006) (see below).
It has been noted that organisms that lack Dmc1 (and Hop2-Mnd1) rely on specific pairing sites that mediate meiotic homolog pairing (such as Drosophila or C. elegans), while organisms that employ these proteins lack such pairing sites (such as budding yeast, mammals). This may suggest that Dmc1 and its cofactors are specifically involved in establishing homolog interactions (Stahl et al. 2004; Villeneuve and Hillers 2001), but a biochemical explanation for the role of Dmc1 in promoting homolog bias is still needed.
2.3.5 Sae3-Mei5: A Meiosis-Specific Mediator Complex for Dmc1
Sae3 and Mei5 form a meiosis-specific complex in budding yeast that is required for Dmc1 focus formation in vivo (Hayase et al. 2004). Cytological and genetic analyses suggest that the mediatorroleofMei5-Sae3isspecific for Dmc1 filament formation, at least in budding yeast (Hayase et al. 2004; McKee and Kleckner 1997b; Tsubouchi and Roeder 2004) (Fig. 4). Like rad51-Δ, dmc1-Δ, rad55-Δ, rad57-Δ and rad52-Δ mutants, sae3-Δ mutants accumulate hyper-resected DSBs. More specifically, sae3-Δ mutants are nearly indistinguishable from dmc1-Δ mutants in their arrest at pachytene, reduced sporulation and spore viability levels, and reduced crossover levels (at 15% recombinant products as opposed to 80% in wild-type cells). As for the relationship of Rad51 and its mediators (Rad52, Rad55-Rad57), sae3-Δ and dmc1-Δ are fully epistatic. In S. cerevisiae, mutations in SAE3 and MEI5 do not affect Rad51 filament formation (Hayase et al. 2004). Dmc1 foci, however, depend on Sae3-Mei5 and the localization of Sae3-Mei5 in turn depends on Dmc1, suggesting mutually dependent localization (Tsubouchi and Roeder 2004).
While the biochemistry of the budding yeast Sae3-Mei5 complex still needs to be developed, the homologous complex from fission yeast, Swi5-Sfr1, has been shown to exhibit mediator function in Rad51 and Dmc1-mediated DNA strand exchange reactions (Haruta et al. 2006). For both DNA strand exchange proteins, Swi5-Sfr1 partially relieved the inhibition imposed by RPA binding to ssDNA. In contrast to S. cerevisiae, the fission yeast counterparts function in both vegetative and meiotic cells (Akamatsu et al. 2003, 2007; Ellermeier et al. 2004). Genetic analysis, in addition to the biochemical results, suggests that Swi5-Sfr1 is not specific for Dmc1 in fission yeast, and support a function for the complex in Rad51 filament formation (Akamatsu et al. 2003, 2007; Haruta etal. 2006). The mechanism by which Swi5-Sfr1 supports Rad51/Dmc1 filament formation on RPA-coated ssDNA remains to be determined.
2.3.6 Hop2-Mnd1: A Complex that Co-evolved with Dmc1
Hop2-Mnd1 form a conserved complex that appears to have co-evolved with the Dmc1 protein, as all organisms identified to have the Dmc1 protein also contain the Hop2-Mnd1 complex, whereas organisms that lack Dmc1 also lack this complex (reviewed in Hunter 2007; Neale and Keeney 2006) (Fig. 4). This pattern may suggest an interaction between Hop2-Mnd1 and Dmc1 specific to a mechanism in meiosis, but biochemical results show that the mammalian and fission yeast complex can functionally interact with both Dmc1 and Rad51 (Chi et al. 2007; Enomoto et al. 2006; Petukhova et al. 2005; Ploquin et al. 2007). hop2 and mnd1 mutants in budding yeast, like dmc1, arrest at pachytene and sporulate only poorly (1.3% for hop2 and 1.5% for mnd1, with no viable spores) (Leu et al. 1998; Tsubouchi and Roeder 2002). Unusual for hop2 and mnd1 among meiotic mutants is the failure of most chromosomes to properly pair with their homolog; instead, most chromosomes pair with non-homologous partners or are folded over as though synapsed with ectopic sites on the same chromatid. This anomalous pairing does not appear to be mediated at the level of DNA strand exchange intermediates, however, as joint molecules and COs are not detectable in the mnd1 mutant (Gerton and DeRisi 2002). Hop2-Mnd1 may therefore function at or prior to DNA strand exchange to promote appropriate nucleoprotein filament interactions with a homologous target.
While the biochemical work is now focused on the Hop2-Mnd1 complex, Hop2 alone has been shown to catalyze ATP-independent D-loop formation. This activity is attenuated in the Hop2-Mnd1 complex (Petukhova et al. 2005; Pezza et al. 2006). The biological significance of this Hop2 activity remains unclear, because the genetic evidence suggests that Hop2-Mnd1 function as an obligatory heterodimer (Tsubouchi and Roeder 2002). The Hop2-Mnd1 complex stimulates the in vitro recombination activity of Dmc1 and Rad51, where tested (reviewed in Hunter 2007; Neale and Keeney 2006). Recent biochemical analysis identified two distinct mechanisms by which Hop2-Mnd1 enhance the function of Dmc1 and Rad51 (Chi et al. 2007; Pezza et al. 2007). First, Hop2-Mnd1 stabilized Rad51- or Dmc1-presynaptic filaments against disassembly. Second, Hop2-Mnd1 enhanced the ability of the Dmc1 or Rad51 presynaptic filament to capture duplex DNA in a homology-independent manner. The first function is akin to a mediator protein, and would predict an effect of hop2/mnd1 mutants on Dmc1 or Rad51 focus formation. However, meiotic Rad51 and Dmc1 foci form normally in the mnd1 and hop2 mutants, and Mnd1 does not colocalize with Rad51 foci (Henry et al. 2006; Leu et al. 1998; Zierhut et al. 2004). Hence, there is no direct in vivo evidence that would support a role of Hop2-Mnd1 in presynapsis, although it is possible that the filaments formed in the absence of Hop2-Mnd1 are somehow different from those formed in wild-type cells. The second biochemical function of Hop2-Mnd1 in the homology search process is supported by the in vivo phenotype of the mutants, which display a complete homologous pairing defect. However, the absence of Mnd1 staining at recombination sites (Zierhut et al. 2004) is puzzling, and additional analysis will be needed to reconcile the biochemical, genetic, and cytological data.
2.3.7 The Breast Cancer Tumor Suppressor Protein BRCA2
The breast cancer tumor suppressor protein BRCA2 plays a role in HR in metazoans, plants, and at least one microbe (Ustilago maydis), but an obvious homolog is absent in budding and fission yeast (Fig. 4). BRCA2 is required for DNA damage-induced Rad51 focus formation (Tarsounas et al. 2003), and biochemical analysis of the Ustilago Brh2 protein and fragments of the human BRCA2 proteins have established its mediator function in targeting Rad51 filament formation on RPA-coated ssDNA to the ssDNA–dsDNA junction (Yang et al. 2002, 2005). Genetic evidence in A. thaliana implicates BRCA2 in meiosis, as silencing BRCA2 by RNAi caused meiotic defects and sterility (Siaud et al. 2004). BRCA2 was found to interact both with Rad51 and Dmc1 in A. thaliana and humans (Dray et al. 2006; Thorslund et al. 2007), suggesting that BRCA2 might play a similar mediator function for Dmc1 as it does for Rad51.
2.4 Formation of Heteroduplex DNA by Rad51 and Dmc1: Cofilaments or Asymmetry
Once assembled as functional filaments on ssDNA, both Rad51 and Dmc1 are homology search and DNA strand exchange proteins capable of DNA strand invasion. The efficiency and robustness of their in vivo reactions as well as the timing and routing (sister/homolog) are likely regulated by the various specific and common cofactors. Whereas only Rad51 functions in recombination in vegetative cells, both proteins are required for meiotic recombination and CO formation in most eukaryotes (Hunter 2007; Krogh and Symington 2004). How do Rad51 and Dmc1 perform their tasks during meiotic recombination? And why are two RecA homologs employed for meiosis?
Four models can be envisioned to explain how Dmc1 and Rad51 cooperate during meiotic recombination:
Rad51 and Dmc1 form mixed filaments
Cofilaments of Rad51 and Dmc1 patches on each resected DSB end
Rad51 and Dmc1 form asymmetric filaments, Rad51 on one end and Dmc1 on the second end of the DSB
Rad51 and Dmc1 consecutively load to form individual filaments on the same DNA at different times during meiosis (see Fig. 5)
The structural similarity of the presynaptic filaments formed by the RecA-like proteins (Egelman 2003) opens a possibility for the Rad51-Dmc1 cofilament scenario. Furthermore, in budding yeast, formation of Dmc1 foci is greatly reduced in rad51 mutant cells (Shinohara et al. 1997a). Mouse Dmc1 and Rad51 interact and colocalize at recombination sites on meiotic chromosomes (Tarsounas et al. 1999). These observations seem compatible with a cofilament model. However, the fact that Dmc1 forms dHJs in rad51 mutants argues that Dmc1 can function in vivo in filaments not containing Rad51 (Schwacha and Kleckner 1997), consistent with the Dmc1 biochemistry. On the converse, Rad51 also evidently functions in the absence of Dmc1 during vegetative growth, and may even compensate for loss of Dmc1 function in meiosis under certain genetic conditions. In the dmc1 background, a hed1 mutation relieves Rad51 inhibition and partially rescues the dmc1 spore viability defect, and overexpression of Rad51 similarly partially overcomes a dmc1 defect (Tsubouchi and Roeder 2006). Most importantly, no biochemical evidence demonstrates the formation of mixed Rad51-Dmc1 filaments or cofilaments. An alternative to cofilaments is the formation of asymmetric filaments at the DSB site (Fig. 5, part B1). This was initially proposed on the basis of cytological data showing a close side-by-side localization of Rad51 and Dmc1 foci (Shinohara et al. 2000), but the mechanistic basis for such an asymmetry was difficult to reconcile with the apparent symmetric nature of the two ends of the DSB (see Fig. 2). However, analysis of the products of Spo11 cleavage revealed a surprising asymmetry in the oligonucleotides associated with Spo11 in vivo (Neale et al. 2005) (Fig. 3). This analysis showed that the Spo11 cleavage complex is associated with oligonucleotides of two discrete size ranges, either 10–15nt or 24–40 nt. These oligonucleotide size classes could be the consequence of asymmetric processing of the two ends of a DSB. It was envisioned that the shorter oligo detaches to reveal a dsDNA–ssDNA junction for resection, whereas the longer oligo stays bound, creating a nick or gap for resection to commence (Neale et al. 2005). Such an asymmetry would result in two different substrates presented for end-processing at the DSB (nick versus 3′-overhang), resulting also in different substrates (tail versus gap) for Dmc1 or Rad51 filament assembly in DSBR or Rad52 loading for strand annealing during SDSA (Fig. 2). The two ends of a meiotic DSB are therefore not necessarily inherently symmetric, and a basis for potential asymmetric Rad51 and Dmc1 filament assembly is conceivable (Fig. 5, part C). Alternatively, the asymmetry of the meiotic DSB ends may provide a basis for temporally regulated, successive loading of Rad51 and Dmc1.
2.5 Roles of the Rad54 and Rdh54-Tid1 Motor Proteins in Presynapsis, Synapsis and Postsynapsis
Rad54 and Rdh54/Tid1 are closely related members of the Snf2-like family of dsDNA translocases with a partially overlapping function in meiosis. Biochemical experiments uncovered a surprising versatility of these enzymes, identifying potential functions at all three stages of HR: presynapsis, synapsis, and postsynapsis (Figs. 2, 4, 6), which are discussed in this section. In budding yeast, 30% of rad54-Δ mutants form spores, of which 53% are viable (compared to 88% spore formation in wild-type with 98% viability). Similarly, 10–44% of rdh54-Δ/tid1-Δ mutants form spores, of which 64–82% are viable. The meiotic defect in the rad54 rdh54 double mutant, however, rivals that of the rad51 dmc1 double mutant, virtually eliminating HR. Like the rad51 dmc1 double mutant, the rad54 rdh54/tid1 double mutant accumulates hyper-resected DSBs and produces few recombinants; consequently, spore formation is reduced to 0.5%, of which a mere 1.6% are viable (Klein 1997; Shinohara et al. 1997b). The potential functional overlap could be a consequence of the shared interaction of Rad51 with both Rdh54/Tid1 and Rad54, whereas Dmc1 appears to interact only with Rdh54/Tid1 (Clever et al. 1997; Dresser et al. 1997; Jiang et al. 1996).
Fig. 6.
Rad54 and Rdh54-Tid1: removal of Rad51 from dsDNA and DNA polymerase extension from the 3′ end of heteroduplex DNA. Rad51 and Dmc1 bind readily to dsDNA, unlike their bacterial counterpart RecA. Rad54 and Rdh54/Tid1 may function to dissociate Rad51-dsDNA complexes or Dmc1-dsDNA complexes, in at least two contexts: A non-specific binding to dsDNA (“dead-end” complexes), and B turnover from the heteroduplex dsDNA product of DNA strand exchange to allow access of DNA polymerase to the invading 3′ end. Not all activities depicted here have been experimentally demonstrated, but are inferred from in vivo and in vitro results with these proteins (for details see text)
Rad54 and Rdh54/Tid1 display exceedingly similar biochemical characteristics, although not all experiments have been performed with both enzymes (for review Heyer et al. 2006; Tan et al. 2003). Both proteins are dsDNA-dependent motor proteins, and single molecule studies determined that Rad54 translocates at ~300bp/s in a processive manner on average for 11.5 kb, whereas Rdh54/Tid1 translocates at ~100bp/s for an average of 10kb (Amitani et al. 2006; Nimonkar et al. 2007; Prasad et al. 2007). Comparison of the phenotypes of ATPase-deficient Rad54 mutants with those caused by the gene deletion demonstrates that the ATPase activity is critical for in vivo function (Clever et al. 1999; Petukhova et al. 1999b). Using the same Walker A box ATPase mutation, biochemical and in vivo studies identified ATP-dependent and ATP-independent functions of Rad54 (and possibly by implication Rdh54/Tid1). Together these results show that Rad54 serves motor-dependent and motor-independent roles in HR (see below). Many of these roles may relate directly to an interaction with Rad51, at the level of nucleoprotein filament dynamics (Rad51 stability on ssDNA), DNA strand exchange, heteroduplex DNA extension (branch migration), and DNA repair synthesis from the heteroduplex product of DNA strand exchange (Rad51 dissociation from duplex DNA) (Heyer et al. 2006).
During presynapsis, Rad54 stabilizes Rad51-ssDNA filaments in an ATP-independent fashion (Mazin et al. 2003) (Fig. 4). ChIP experiments provided evidence for the in vivo significance of this stabilization function, demonstrating enhanced association of Rad51 with the proximal end of the resected DNA strand in a strain expressing an ATPase-deficient Rad54 mutant (Rad54-K341R), but not in a rad54-Δ strain (Wolner and Peterson 2005). Association of Rad54 with the presynaptic filament effectively targets Rad54 to the pairing site, where it can exert its motor function on dsDNA (Mazin et al. 2000a; Solinger et al. 2001; Van Komen et al. 2000). A similar function has not yet been directly demonstrated for Rdh54/Tid1.
Synapsis entails homology search and DNA strand invasion (Figs. 2, 7). Rad54 stimulates the Rad51-mediated DNA strand exchange reaction in vitro (circular ssDNA invading linear dsDNA; for a review of biochemical recombination assays see Heyer 2007) and Rad51-mediated D-loop formation (linear ssDNA invading supercoiled dsDNA) (Mazin et al. 2000b; Petukhova et al. 1998). The mechanism of this stimulation has not been elucidated yet, but requires the motor function of Rad54, since it depends on Rad54 ATPase activity (for review Heyer et al. 2006; Tan et al. 2003). In vivo, Rad54 is not absolutely required to target the Rad51 filament to the pairing site as monitored by ChIP experiments (Sugawara et al. 2003), suggesting that homology search can proceed in a Rad54-independent fashion. However, it is nevertheless possible that Rad54 contributes to this process in vivo (Wolner et al. 2003). The ChIP experiments (Sugawara et al. 2003; Wolner et al. 2003) cannot determine whether DNA strand invasion depends on Rad54, as they do not determine the structure of the DNA intermediate bound by the proteins. Rdh54/Tid1 also stimulates Rad51-mediated D-loop formation in vitro (Petukhova et al. 2000), but this activity has not yet been demonstrated with Dmc1.
Fig. 7.
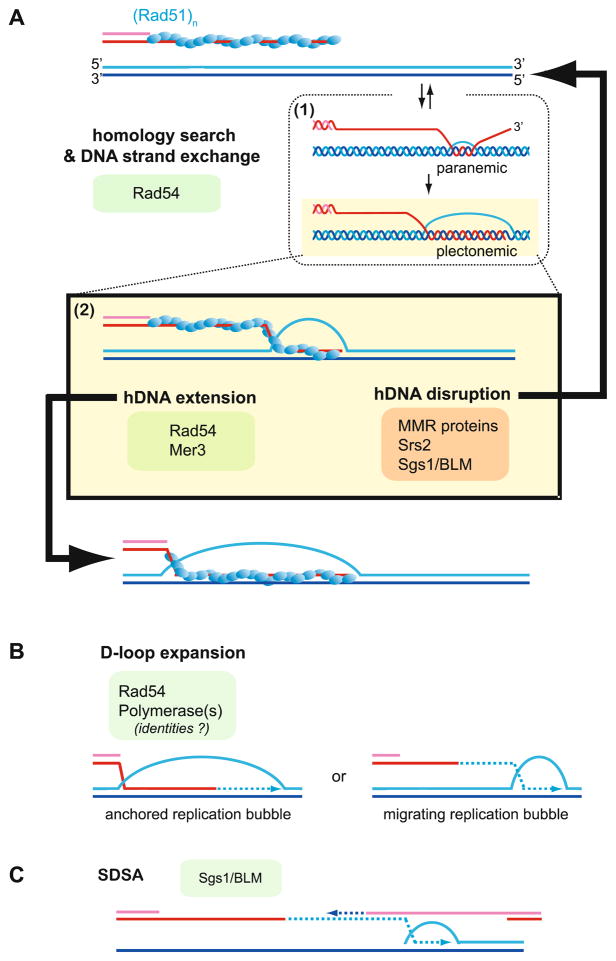
Joint molecule dissociation versus maturation by hDNA extension and D-loop expansion. A Homology search and DNA strand invasion by the Rad51 (or Dmc1, not shown) filament leads to the D-loop intermediate. Strand invasion by an incomplete filament or DNA strand invasion initiating internally (not at end) generates a paranemic joint, where the invading strand is not fully intertwined with the template strand and pairing is protein-mediated. (See box 1 for a representation, although the true nature of a paranemic interaction is uncertain. The Rad51 protein is not shown for simplicity.) Paranemic joints are unstable and may revert, or the pairing is extended to the end, allowing formation of a plectonemic joint with full strand intertwining in which heteroduplex base-pairing is sufficient for stability of the DNA strand exchange product (as explicitly drawn in box 1). Rad51 filament nucleation at the dsDNA–ssDNA junction would increase the probability of interstitial pairing resulting in paranemic joints. The D-loop drawn in box 2 is a plectonemic joint, but for simplicity strand intertwining is not drawn. The D-loop may be disrupted in processes averting recombination or during SDSA (after DNA synthesis, see C) involving MMR proteins, Srs2, and RecQ-like DNA helicases (see text for details). Alternatively, the D-loop can be enlarged by hDNA extension, and the Rad54 motor protein as well as the Mer3 DNA helicase have been implicated in this step. Mer3 plays a key role in CO formation through CO pathway 1 (see text). The extended D-loop is possibly the metastable intermediate SEI that is specific for CO pathway 1 (Hunter and Kleckner 2001). B The initial D-loop may also be expanded by DNA synthesis as an anchored bubble (left) or may become a migrating bubble (right), where its size remains unchanged (Formosa and Alberts 1986). C SDSA is effectively D-loop expansion coupled to regulated hDNA and D-loop disruption. Presumably, D-loop disruption is initiated after homology quality check has sanctioned DNA synthesis. hDNA extension and D-loop expansion are not necessarily stable end-points. DNA strand invasion and SDSA may be dynamic sampling states, where joint molecule formation and disruption occur iteratively, explaining the identification of genetic information obtained from multiple donor sites (Symington and Heyer 2006)
A distinct role of Rad54 after DNA strand invasion (postsynapsis) is indicated by the specific stimulation of the Rad54 ATPase activity at the termini of Rad51-dsDNA filaments (Kiianitsa et al. 2002, 2006; Li et al. 2007). Rad51-dsDNA complexes may represent dead-end complexes caused by the binding of Rad51 to chromosomal (duplex) DNA, or they may constitute the product complex of DNA strand invasion, when Rad51 is bound to the heteroduplex DNA (Fig. 6). Snf2-like proteins remodel a diverse array of protein-duplex DNA complexes, including nucleosomes and transcription complexes (Pazin and Kadonaga 1997). Rad54 remodels the Rad51-dsDNA filament, leading to the dissociation of Rad51 from duplex DNA (Solinger et al. 2002). The low dsDNA ATPase activity of Rad51 (over 100-fold lower than RecA) and the stability of the Rad51-dsDNA complexes even under conditions of ATP hydrolysis (Li et al. 2007; Sung 1994) suggest that Rad54 acts as a turnover factor for Rad51 (Solinger et al. 2002). This activity is likely critical to allow DNA polymerases access to the invading 3′-end, since catalytic turnover of RecA is required for this step (Xu and Marians 2002). This notion is consistent with the finding that meiotic Rad51 foci do not colocalize with recombination-dependent DNA synthesis (Terasawa et al. 2007), suggesting that Rad51 needs to dissociate from the hDNA before DNA synthesis can extend the D-loop. In vivo experiments demonstrate that in Rad54-deficient cells no DNA synthesis takes place at the pairing site (Sugawara et al. 2003), although the lack of DNA synthesis could be a downstream consequence of a role of Rad54 in forming the DNA pairing intermediate (D-loop) required for extension. Rdh54-Tid1 has also been demonstrated to dissociate Rad51-duplex DNA filaments (Chi et al. 2006). This activity has not yet been demonstrated for Dmc1-dsDNA complexes, although the biochemical properties of Dmc1 (low dsDNA-dependent ATPase, dsDNA binding) suggest that Dmc1 might also form dead-end complexes on duplex DNA and remain stuck on the heteroduplex DNA after DNA strand exchange. Indeed, elegant in vivo experiments demonstrated that in tid1/rdh54 mutants Dmc1 accumulates at non-DSB sites, suggesting a function of Rdh54/Tid1 in dissociating dead-end complexes of Dmc1 on duplex DNA during meiosis (Holzen et al. 2006).
A second potential role of Rad54 in postsynapsis is in branch migration (Fig. 7), and Rad54 motor activity was shown to enhance branch migration in Rad51-mediated in vitro recombination reactions (Bugreev et al. 2006; Solinger and Heyer 2001). Stimulation of branch migration was observed during Rad51 -mediated DNA strand exchange and on protein-free junctions. The in vivo significance of these biochemical data remains uncertain. Overexpression of wild-type Rad54 protein leads to a reduction in conversion tract length, whereas overexpression of an ATP-deficient mutant Rad54 protein increased conversion tract length (Kim et al. 2002). These results appear inconsistent with a role for Rad54 in driving branch migration (defined as heteroduplex extension), which would be expected to lead to an increase in conversion tract length. However, this capacity to move DNA junctions has also been proposed to lead to the disruption of recombination intermediates (see below) enabling second end capture (Bugreev et al. 2007a), which would curtail conversion tract length. Similar experiments have not yet been conducted with Rdh54/Tid1 protein.
As members of the Snf2-like protein family (Flaus and Owen-Hughes 2004), Rad54 and Rdh54/Tid1 are related to prominent chromatin remodeling factors but also to other factors that have non-chromatin remodeling targets like Mot1, which dissociates TBP from the TATA-box (Sprouse et al. 2006). The Snf2-like chromatin remodeling factors function as a single sub-unit in large hetero-multimeric assemblies, whereas Rad54 and Rdh54/Tid1 form homo-multimeric assemblies, possibly hexameric or double-hexameric rings as suggested by the processivity of their translocation (Amitani et al. 2006; Flaus and Owen-Hughes 2004; Kiianitsa et al. 2006; Nimonkar et al. 2007; Prasad et al. 2007). Rad54 slides mono-nucleosomes in vitro (Alexeev et al. 2003; Jaskelioff et al. 2003) and enables DNA strand invasion on nucleosomal templates (Alexiadis and Kadonaga 2003; Zhang et al. 2007). Whether Rad54 is required for chromatin remodeling in vivo remains to be demonstrated. Direct analysis of a positioned nucleosome at the recombination target during mating-type switching did not reveal a function of Rad54 in chromatin remodeling during HR (Wolner and Peterson 2005). The activity of Rdh54/Tid1 on nucleosomal substrates remains to be tested.
As for Rad51 and Dmc1, Rad54 and Rdh54/Tid1 share key biochemical properties. The nature of the specialization of Dmc1 and Rdh54/Tid1 for meiotic recombination therefore remains to be satisfactorily explained, as does the likely regulation of Rad51 and Rad54 in association with their meiotic paralogs. While Rad54 is as essential for recombination in mitotic cells as Rad51, in meiosis Rad54 is more involved in sister chromatid repair than interhomolog recombination (Arbel et al. 1999). Rdh54, in contrast, plays little role in mitotic recombination, but is more critical in meiosis (Klein 1997; Shinohara et al. 1997b). The important role of Rdh54 in interhomolog recombination is likely mediated by its interaction with Dmc1 (Dresser et al. 1997).
2.6 DNA Synthesis: Involvement of the PCNA/RFC-Dependent Polδ and Possibly Polλ
Whereas factors associated with Rad51 and Dmc1 filament assembly and DNA strand exchange have been identified, little is known about the proteins and the mechanisms involved in extending the invading 3′-end in the D-loop (Fig. 7, part B). This makes it difficult to anticipate variations unique to meiotic DNA repair synthesis, as so little is known about factors that accomplish DNA repair synthesis during recombination in vegetative cells. The multitude of nuclear DNA polymerases (Rattray and Strathern 2003) and the likely involvement of polymerase processivity factors such as PCNA and RFC provides for significant complexity at this step. Genetic evidence implicates DNA polymerase δ in meiotic DNA repair synthesis, as a hypomorphic allele of the catalytic subunit encoded by the POL3 gene displays reduced meiotic conversion and lower CO frequency (Maloisel et al. 2004). Since Polδ is a PCNA-dependent DNA polymerase, it is expected that the processivity clamp and the RFC clamp loader are required as well. Estimates for the extent of resection (on average 500 nucleotides per break end) (Sun et al. 1991) provide a minimum for the new DNA synthesis required. This estimate also supports the involvement of a processive, PCNA-dependent polymerase.
Another DNA polymerase with a possible role in meiotic recombination is Polλ, a nonessential, Polβ-like enzyme encoded by the POL4 gene of S. cerevisiae (Shimizu et al. 1993). Mutants in POL4 display a fivefold elevated frequency of intragenic recombination and a several-fold increase in the steady state level of Spo11-induced meiotic DSBs (Leem et al. 1994). This suggests a function in recombination downstream of DSB formation. The POL4 locus expresses a meiosis-specific transcript, in addition to a constitutive transcript (Leem et al. 1994), and also the protein level appears to be increased in meiosis (Shimizu et al. 1993). Nevertheless, spore viability is normal in pol4 mutants. The biochemical characteristics of Polλ purified from vegetative (mitotic) and meiotic cells were reported to be nearly identical (Shimizu et al. 1993). Polβ typically inserts one to few nucleotides, for example during base excision repair (Lindahl and Wood 1999), but processivity has not been tested with the budding yeast Polλ enzyme.
Recent evidence suggested the involvement of DNA polymerase η in D-loop extension reconstituted in vitro with human proteins and in certain recombination events in chicken DT40 cells (Kawamoto et al. 2005; McIlwraith et al. 2005). Polη has a well-established role in bypassing UV photoproducts and there is presently no genetic or biochemical evidence linking Polη in S. cerevisiae to HR. Considering functional overlap with other polymerases, however, such a role cannot be ruled out presently.
2.7 D-Loop Dissolution and Strand Annealing in SDSA
In addition to being the DNA structure in which DNA repair synthesis initially occurs, the D-loop is in fact a key intermediate at which meiotic “choices” for CO and NCO pathways are thought to be made (Figs. 2, 7). Most DSBs generated by Spo11 are processed to NCO, likely involving an SDSA mechanism. Some DSBs (estimated to be about one third) mature along a CO-designated pathway, primarily envisioned as a variant of the DSBR model. The recombination pathways as depicted in Fig. 2 bifurcate at the D-loop intermediate to promote either (1) second end capture and dHJ formation (DSBR pathway; see below) or (2) disengagement of the interaction by D-loop dissolution and annealing of the newly synthesized strand to the second end (SDSA pathway). It has been proposed that the SEI intermediate identified in physical assays is a D-loop with extended heteroduplex stabilized by branch migration, but not necessarily expanded by DNA repair synthesis from the invading end. The SEI represents an early CO-dedicated intermediate; as dHJs are also CO-dedicated, SEIs presumably develop along the dHJ pathway (Borner et al. 2004; Hunter and Kleckner 2001) (reviewed in Hunter 2007). The consequence of pathway divergence at the level of the D-loop would be that the D-loops in the DSBR and SDSA pathways are somehow distinct, possibly with different physical properties. What determines these early distinctions is currently unknown.
Dissolution of D-loops is the distinguishing reaction of the SDSA pathway (Figs. 2, 7). From biochemical analysis, the primary candidates for this activity are the RecQ-like DNA helicases. The biochemistry with the budding yeast RecQ helicase, Sgs1, is underdeveloped due to challenges purifying the enzyme, but the human BLM and WRN DNA helicases were shown to dissolve D-loop substrates (Bugreev et al. 2007b; Orren et al. 2002; van Brabant et al. 2000). In the case of BLM, D-loops appear to be the preferred substrate (Bachrati et al. 2006). The involvement of BLM in D-loop dissolution has also been proposed on the basis of genetic results in Drosophila (Adams et al. 2003; McVey et al. 2004a,b). Genetic evidence for such a function of Sgs1 in budding yeast is lacking, but an analysis of mitotic recombination events implicated the unrelated Srs2 helicase in D-loop dissolution during SDSA (Ira et al. 2003). A possible functional overlap of both types of DNA helicase is indicated by the observation that overexpression of Sgs1 can rescue the MMS-and HU-sensitivity of an srs2 mutant (Mankouri et al. 2002). However, it is unclear whether this can be interpreted as functional equivalence or compensation of a pathological situation. No Srs2 homolog has been identified in metazoans yet, opening the possibility that one of the RecQ-like helicases has usurped the D-loop dissolution function. The biochemical analysis of Srs2 identified a novel mechanism of anti-recombination distinct from D-loop dissolution: disassembly of the Rad51 presynaptic filament (Krejci et al. 2003; Veaute et al. 2003). This mode of action is consistent with, and in fact was suggested by, the genetic analysis of semidominant RAD51 suppressors of the srs2 phentoype (Aboussekhra et al. 1992). Also, the mammalian BLM and RECQL5 DNA helicases were demonstrated to dissociate Rad51 from ssDNA (Bugreev et al. 2007b; Hu et al. 2007). The biochemical experiments (Krejci et al. 2003; Veaute et al. 2003) also examined the possibility that Srs2 disrupts D-loops, but Srs2 was unable to dissociate D-loops once formed. This suggests that Srs2 might need another cofactor or different reaction conditions to catalyze D-loop dissolution. In sum, the genetic evidence favors Srs2 as the helicase that dissolves D-loops in budding yeast, but the biochemical support for this proposal still needs to be forthcoming.
Like the RecQ helicases, human Rad54 was also reported to dissociate D-loops in vitro (Bugreev et al. 2007a). A role of Rad54 in D-loop dissociation appears counterintuitive, because of its stimulation of Rad51-mediated D-loop formation in the yeast and human systems (Mazin et al. 2000b; Sigurdsson et al. 2002). However, when the reaction is staged in vitro such that Rad54 is added after D-loop formation, Rad54 dissociates deproteinized D-loops in an ATPase-dependent fashion (Bugreev et al. 2007a). The in vivo significance of these biochemical results is uncertain. Such an activity would predict an anti-CO function for Rad54, which has not been identified in budding yeast (Krogh and Symington 2004; Paques and Haber 1999). Moreover, extensive in vivo analysis by ChIP and PCR assays has identified an absolute defect in rad54 mutants to extend (or make) D-loops (see discussion above) (Sugawara et al. 2003; Wolner et al. 2003). Since D-loop extension precedes D-loop dissolution in the SDSA model, D-loop dissociation cannot be the first rate-limiting Rad54 function, at least in budding yeast. It will be difficult to genetically test whether Rad54 might have a role at this step, as it will require specific mutants that can separate the earlier function (D-loop formation/extension) from the proposed later function (D-loop dissociation).
D-loop dissolution during SDSA is likely coordinated with the release of the newly synthesized DNA from its template, to allow annealing of the disengaged, extended single strand with the second resected DSB end (Fig. 7, part B). DNA strand annealing between these ssDNA species involves RPA and Rad52, possibly involving a contribution by Rad59 protein. A role for Rad52 in annealing complementary ssDNA in fact is supported for both SDSA and DSBR, suggesting that strand annealing is a common feature of both NCO and CO meiotic repair pathways (Lao et al. 2007; Sugiyama et al. 2006). It follows that a primary difference in SDSA and DSBR pertains not to the fate of the second end, but to the fate of the first invading end (see Sect. 2.8). In the nuclear context, newly generated ssDNA can be thought of as an ssDNA–RPA complex, because of the high affinity of RPA binding to ssDNA (Wold 1997). RPA binding provides an effective barrier against spontaneous reannealing of ssDNA. Rad52, like its bacterial counterpart RecO, is special in its ability to reanneal ssDNA complexed to the cognate ssDNA binding protein, RPA in eukaryotes (Kantake et al. 2002; Mortensen et al. 1996; Sugiyama et al. 1998, 2006). This activity is also key for SSA, a DSB repair pathway that is not further discussed here (see Paques and Haber 1999). The unique role of Rad52 in annealing and its mediator activity in Rad51 filament formation (see above) provide the mechanistic explanation for why rad52 mutants display the most extreme recombination defect in budding yeast. The RAD59 gene was identified in a screen for recombination factors acting in a Rad51-independent fashion, and is likely specific for the SSA pathway (Bai and Symington 1996). Rad59 is a Rad52 paralog, sharing Rad52’s N-terminal DNA binding domain but lacking the C-terminal Rad51 interaction domain. The protein functions in conjunction with Rad52, as indicated by the observed suppression of rad59 mutants by Rad52 overexpression and their mutual physical interaction (Bai and Symington 1996; Davis and Symington 2001, 2003). Like Rad52, Rad59 can reanneal DNA (Davis and Symington 2001; Petukhova et al. 1999a), but not in the presence of RPA (Wu et al. 2006b), suggesting that Rad59 is unlikely to function in a Rad52-independent fashion during strand annealing in vivo. The specific mechanistic contribution of Rad59 to Rad52-mediated annealing remains to be determined, and it is unclear whether Rad59 becomes part of the Rad52 ring or how it associates with the Rad52 ring structure.
2.8 Second End Capture in DSBR
Although SDSA is thought to process the majority of DSBs induced during meiosis, it has no contribution to CO or to chiasmata (Fig. 2). Its NCO outcome does, however, contribute to meiotic homolog segregation because it achieves the physical repair of most DSBs and effectively limits the number of COs per chromosome interval. Too many COs may be as detrimental as too few COs. An excess of COs may interfere with homolog disjunction during meiosis I by making physical segregation too difficult; too closely spaced COs may leave insufficient sister cohesion in place to stabilize the chiasma. The primary CO pathway in budding yeast (and by implication of the genetic requirements in C. elegans and mammals) is defined by dHJs. dHJs develop from a minority of the meiotic DSBs and require engagement of the two DSB ends, which can occur by at least two different mechanisms. Both ends could invade independently of each other, or the second end could be captured by the displaced strand of the D-loop. As in the SDSA pathway, the second end capture model implies an asymmetry between the invading end (presynaptic filament) and the second end (RPA-Rad52) (see Sect. 2.7). The displaced strand of the D-loop is likely bound by RPA (Eggler et al. 2002), and capture of the second end involves Rad52 (Lao et al. 2007; Sugiyama et al. 2006) and possibly Rad59. The reaction is similar but not identical to the reannealing step in SDSA. The basic biochemistry of these proteins and the reannealing reactions they catalyze were summarized above. It is unclear whether the specific topology in the D-loop provides constraints in the annealing reaction that require additional factors (Rad59?). Physical evidence from budding yeast is consistent with a second end capture mechanism promoted by Rad52: analysis of a rad52-327 separation of function allele that is deficient in Rad51 mediator function but proficient in annealing of complementary ssDNA showed that Rad52 promotes the transition from SEIs to dHJs (Lao et al. 2007). Capture of the second end by an annealing mechanism is not easily matched with the proposal that Dmc1 and Rad51 form distinct filaments each on one end of the DSB (discussed under Sect. 2.4). A possible solution is that these processes occur sequentially, with initial Rad51 end invasion into the sister, followed by dissolution of the joint, and subsequent second-end capture to form an interhomolog dHJ (see Hunter 2007; Oh et al. 2007).
Although second end capture is viewed as an intermediate to the formation of dHJs and ultimately COs during meiotic HR (Mazina et al. 2004; Sugiyama et al. 2006), this notion has been challenged on the basis of biochemical experiments with human Rad54 protein (Bugreev et al. 2007a). Elegantly designed biochemical experiments reconstituted major steps of DSB repair in vitro. These included Rad51-mediated strand invasion (tailed DNA with supercoiled dsDNA) and second end capture mediated by Rad52, resulting in double-D-loop structures that are equivalent to dHJs prior to ligation of the nicked strands (see Fig. 2 intermediate 5a; Fig. 11) (Bugreev et al. 2007a). Staged addition of Rad54 to these intermediates resulted in their dissolution, leading to the proposal that second end capture is not necessarily a CO-dedicated intermediate in HR (Bugreev et al. 2007a). The biological significance of this biochemical activity of Rad54 is uncertain, since the predicted anti-CO function for Rad54 has not been identified (Krogh and Symington 2004; Paques and Haber 1999). Moreover, a sole function of Rad54 at this step is contradicted by in vivo data, showing a Rad54 requirement for strand invasion and/or D-loop extension (see above) (Sugawara et al. 2003; Wolner et al. 2003).
Fig. 11.
Double-Holliday junction resolution and dissolution: CO and NCO outcomes. A dHJ resolution: dHJ incision by a DNA structure-specific endonuclease can yield CO or NCO outcome, depending on whether the same strands or different strands are cut at each junction. dHJs are recognized as intermediates of CO pathway 1 and are thought to be resolved primarily, if not exclusively, to CO outcome. Inset: HJ in open planar configuration, showing symmetric incision across its core. Yellow bubbles represent the two phospho-diester bonds hydrolyzed during HJ resolution. B dHJ dissolution: dHJ can be dissolved to NCO outcome by coordinated convergent migration of the Holliday junctions followed by their decatenation, a mechanism demonstrated for BLM-TOPOIIIa-BLAP75. Inset: Single-strand DNA passage at a hemicatenane (collapsed dHJs) accounts for the separation to NCO. The yellow bubble represents the single phospho-diester bond hydrolyzed during DNA strand passage. Although the phospho-diester bond hydrolysis penultimate to strand passage probably occurs once at the single hemicatenane, topoisomerase nicking likely occurs repeatedly during dHJ convergent migration to relieve torsional strain associated with branch migration
2.9 Branch Migration in D-Loops and Double Holliday Junctions
Branch migration can occur at structural intermediates such as D-loops, single HJs, or dHJs, and may promote the interconversion of 3′ to 5′ flap structures. In D-loops, active and directed branch migration by an activity dedicated to the movement of a specific joint molecule species either enlarges or dissolves the structure (heteroduplex extension or D-loop disruption; Fig. 7) and may have key consequences for the stability of a DNA strand exchange product and its ultimate maturation along a CO- or NCO-designated path-way. An alternative application for branch migration entails migration of the D-loop bubble in conjunction with DNA synthesis (D-loop expansion), as suggested for T4 recombination (Formosa and Alberts 1986). In either case, branch migration refers to the transplacement of a joint molecule relative to the initial position of DNA strand exchange, and may occur as an active process of joint molecule migration, or as an indirect consequence of DNA synthesis within the D-loop. In dHJs, branch migration may either coordinately move the double junction or alter the distance between the component junctions (for further discussion, see Hunter 2007). Although there may be no change in net base pairing, branch migration is likely driven by specific motor proteins and may also require topoisomerase contributions to relieve super-helical strain. The paradigm for a branch migration protein is the bacterial RuvAB complex, which enforces a square planar configuration on a HJ and moves the junction by pumping DNA through two appropriately positioned hexameric rings that are coordinated by a RuvA tetramer (West 1997). This branch migration enables the sampling of DNA sequences for preferred incision sites cleaved by the RuvAB-associated HJ resolvase, RuvC. Eukaryotes do not have obvious sequence homologs of the RuvABC complex, and whether a similar branch migration-resolution mechanism applies to eukaryotic recombination is not known. At any rate, HJ migration to extend hDNA may not be needed to explain heteroduplex tract lengths in meiosis. DSB resection extends on average 500 nucleotides from each break end, a span that is close to estimates of the average gene conversion tract lengths associated with CO (Paques and Haber 1999; Sun et al. 1991). Resection might therefore be sufficient to define the extent of the hDNA. Here, we discuss the DNA helicase Mer3 that is able to promote branch migration of D-loops. In addition, the function of additional pro-CO factors, Msh4-Msh5 and Mlh1-Mlh3, and their relationship to dHJs will be considered.
2.9.1 The Meiosis-Specific Mer3 DNA Helicase
Mer3 is a meiosis-specific DexH-box-type 3′–5′ DNA helicase that functions in the SEI-dHJ CO pathway (Figs. 2, 7), together with Msh4-Msh5, Mlh1-Mlh3 and Exo1 (Borner et al. 2004; Hunter 2007; Nakagawa and Kolodner 2002a; Nakagawa and Ogawa 1999). mer3-Δ mutants have reduced sporulation (~24% relative to 61% in wild-type) with reduced viability (20–40% relative to 95% in wild-type), probably explained by the reduction in COs in the mutant (Nakagawa and Ogawa 1999). In five intervals studied on two different chromosomes, COs are reduced on average 2.4-fold relative to wild-type and result in an increased incidence of two- and zero-spore tetrads, consistent with meiois I non-disjunction. Moreover, the COs that remain in the mer3-Δ mutant are distributed randomly, indicating an absence of crossover interference that suggests that CO pathway 1 in budding yeast depends on MER3 (Nakagawa and Ogawa 1999). Interestingly, mer3 alleles with mutations in the Walker A-type ATPase boxes reduce CO and interference in some, but not all, of the loci tested (Nakagawa and Kolodner 2002a).
Biochemical analysis of purified Mer3 protein demonstrated 3′–5′ DNA helicase activity, preferentially unwinding substrates with a 3′ tail but also HJs assembled from oligonucleotides (Nakagawa et al. 2001; Nakagawa and Kolodner 2002a,b). HJ unwinding in vitro is challenged, however, by the addition of NaCl (to 150 mM) in the reaction, which does not affect the activity of Mer3 on other DNA substrates. The salt effect may reflect a stabilization of blunt ends and suggests HJ disruption may not represent a normal biochemical property of the protein (Nakagawa and Kolodner 2002b). Rather the physiological target of Mer3 is likely a D-loop as discussed below.
In Rad51-catalyzed DNA strand exchange, Mer3 stimulates hDNA extension when strand exchange is initiated in a 3′ to 5′ direction with reference to the invading strand (equivalent to enlarging the D-loop after 3′ end invasion; see Fig. 7), whereas it inhibits DNA strand exchange in the opposite direction (Mazina et al. 2004). Rad51 -catalyzed DNA strand exchange has been reported to proceed in either a 3′ to 5′ or 5′ to 3′ direction in vitro (Namsaraev and Berg 1998). However, it is widely believed that the 3′ end invades to prime DNA synthesis and that filament formation is initiated at the dsDNA-ssDNA junction, biasing filament growth 5′ to 3′ and therefore DNA strand exchange 3′ to 5′ (Fig. 2). As a consequence, Mer3 is envisioned to enlarge the D-loop by translocating on the displaced strand and to counteract nonproductive invasion of the 5′ end (Mazina et al. 2004). Kinetic analysis showed that Mer3 did not enhance joint formation, but specifically stimulated nicked circle formation, an indicator of completed DNA strand exchange. This biochemical function to stabilize nascent DNA strand invasions is consistent with a pro-CO role of Mer3 in generating dHJ intermediates (Borner et al. 2004; Hunter 2007). Although tested in vitro with Rad51, the role of Mer3 in meiotic heteroduplex extension may also (or more importantly) extend to branch migration of D-loops catalyzed by Dmc1. This has not been tested yet in vitro for Dmc1 or mixed Dmc1-Rad51 reactions, but the requirement for Dmc1 in homolog-directed SEIs in vivo indicates that Dmc1-associated heteroduplex tracts are the targets for extension along the dHJ-associated CO pathway.
2.9.2 The Meiosis-Specific MutS Homolog Complex Msh4-Msh5 and dHJ Stabilization
Eukaryotic homologs to the bacterial MutS and MutL proteins function in heterodimeric assemblies (in S. cerevisiae Msh2-Msh3, Msh2-Msh6, Msh4-Msh5 and Mlh1-Mhl2, Mlh1-Pms1, Mhl1-Mlh3, respectively; see Sect. 2.10, Table 1). Besides their characteristic function in postreplicative MMR, these complexes, with the exception of Msh4-Msh5, function also in the processing of mismatches in heteroduplex DNA formed during meiotic recombination (Hoffmann and Borts 2004; Schofield and Hsieh 2003) (see below and Fig. 8). Msh4-Msh5 has a unique role to promote meiotic COs by the dHJ pathway (Figs. 2, 9). Similar to MMR, the Msh4-Msh5 heterodimer functions in conjunction with a MutL heterodimer, namely Mlh1-Mlh3, in the major CO pathway in budding yeast involving the SEI-dHJ intermediates (Hoffmann and Borts 2004).
Fig. 8.
Applications of mismatch repair to meiotic recombination. A Signals in the parental versus daughter DNA strands distinguish a newly replicated DNA strand from its template strand, and are used by mismatch repair proteins to target excision repair to the new strand and its misincorporated nucleotide. (1) Prokaryotic mismatch repair capitalizes on the hemimethylated state of newly replicated DNA; MutS (indicated as S) scans the DNA for discontinuities, to which it binds and in association with MutL (indicated as L), presents to the endonuclease MutH (indicated as H). (2) Eukaryotic mismatch repair, involving MutS and MutL homologs (MSH, MHL), identifies the newly replicated strand not based on an absent signal (absence of methylation), but more likely on the presence of a nick associated with Okazaki fragment processing during lagging strand DNA synthesis. As in prokaryotic MMR, the nick defines the starting point for an exonuclease that travels to the mismatched region and removes the misincorporated nucleotide(s). (3) DNA repair synthesis and ligation restores the helix. B The basic properties of mismatch repair (mismatch binding, structural recognition, DNA strand discrimination, and endonuclease instruction) maybe employed for specific objectives of meiotic recombination. Several possibilities are shown here. (1) MMR factors bound to mismatches within heteroduplex DNA likely report on the degree of homeology or homology, which may distinguish ectopic from allelic targets. How MMR factors engage with a Rad51 or Dmc1 filament on hDNA is unknown and how they communicate to sanction hDNA extension or promote hDNA disruption is also unknown. (2) Repair of mismatches by MMR pathways is responsible for gene conversion. (3) The dHJ intermediate in budding yeast contains contiguous strands (Schwacha and Kleckner 1995), but a prior nick or another feature associated with DNA repair synthesis in CO pathway 1 may inform the placement of strand incisions by a dHJ resolvase, enforcing a CO outcome without a need for the two resolution events to be directly coordinated
Fig. 9.
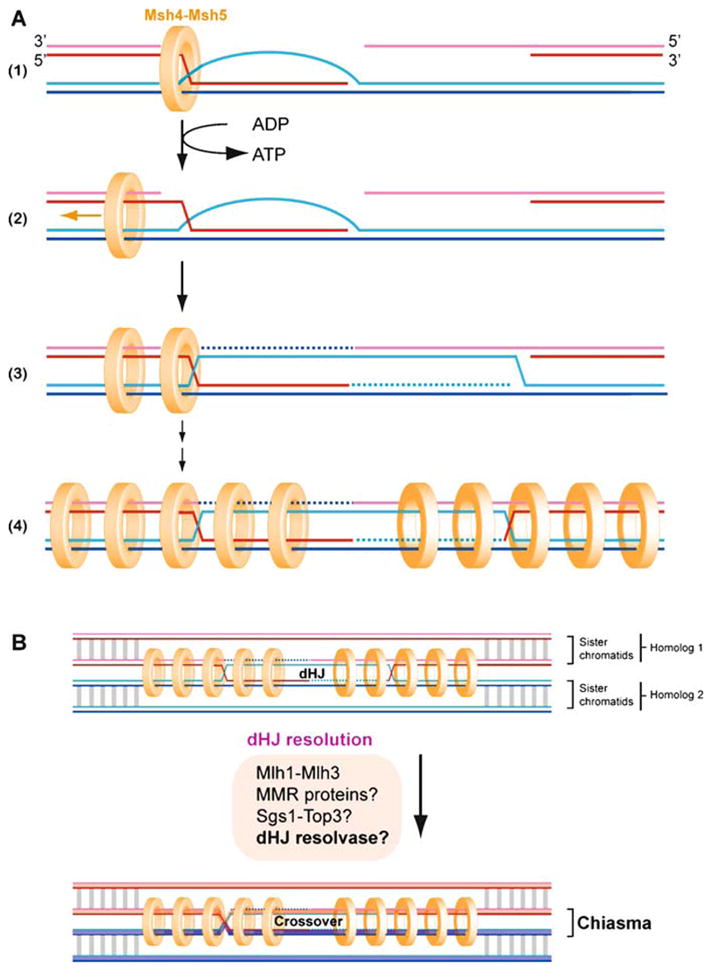
Msh4-Msh5 promotes crossovers by stabilizing joint molecules associated with single-end invasions. A Msh4-Msh4 loads at a joint (single-end invasion). An ADP→ATP switch induces Msh4-Msh5 to slide on duplex DNA, embracing both homologs within a ring-like complex. Iterative loading of Msh4-Msh5 complexes and their subsequent diffusion is thought to tether the homologs (Snowden et al. 2004). Loading at only one joint is shown, and it is unclear whether Msh4-Msh5 loads at the invasion intermediate or at both nicked Holliday junctions. Ligation of the nicked junctions results in covalently sealed dHJs, the intermediates presently believed to precede resolution to CO outcome in CO pathway 1 (Schwacha and Kleckner 1995). In association with Mlh1-Mlh3, MMR proteins, Sgs1-Top3, and/or an unidentified dHJ resolvase, dHJ resolution is directed or enforced to CO. B The Msh4-Msh5 dHJ and CO resolution to a chiasma is shown in the context of the bivalent; cohesion is depicted by gray lines and may also be rings encircling the chromatids rather than the homologs as by Msh4-Msh5. The loss of cohesion associated with Msh4-Msh5 loading on homologs may partially account for CO interference in CO pathway 1 (see Fig. 1), as loss of cohesion over long tracks associated with closely spaced Msh4-Msh5 COs may risk premature loss of sister chromatid cohesion
Msh4 was identified in a screen for meiosis-specific transcripts; its budding yeast mutants are normal for mismatch correction and gene conversion, but show 30–50% reduction in meiotic COs and consequently meiosis I non-disjunction, resulting in reduced spore viability (Ross-Macdonald and Roeder 1994). Msh5 was identified in a genetic screen for mutants deficient in recombination between, but not within, homologs (Hollingsworth et al. 1995). Like other Msh heterodimers, Msh4-Msh5 responds to target structures in DNA by exchanging ADP for ATP, a biochemical switch that induces clamplike binding to the DNA (Schofield and Hsieh 2003; Snowden et al. 2004). Once loaded on DNA, the ATP-charged complex diffuses or slides along the duplex away from the initial site of binding (Fig. 9). In the case of MutS and its orthologs, iterative loading and sliding events promote the accumulation of MutS complexes in the vicinity of the single mismatch, amplifying the signal to downstream effectors and probably contributing to a local state that defines the extent of subsequent excision and resynthesis (Schofield and Hsieh 2003). Unlike Msh2-Msh3 or Msh2-Msh6, however, Msh4-Msh5 shows little binding to a mismatch in vitro. Instead, its target DNA structural features are found in joints between DNA duplexes (D-loops and HJs) and not simply irregularities within one duplex (Snowden et al. 2004). This sets Msh4-Msh5 apart from its vegetative paralogs, such as Msh2-Msh6, which bind a more degenerate range of junctions that includes splayed Y and duplex DNA with a G/T mismatch. Indeed, the Msh4-Msh5 preference for binding recombination-associated joint molecules is probably a direct reflection of its in vivo contribution to CO.
Based on these biochemical properties, Msh4-Msh5 is proposed to promote COs by the key property of encircling both homolog duplexes (Snowden et al. 2004) (Fig. 9). Topological tethering of homologous duplex arms to one another in the vicinity of a D-loop or HJ may promote CO formation by stabilizing early intermediates (such as SEIs) or imposing structural conformations required for hand-off to later pathway components. It is not known how many Msh4-Msh5 complexes are likely to be loaded at a designated CO site in vivo, but repeated Msh4-Msh5 loading and clamp sliding may be necessary or sufficient to enforce a local structural property along a homolog axis that (1) may explain CO resolution and (2) may partially account for CO interference. In other words, Msh4-Msh5 may contribute to the CO resolution bias for dHJs by defining their relative orientation such that incision factors can only access each joint molecule in a manner that results in CO outcome.
2.9.3 Mlh1-Mlh3: Working in Succession to Msh4-Msh5 During Crossover Formation
In addition to defining a putative DNA junction-specific homolog tether that promotes maturation along a CO pathway, Msh4-Msh5 is probably important for recruiting subsequent components of the CO pathway (Fig. 9). Genetic relationships between msh4, msh5 and mlh1, mlh3 plus analogy to the E. coli MutS-MutL system predict that Msh4-Msh5 heterodimers recruit Mlh1-Mlh3 heterodimers to the site of their structure-specific loading to DNA. mlh1 msh4 and msh4 mutants have a more severe phenotype than mlh1, suggesting that Mlh1 functions downstream of Msh4-Msh5 (Hunter 2007; Hunter and Borts 1997). Furthermore, Msh4 foci appear before Mlh1 foci in mice (Baker et al. 1996). Mlh3−/− mice in turn are deficient in the formation of Mlh1 foci, indicating that Mlh3 recruits Mlh1 or that both proteins are needed for complex stability. What is the biochemical function of Mlh1-Mlh3?
Little is known about the biochemical properties of the eukaryotic complex, and most of what we anticipate for Mlh1-Mlh3 functions derives from analogy to the E. coli MutL homodimer, which coordinates factors such as helicases and nucleases downstream to mismatch recognition in MMR. Complexes containing Sgs1 and Mlh1-Mlh3 can be recovered from meiotic extracts, which may suggest a role for Mlh1-Mlh3 in recruiting the helicase/structure-migrating and decatenation activities of Sgs1-Top 3 to CO-dedicated dHJs (Wang and Kung 2002). Unexpectedly, a latent endonuclease activity has been identified in the human MutLα heterodimer (Mlh1-Pms2; homologous to Mlh1-Pms1 in budding yeast) (Kadyrov et al. 2006). The nuclease active site was found in the Pms2 (S. cerevisiae Pms1) subunit, and the sequence is conserved in Mlh3 but not Mlh1, Mlh2 or bacterial MutL. Incision of a nicked heteroduplex requires RFC, PCNA, MutSα (Msh2-Msh6) and ATP, and is directed primarily to the discontinuous strand for subsequent exonucleolytic removal of the region between the two nicks by Exo1 (Kadyrov et al. 2006). Presumably, the nicks that define the discontinuous strand in meiotic recombination derive from DNA strand exchange or second end capture directly (dsDNA-ssDNA junction in a D-loop or at an unligated Holliday junction), or may be associated with DNA repair synthesis. If endonuclease activity is confirmed for the Mlh1-Mlh3 complex, these intriguing observations suggest that not only have MMR functions of hDNA identification been co-opted for purposes of meiosis, but also that endonuclease activity associated with pathway members may figure in resolution of recombination intermediates. Whether a role in resolution is direct (in nick catalysis) or indirect (in guiding strand choice by an endonuclease) remains to be determined. At any rate, Mlh1 foci are the most consistent late markers for COs in mice, consistent with the mechanistic possibility that these MMR factors in some fashion direct resolution to CO.
2.10 Meiotic MMR
2.10.1 Mismatch Repair of hDNA and hDNA Rejection
Because the interacting DNA regions of heteroduplex are not identical nucleotide for nucleotide, hDNA is characterized by regions of unpaired bases. These mismatches may be dispersed at the single nucleotide level or occur over several bases as small bubbles. As substrates for meiotic MMR, these are the basis of gene conversion and fundamental to recombination models (Holliday 1964; Szostak et al. 1983) (see contribution by J.E. Haber, this BOOK; Fig. 8). The biochemistry underlying all MMR systems is well understood and based on a three-part logic: (1) a locally unpaired duplex region is bound by a protein complex that recognizes structural deformity associated with the mismatch, (2) a secondary protein joins the mismatch-bound complex, presumably stabilizing the first and communicating a further level of sanction that (3) recruits an endonuclease and instructs the position of its incision in one of the two duplex strands near the mismatch or utilizes a pre-existing nick for resection/resynthesis. Excellent reviews have covered the mechanistic details of MMR (Hoffmann and Borts 2004; Jiricny 2006; Modrich 2006; Schofield and Hsieh 2003). We will briefly allude to another role of MMR in quality control of recombination by rejection of hDNA, a critical function to avoid non-allelic interaction during meiotic recombination.
A mechanism to assess hDNA quality is presumably important to meiotic recombination, because only COs that occur at allelic positions form useful chiasmata that direct meiotic chromosome segregation. At non-allelic sites, COs pose a risk for inadvertent chromosomal translocations, inversions, or deletions. Moderate homology (homeology) is therefore not sufficient to authorize the maturation of a DNA strand invasion intermediate along a CO pathway. How is homology best detected during recombination, and how are off-target intermediates reversed or processed as NCOs?
Genetic experiments in bacteria and eukaryotes have clearly implicated MMR as a barrier to homeologous recombination, preventing ectopic interactions (Hunter et al. 1996) (reviewed in Schofield and Hsieh 2003). Biochemical experiments with the bacterial proteins have shown that MutS and MutL reduce the extent of hDNA formation by RecA and inhibit RuvAB-mediated branch migration (Fabisiewicz and Worth 2001; Worth et al. 1994). These data favor an hDNA rejection model, akin to D-loop dissolution during SDSA. However, how MMR communicates with the meiotic recombination machinery at the level of hDNA and which DNA helicases/translocases might be involved is unknown. The RecQ-like helicase Sgs1 is one likely candidate for a function at this stage (Myung et al. 2001).
2.10.2 Involvement of the Rad1-Rad10 Endonuclease in Meiotic MMR of Large Insertion/Deletions
Supplemental to the typical complement of MMR proteins, Rad1-Rad10 (XPF-ERCC1 in mammals) plays a specific role in the repair of long insertion/deletion loops during meiotic recombination (Kirkpatrick and Petes 1997). Rad1-Rad10 is required for the repair of loops that are 26 bases or more in size, and unpaired loops as large as 5.6 kb are compatible with assimilation into hDNA (Kearney et al. 2001). These observations suggest significant tolerance for insertions/deletions in heteroduplex so long as other requirements of homology are met in flanking regions. The conversion events associated with large deletions or insertions tend to restore sequence where there was a deletion. In other words, conversion is biased for the retention of sequence where there is disparity in a heteroduplex region. To achieve this bias, Rad1-Rad10 is proposed to cleave the bottom strand (as drawn in Fig. 10), opposite the extruded ssDNA loop (Jensen et al. 2005). Incision at this position opens the loop so that its ssDNA becomes a gap flanked by duplex DNA. Rad1-Rad10 is a DNA structure-selective endonuclease complex best characterized for its role in the processing of UV photoproducts (nucleotide excision repair/NER) and in SSA (reviewed in Heyer et al. 2003). In NER and SSA, Rad1-Rad10 cuts 5′ to a transition between dsDNA and ssDNA, where the ssDNA is directed 5′ to 3′ Incision opposite the loop rather than adjacent to the loop therefore departs from the standard biochemical behavior observed for Rad1-Rad10 incision on DNA bubble substrates in vitro. Kearney et al. (2001) propose that RPA bound to the ssDNA loop directs Rad1-Rad10 incision to the bottom strand, rather than ssDNA in the loop. This unconventional incision site probably involves an additional interaction with the MMR complex Msh2-Msh3, which may promote Rad1-Rad10 incision appropriate to the long loop repair context. Neither of these scenarios has been tested with a long loop substrate in vitro. This example illustrates how endonucleolytic incision might be dictated or sanctioned by the specific substrate context and other bound proteins.
Fig. 10.
XPF-ERCC1 promotes long-tract gene conversion by loop incision in a manner different from its incision during nucleotide incision repair. A XPF-ERCC1 is biochemically responsible for “upper-strand incision” 5′ to a single-stranded DNA lesion during nucleotide excision repair (NER). RPA promotes cleavage of the bubble substrate. B In contrast to its behavior in NER, XPF-ERCC1 is proposed to incise the “bottom strand” of a looped substrate associated with a long-tract heterology during gene conversion (Jensen et al. 2005). This incision site is genetically implicated but remains to be biochemically demonstrated in association with the factors that might direct or promote its incision in this context (such as Msh2-Msh6). C The predicted incision site for XPF-ERCC1 in long-tract gene conversion promotes the preservation of long insertions in otherwise homologous sequences
In sum, MMR and long-loop repair processes account for the gene conversion products that are fundamental to meiotic recombination. MMR (and possibly long loop repair proteins) are also likely to perform key roles in communicating hDNA quality. How they achieve this for purposes of HR and its fidelity are questions that remain broadly open for biochemical exploration.
2.11 Double Holliday Junction Processing: Roads to Crossover and Non-Crossover
The dHJ is a key intermediate in the DSBR pathway of HR (Szostak et al. 1983) (Figs. 2, 11). Physical analysis of meiotic recombination intermediates in S. cerevisiae has demonstrated the existence of dHJs as paired and fully ligated interhomolog joint molecules consisting of contiguous strands, where the invading ends have been ligated (Schwacha and Kleckner 1994, 1995, 1997). Combined genetic and physical analysis defined a major meiotic CO pathway in S. cerevisiae (termed CO pathway 1, Fig. 12) that genetically depends on Msh4-Msh5, Mlh1-Mlh3, Exo1, and Mer3 (the Zip 1-4 components are not discussed here; see for review Cromie and Smith 2007; Hunter 2007). This CO pathway posits the maturation of SEI intermediates into dHJs (Fig. 2), which appear to be dedicated to become COs that exert chiasma interference (Allers and Lichten 2001; Borner et al. 2004; Hunter and Kleckner 2001) (Figs. 1 part C, 12). This CO-specific model represents a significant deviation from the original DSBR model (Szostak et al. 1983), which envisioned an equal likelihood of dHJ resolution into CO and NCO products to account for roughly 50% association of COs with meiotic gene conversion. While endonucleolytic resolution has been consistently envisioned to resolve dHJs into CO or NCO products, recent biochemical experiments using the BLM DNA helicase in conjunction with TOPOIIIα provided biochemical support for an alternative model of dHJ processing originally proposed to explain budding yeast mating-type switching (Nasmyth 1982; Wu and Hickson 2003). In a process termed dissolution, active migration of the two junctions towards each other and decatenation of the resulting hemicatenane yields exclusively NCO products (Wu and Hickson 2003) (Fig. 11). While the genetic data in S. cerevisiae argue that a majority, if not all, of the meiotic dHJs are processed to COs, it is possible that dHJ dissolution to NCOs might be relevant for meiosis not only in the context of homolog but also sister interactions.
Fig. 12.
Pathways to generate crossovers. A Symmetric endonucleolytic resolution of dHJs (a) (Szostak et al. 1983) and single HJs b′ (Holliday 1964) have long been considered to represent two mechanisms of generating crossovers. dHJ resolution represents CO pathway 1. CO pathway 2 is currently defined by Mus81-Mms4/Eme1, although the physical intermediates of this pathway are presently uncertain. A single HJ can be generated by endonucleolytic cleavage 3′ to an extended D-loop (b), if the displaced strand of the D-loop reanneals with the second end b′. Two successive rounds of D-loop cleavage, or more specifically D-loop incision at the bottom strand (c) and nicked HJ incision c′, can generate a CO without the involvement of an intact HJ intermediate (Heyer et al. 2003; Osman et al. 2003). Finally, consecutive incision of nicked dHJs (d) can yield a CO product. B Taxonomic distribution of CO pathways. CO pathway 1 is defined by a collection of factors associated with Msh4-Msh5, including Mer3, Mlh1-Mlh3, and synaptonemal complex factors not discussed here. CO pathway 2 is defined by the XPF paralog Mus81-Mms4/Eme1, and CO pathway 3 by the XPF ortholog Mei9 (pathway 3). The size of the checked box indicates the relative use of the pathway in the given organism. (*) Mus81-Mms4/Eme1 and Mei9-Mus312 are the endonucleases responsible for the incisions believed to be associated with CO generation in CO pathways 2 and 3, but Msh4-Msh5 is not an endonuclease and the endonuclease responsible for dHJ resolution in CO pathway 1 remains to be identified. CO pathway 1 displays CO interference (see Fig. 1). Interference is not intrinsic to chiasmata, as COs in pathway 2 do not display interference (de los Santos et al. 2003; Munz 1994). In Drosophila melanogaster, most if not all COs depend on pathway 3 (Sekelsky et al. 1995), implying that COs in this pathway are associated with interference (Muller 1916; Sturtevant 1915)
2.11.1 Sgs1-Top3-Rmi1 and dHJ Dissolution: Inferences from BLM-TOPOIIIα-BLAP75
Extending earlier observations that BLM helicase can migrate model HJs (Karow et al. 2000), elegant biochemical studies of human and Drosophila BLM-TopoIIIα on model dHJs demonstrate that the combined activities of the BLM helicase and TOPOIIIα topoisomerase can converge model dHJs and decatenate them to a NCO outcome (Plank et al. 2006; Wu and Hickson 2003). Work with human BLM-TOPOIIIα on oligonucleotide-based dHJs demonstrated that dissolution activity was specific for an interaction between BLM and TOPOIIIα, and no other helicase or topoisomerase can substitute in the relationship (Wu and Hickson 2003). dHJ dissolvase activity requires the catalytic activities of both proteins. ATP hydrolysis is required, suggesting that BLM helicase activity, and not DNA melting by binding alone, catalyzes convergent migration of the two HJs toward one another (Wu and Hickson 2003). This mechanism was authoritatively borne out by reactions with Drosophila BLM-TOPOIIIα on large, circular dHJ substrates (Plank et al. 2006). Dissolution of these dHJs, separated by up to 165 bp of homologous sequence, demonstrated that BLM-TOPOIIIα can migrate and decatenate the junctions without obligatory ssDNA. RPA stimulates the BLM-TOPOIIIα reaction in a species-specific manner (the bacterial and T4 ssDNA binding proteins SSB and gp32 cannot substitute), suggesting that a functional complex entails BLM, TOPOIIIα, and RPA (and therefore that some measure of ssDNA is generated by the complex and involved in the reaction mechanism). Further support for the role of convergent migration in dHJ dissolution was supplied by limiting junction migration in the model substrates. The bacteriophage HJ resolvase T7 endo I processes dHJs in the presence of interstrand crosslinks, but BLM-TOPOIIIα-RPA dissolution is inhibited (Plank et al. 2006). Additional substrates that were engineered with discrete mismatches underwent exchange of complementary strands to generate novel restriction sites. These observations suggest that duplex DNA is unpaired ahead of the migrating junctions and re-annealed behind them, consistent with convergent migration of the junctions toward one another. In reactions with Drosophila BLM-TOPOIIIα, up to 30 linkages between the dHJs were dissolved. This indicates a mechanism in which the complex resides at each HJ and performs iterative strand passage events at each step position of convergent migration (Plank et al. 2006).
At present, it is not clear how a directionality of HJ migration compatible with dissolution is enforced, because in principle the junctions can be migrated toward or away from one another. One possibility relates to a factor that may target BLM-TOPOIIIα to dHJs. Rmi1 (BLAP75/RMI1 in mammals) is an OB-fold protein identified as a coimmunoprecipitating member of Sgs1-Top3 and BLM-TOPOIIIα complexes (Chang et al. 2005; Mullen et al. 2005). Human BLAP75/RMI1 and budding yeast Rmi1 bind weakly to ssDNA on their own, but stimulate Top3 ssDNA binding by approximately fivefold and enhance Top3-catalyzed relaxation of supercoiled DNA (Chen and Brill 2007; Wu et al. 2006a). dHJ dissolution by human BLM-TOPOIIIα under conditions of physiological ionic strength depends on BLAP75/RMI1 (Raynard et al. 2006), highlighting a specific relationship not observed with TOPOIIIα and WRN or E. coli RecQ (Wu and Hickson 2003). Whether BLAP75/RMI1 promotes a conformational change in TOPOIIIα-BLM that promotes DNA binding, or whether it helps melt DNA structural regions that enhance TOPOIIIα binding, is unclear. How BLAP75/RMI1 promotes the helicase-topoisomerase cooperation for convergent migration of dHJs is also unclear, although it could possibly determine the orientation with which the complex loads on the DNA.
dHJ dissolution has not been demonstrated for S. cerevisiae Sgs1-Top3 due to the challenges in purifying the proteins. Whether Rmi1 is needed for or enhances Sg1-Top3 activities is also not known. Nevertheless, the observations that mutations in rmi1 mimic those of sgs1 and top3 strains for slow growth, hyper-recombination, reduced sporulation and genotoxin sensitivity indicate that a potential targeting factor may be absolutely required for the in vivo execution of a biological activity under normal protein expression levels (Mullen et al. 2005).
Does dHJ dissolution explain the genetic requirements for Sgs1-Top3 during meiosis? Genetic observations for Sgs1 in mitotic cells are consistent with a role in CO suppression, and/or promotion of NCO dissolution of recombination intermediates. Whether the NCO contribution in vivo is by dHJ dissolution, however, is difficult to deduce and not likely to be the only mechanism by which Sgs1-Top3 promotes NCO. Ira et al. (2003) observed in an ectopic DSB-induced recombination assay that COs were rare in mitotic cells (5%) and that disruption of sgs1 increased CO two- to threefold. Absence of Sgs1 did not affect the DSB repair efficiency or kinetics, suggesting that Sgs1 does not curtail DSB repair events but influences their outcome to result in NCOs (Ira et al. 2003). These genetic data fit well with the proposal that Sgs1-Top3 functions in the dissolution of dHJs in vegetative S. cerevisiae cells (Ira et al. 2003), although the existence of dHJs in mitotically growing cells has not been physically demonstrated yet. Sgs1-deficient cells also display elevated CO frequencies in meiosis, although the effect is rather subtle (<twofold) (Jessop et al. 2006; Oh et al. 2007; Rockmill et al. 2003). An early role of Sgs1 in meiotic recombination may be in the turnover of pairing intermediates generated during a mechanism that queries homology (Myung et al. 2001). A role for Sgs1 in hDNA rejection during SDSA in vegetative cells has also been described (Sugawara et al. 2004). Consistent with this possibility, human BLM promotes dissolution of D-loops, as mentioned earlier (Bachrati et al. 2006; Bugreev et al. 2007b; Orren et al. 2002; van Brabant et al. 2000) (see above Sect. 2.6). Like Mer3, BLM helicase has a 3′ ↔ 5′ polarity for translocation, but presumably a different loading position achieves heteroduplex disruption rather than extension (see Sect. 2.9; Fig. 7). The role of Sgs1 in meiotic CO suppression has been further clarified by genetic analysis of double mutants and physical analysis of recombination intermediates. Mutants in the CO pathway 1 of S. cerevisiae (Fig. 2; SEI-dHJ), in particular mlh3 and msh5, show a significant decrease in CO, which is completely suppressed by Sgs1 deficiency (Jessop et al. 2006; Oh et al. 2007). This could suggest that the dHJ clamp Msh4-Msh5 protects the dHJ from dissolution by Sgs1, in support of the notion that dHJs are CO-dedicated intermediates (Allers and Lichten 2001; Borner et al. 2004; Hunter and Kleckner 2001). Careful genetic analysis identified that Sgs1 specifically suppresses closely spaced double COs, and that the increase in COs in the sgs1 mutant can be primarily explained by a transition from single COs to closely spaced double COs (Oh et al. 2007). Physical analysis of the recombination intermediates provided a mechanistic basis for this genetic outcome, by showing that the two ends of the DSB become disco or dinated in sgs1 cells, leading to the accumulation of joint molecules with three or four partners at the expense of joints with the canonical two partners (Oh et al. 2007). This function of Sgs1 is unlikely to be explained by dissolution of dHJs generated by second end capture, but rather pertains to the independent joints formed by the second end.
Do all meiotic functions of Sgs1 take place in association with Top3, or are only specific functions associated with the strand passage activity of the topoisomerase? This question is difficult to answer at the moment. Top3 function is essential in S. cerevisiae meiosis, and the phenotypes of the top3 mutant are more extreme than those of an sgs1 mutant. Few top 3 cells are able to complete meiosis I, and spore viability is zero. However, physical analysis reveals that DSB levels and the kinetics of DSB processing at the CYS3 and ARG4 meiotic recombination hotspot loci resemble wild-type in the top3 mutant, suggesting that the defect occurs late and may entail HJ resolution or dissolution (Gangloff et al. 1999). Rather than mirroring the top3 phenotype for meiosis, inactivation of SGS1 in fact relieves the severity of the top3 defect (spore viability of sgs1 is the same as sgs1 top3, at 67%). Also, Top3 may function outside the context of the Sgs1-Top3-Rmi1 complex.
In sum, dHJs are concrete intermediates of the CO pathway 1 in S. cerevisiae, but details of their biochemical processing in meiosis remain a crucial deficiency in our understanding of eukaryotic meiotic recombination (see also below). The original proposal of the DSBR model, that CO and NCO are alternative outcomes of dHJ resolution, can no longer be maintained. dHJs develop from stable SEI intermediates, which are determined from early strand exchange to mature along a CO-designated pathway. These observations make less certain the role of dHJ dissolution by Sgs1-Top3-Rmi1 in meiosis, although this mechanism may present an alternative to SDSA in the generation of NCO from intermediates aborted early in meiosis or in sister interactions.
2.11.2 Double Holliday Junction Resolution: The Holy Grail and Resolvase A
Endonucleolytic resolution of HJs is a central tenet of recombination models to explain CO formation (Holliday 1964; Szostak et al. 1983) (Figs. 2, 11). The physical demonstration of their existence and the identification of enzymes that are highly selective for HJs in bacteria (RuvC), Archaea (Hjc, Hje), and eukaryotic mitochondria (Cce1) provided experimental support for this model (reviewed in Heyer et al. 2003; West 1997). In particular, the elegant work on bacterial RuvC has set a paradigm for a eukaryotic nuclear HJ resolvase and its reaction mechanism of delivering a symmetric pair of cuts across the junction that allows direct religation of the products (West 1997). None of the known HJ resolvases has an obvious sequence homolog in eukaryotes. However, such a relationship may be difficult to discern. As an example, the similarity between archaeal Hjc and the eukaryotic XPF family, and their evolutionary relationship to eubacterial type II restriction endonucleases, was not recognized until structural comparisons revealed a common architecture and configuration of a small set of active site residues (Bond et al. 2001; Nishino et al. 2003). This suggests that resolvases with similar architecture to bacterial or archaeal HJ resolvases cannot be categorically discounted in eukaryotic genomes from sequence analysis alone. The identity of the eukaryotic nuclear resolvase is still unknown, although significant progress in this direction has been made for the mammalian Resolvase A activity.
A mammalian activity that symmetrically cuts model HJs has been partially purified from Chinese hamster ovary cells, human HeLa cells, and several DNA repair-deficient cell lines (Constantinou et al. 2001, 2002; Hyde et al. 1994; Liu et al. 2004). Fractionation of cell extracts over several columns consistently yields activity that generates duplex products that can be religated. In a compelling analogy to the RuvABC paradigm, the incision activity copurifies extensively with an ATP-dependent junction-migration activity (Constantinou et al. 2001). Nevertheless, the protein factor(s) responsible for branch migration and DNA incision continue to elude identification. The incision activity was called Resolvase A and can be separated from Mus81-Mms4/Eme1, a DNA structure-selective endonuclease also implicated in the processing of recombination-associated joint molecules (see Sect. 2.12. Re-solvase A shows a greater preference for HJ structures relative to the three-way, replication fork-like structures preferred by Mus81 (Constantinou et al. 2002). Most recently, it was reported that the Rad51 paralogs Rad51C and Xrcc3 were required components of the HJ incision associated with a partially purified mammalian fraction (Liu et al. 2004). Immunodepletion of Rad51C from extracts reduced model HJ incision in vitro, but addition of recombinant Rad51C restored junction cleavage. Recombinant Rad51C-Xrcc3 was devoid of resolvase activity, suggesting that the endonuclease activity is associated with a different protein. Furthermore, Rad51C localizes to the obligatory CO site during XY pairing in mouse cells, in a temporal sequence that follows Mlh1 arrival (Kuznetsov et al. 2007; Liu et al. 2007) (see Sect. 2.9.3). These data may be consistent with a late role for Rad51C-Xrcc3 associated with meiotic CO sites, but a mechanistic explanation to link Rad51C-Xrcc3 to the resolvase responsible for resolution in vitro and in vivo is still needed.
2.12 Other Junctions and Alternative Mechanisms for Crossover Formation: Possible Roles of Mus81-Mms4 and XPF
dHJs are the key intermediate for the meiotic CO pathway 1 of S. cerevisiae that depends on Mer3, Msh4-Msh5, Mlh1-Mlh3, and other proteins (Figs. 11 and 12). However, disabling this pathway reduces CO frequencies by only about twofold in budding yeast. A second meiotic CO pathway in budding yeast is defined by the Mus81-Mms4 endonuclease. There is significant evidence, discussed below, that this pathway does not involve dHJs in the formation of COs. The fission yeast S. pombe lacks CO pathway 1 and the proteins associated with its mechanism; most, if not all, meiotic COs depend on Mus81. As a mirror image, Caenorhabditis elegans exclusively relies on CO pathway 1 for meiotic CO formation (Zalevsky et al. 1999) as all COs appear to depend on Msh4-Msh5. Nonetheless, this organism has a Mus81 homolog but genetic analysis of the C. elegans mus81 mutant in meiosis remains to be reported (Interthal and Heyer 2000) (for review Hollingsworth and Brill 2004). In Drosophila, meiotic COs are largely controlled (>90%) by yet another pathway, involving the XPF (Drosophila mei-9) endonuclease function. Drosophila mus81 mutants show no meiotic CO defect (Trowbridge et al. 2007), and crossover pathway 1 may be absent, as indicated by the lack of Msh4/Msh5 homologs in Drosophila (Sekelsky et al. 2000a) (Fig. 12). Clearly, alternatives to dHJ resolution-mediated CO formation exist (Fig. 12), but what are the enzymes involved?
2.12.1 The Structure-Selective DNA Endonuclease Mus81-Mms4/Eme1
Mus81-Mms4/Eme1 is a well-conserved heterodimeric XPF family DNA endonuclease consisting of a catalytic subunit (Mus81) and a non-catalytic subunit. (Mms4 in budding yeast/Eme1 in fission yeast and mammals; therefore the complex is frequently referred to as Mus81-Mms4/Eme1. In this text, “Mus81” is also used to refer to the complex from any organism.) Mus81 functions as a context-specific factor in recombination in vegetative cells and defines the meiotic CO pathway 2 in budding yeast and the major, if not only, CO pathway in fission yeast (reviewed in Cromie and Smith 2007; Heyer et al. 2003; Hollingsworth and Brill 2004). Mus81 was identified by a series of genetic screens that implicated the protein in recombination associated with replication fork support. Mus81 was initially isolated in S. cerevisiae by a two-hybrid screen for proteins interacting with the Rad54 motor protein and was shown to be a member of the RAD52 epistasis group, although the single mutant lacks sensitivity to DSBs that typifies this group (Interthal and Heyer 2000). Independently, Mus81 was identified in a synthetic lethal screen with sgs1, demonstrating the need for the Mus81-Mms4 complex in cells lacking Sgs1, Top3, or Rmi1 (Mullen et al. 2001). In fission yeast, Mus81 was also found as a two-hybrid interactor with the S-phase checkpoint kinase Cds1 (Rad53 in budding yeast), and shown to be particularly required for replication fork support (Boddy et al. 2000). While Mus81 plays an important role in recombination pathways that support DNA replication, the discussion here will focus on its role during meiotic recombination.
What is the substrate by which the endonuclease activityof Mus81 promotes meiotic CO? One clear possibility is that Mus81-Mms4/Eme1 cleaves HJs. Biochemical studies suggested that the endogenous fission yeast and human Mus81 complexes resolve model HJs to linear duplex products, with incision targeted to the homologous core of the structure (Boddy et al. 2001; Chen et al. 2001; Gaillard et al. 2003). However, HJ incision has been difficult to observe for recombinant enzyme from any organism and, where observed, has been primarily restricted to incision by partially purified preparations from eukaryotic expression sources (Blais et al. 2004; Boddy et al. 2001; Chen et al. 2001). In fact, biochemical studies with budding yeast, fission yeast, and human enzyme complexes produced and isolated from expression in E. coli mostly show negligible incision on model HJs in vitro (Doe et al. 2002; Gaskell et al. 2007; Kaliraman et al. 2001; Whitby et al. 2003) (reviewed in Haber and Heyer 2001; Hollingsworth and Brill 2004). At first this was attributed to a possible need for post-translational modifications to the complex that occur only in eukaryotic expression sources but not bacterial sources, or to a need for an unidentified cofactor present in partially purified eukaryotic preparations and not in homogenous recombinant preparations. However, purified preparations of budding yeast Mus81-Mms4 from the cognate host and with endogenous modifications are also not competent in HJ incision in vitro (Ehmsen and Heyer 2008). Given that nicked substrates are processed much more readily than intact junctions, Gaillard et al. (2003) proposed that endogenous Mus81-Mms4/Eme1 resolves HJs by a nick-counternick mechanism. The first incision is proposed to be rate-limiting, but when successful, directs subsequent incision across the branch point. The incongruity in HJ activity by endogenous and recombinant preparations remains to be satisfactorily explained.
Aside from the difficulty in demonstrating robust HJ incision activity, model HJ cleavage by Mus81 under certain in vitro conditions takes place in a manner unlike HJ resolvases characterized to date. RuvC shows coordinated incisions on opposing strands of like polarity, in a manner that generates defined nicks in the product duplexes (West 1997). The product nicks are substrates for ligase in the final step of repair, indicating that the two phosphodiester bonds hydrolyzed during junction resolution occurred symmetrically across the junction core. Analysis of Mus81 HJ incision products suggests that the cuts are offset, because only a small fraction of the incisions can be directly ligated (Boddy et al. 2001; Constantinou et al. 2002; Gaskell et al. 2007). At least three possibilities can account for these observations, but the explanation is unclear with the present data sets: (1) the criteria to call an enzyme a HJ resolvase may need to be reframed, (2) the in vitro preparations are missing components important to the placement of the endonuclease and enforcement of symmetric incisions, or (3) the HJ incision observed represents the uncoordinated products of two independent incision events. Since the cell is equipped to resolve gaps and flaps, an asymmetric mechanism of HJ resolution cannot be dismissed categorically.
Quantitative substrate analysis with the Mus81-Mms4/Eme1 heterodimer demonstrated that the nuclease is not DNA structure-specific in vitro, but rather is structurally selective for substrates that have a branch point coincident with a strand discontinuity (nick) (Ehmsen and Heyer 2008). The biochemical preferences of Mus81 preparations in vitro are consistent with a number of substrates that may be valid physiological alternatives to intact HJs. 3′-flapped structures, D-loop structures, nicked three-way junctions, various replication fork-like substrates, and nicked four-way junctions are processed efficiently by all heterodimer preparations (Doe et al. 2002; Ehmsen and Heyer 2008; Fricke et al. 2005; Gaillard et al. 2003; Gaskell et al. 2007; Kaliraman et al. 2001; Osman et al. 2003; Whitby et al. 2003). Mus81-Mms4 incision of joint molecules appears to take place five nucleotides 5′ to the end of a duplex flush with the branch point, suggesting that the 5′ position of a nick in the DNA is a defining feature that directs Mus81 incision (Bastin-Shanower et al. 2003).
Evidently, it is difficult to extrapolate the biochemical behavior of Mus81 to a most likely in vivo joint molecule substrate. Are genetic observations consistent with HJ resolution? S. cerevisiae mus81 and mms4 mutants show a modest (twofold) reduction in meiotic CO, and 40% spore viability (de los Santos et al. 2001,de los Santos et al. 2003; Interthal and Heyer 2000). Physical analysis of recombination intermediates in mms4 cells identified a reduction in SEI and dHJ intermediates, which define the meiotic CO pathway 1 (de los Santos et al. 2003). Hence it is unlikely that Mus81 processes these intermediates. Physical analysis failed to identify other junction intermediates that accumulate in mms4 mutants and might otherwise have revealed its in vivo substrate. These data suggest a biochemical role for Mus81 that is independent of dHJs. CO formation can be achieved in ways that do not involve dHJs, for example by consecutive incision of D-loops results in CO formation (Heyer et al. 2003; Osman et al. 2003) (Fig. 12). A D-loop/nicked HJ model combines the in vitro substrate preference of Mus81-Mms4 for D-loop and nicked four-way junctions with a genetic outcome that always leads to CO (Fig. 12).
In severe contrast to S. cerevisiae, the meiotic phenotype of fission yeast mus81 and eme1 mutants shows a massive failure to segregate chromosomes due to unresolved recombination intermediates (Boddy et al. 2000; Osman et al. 2003). Spore viability is reduced to less than 1% (relative to 80% in wild-type cells), and most DNA is relegated to one large spore product with three small spores nearly devoid of DNA. Total CO is reduced 20- to 100-fold (Osman et al. 2003; Smith et al. 2003). This DNA segregation defect contrasts the phenotype of rhp51 mutants, which accumulate DSBs but nevertheless segregate DNA rather equitably to the four spore products. When Spo11-catalyzed initiation of meiotic recombination is blocked by additional mutations in rec6 or rec12, viability of mus81 mutants is improved to 20%, a value that is close to that expected from random segregation in fission yeast meiosis (Boddy et al. 2000). Genetic analysis in fission yeast shows that mus81 mutants generate heteroduplex DNA and achieve normal levels of gene conversion, but with severely reduced CO levels (19–90% reduction over three genetic intervals tested) (Osman et al. 2003; Smith et al. 2003). This level of spore viability is less than expected for random segregation of three homologs at meiosis I. Together these observations make a compelling case for a late role for Mus81-Mms4/Eme1 and that recombination intermediates accumulate in mus81 mutants that impede chromosome segregation. Physical analysis of recombination intermediates during fission yeast meiosis identified single HJs as a prominent class, and the authors suggested that single HJs rather than dHJs are the key intermediates for CO formation in fission yeast (Cromie et al. 2006). The accumulation of single HJs in mus81 mutants is consistent with a single HJ being a Mus81 substrate, although it is also possible that the Mus81 substrate is different but is processed to an HJ in the mutant. While the biochemical data strongly favor a nicked HJ as a potential Mus81 substrate, the genetic data imply that the observed junctions are likely to be unnicked. Fission yeast has relatively rare DSB sites but an evenly spaced genetic map, which appears to mandate that CO-dedicated junctions migrate over significant distances from the DSB initiation site (Young et al. 2002). Such branch migration of HJs would remove possible nicks left from their initiation as DNA strand exchange products.
In sum, it is clear that Mus81-Mms4/Eme1 catalyzes CO formation in a subpathway of meiotic recombination in budding yeast, which is apparently the primary or exclusive pathway of meiotic recombination in fission yeast. What remains unclear is the identity of the substrate underlying the CO outcome. Although this may ultimately find more unequivocal support in the form of a traditional HJ, it is very possible that an alternative junction, characterized by a nick inherent to the branch point, may be the substrate in question for Mus81-Mms4/Eme1.
2.12.2 XPF Controls Meiotic Crossovers in Drosophila
Drosophila shows reliance on neither the Mus81 nor Msh4-Msh5 pathways for meiotic CO formation, but instead relies on its XPF ortholog, Mei-9 (Sekelsky et al. 1995; Yildiz et al. 2004). Mei-9 is required for 90–95% of CO in meiosis, very different from budding and fission yeast where the XPF orthologs are not involved in meiotic CO formation (Fleck et al. 1999; Paques and Haber 1999; Sekelsky et al. 1995; Yildiz et al. 2004). Mei-9 disruption depletes COs, but not gene conversion, suggesting that it acts at a late recombination step that may be HJ resolution (Radford et al. 2007). In nucleotide excision repair and SSA, Mei-9 interacts with ERCC1 (Sekelsky et al. 2000b). In meiosis, however, Mei-9 also associates with a novel partner, Mus312 (Yildiz et al. 2002). Surprisingly, ercc1 mutants are not as CO-defective as either mei-9 or mus312, suggesting that Mei-9 may partner with alternative subunits in different contexts (Radford et al. 2005). Although it is still relatively unclear how ERCC1 contributes to the enzymatic activity or structural selectivity of the XPF-ERCC1 complex, ERCC1 binds ssDNA and may define substrate recognition and protein interaction properties of the complex (Tsodikov et al. 2005). Mus312 may similarly target Mei-9 to a branched DNA structure or alter HJ presentation to Mei-9 (or Mei9-ERCC1) to effect junction resolution. This scenario resembles BLAP75/RMI1 targeting of BLM-TOPOIIIα to a DNA joint molecule and may provide another example of a manner by which the in vivo selectivity of a catalytic entity is narrowed to a specific application in sanctioned contexts, by association with a context-specific partner subunit. Whether the Drosophila situation reflects a third mechanism for CO formation (distinct from either the Msh4-Msh5 or Mus81-Mms4/Eme1 mechanisms) or merely the application of a different XPF paralog to a similar mechanism as Mus81-Mms4/Eme1 (more basically an “XPF paralog” mechanism) remains to be better defined (Fig. 12). However, unlike the Mus81-dependent CO pathway 2, which lacks interference in fission yeast (Munz 1994) and budding yeast (de los Santos et al. 2003), the Mei-9 pathway in Drosophila generates COs with interference (Muller 1916; Sturtevant 1915), and hence is termed CO pathway 3 here.
What is the substrate for Mei-9 in CO generation – is it possibly the substrate most frequently implicated in a CO defect, the HJ? Genetic experiments led to the proposal that Mei-9 acts on dHJs in Drosophila meiosis, but the physical nature of these junctions (nicked or ligated dHJs) remains undetermined (Radford et al. 2007). Although biochemical analysis of Mei-9 remains to be reported, extrapolations from XPF orthologs may suggest HJ incision is at least available as a biochemical option. In a biochemical role distinct from its incision at dsDNA–ssDNA transitions, S. cerevisiae Rad1 (XPF homolog) can bind and incise model HJs in vitro (Habraken et al. 1994). Junction binding and incision occurred even in the absence of Rad10 (ERCC1 homolog) and was not stimulated by Rad10. Although Rad1 bound a model HJ whether it had a homologous core or not, it incised only junctions with a homologous core and did so over a number of asymmetric positions near the core. It was suggested, however, that this in vitro behavior of Rad1 and its dependence on a homologous core was an artifact of local “breathing” at the branch point that presented a dsDNA–ssDNA transition for Rad1 incision (West 1995). Alternatively, XPF-ERCC1 (Mei-9-Mus312 +/− ERCC1) may process D-loops in a fashion similar to its proposed role in gene targeting (Niedernhofer et al. 2001), which will result in obligatory COs (see Fig. 12). D-loop cleavage may be an activity common to XPF paralogs in certain contexts, and may represent a means to produce COs in a mechanism that does not involve HJs.
3 Conclusions and Outlook
Much progress has been made in identifying and characterizing core recombination factors and some of the meiosis-specific components of the HR machinery. However, the mechanistic basis for the features that distinguish meiotic recombination from recombination in vegetative (mitotically cycling) cells remains unknown. Genetic and cytological data provide a framework to unravel the mechanisms involved in the targeting of meiotic recombination to homologs, as well as the control of CO levels and distribution (Cromie and Smith 2007; Gerton and Hawley 2005; Hunter 2007; Roeder 1997; Zickler and Kleckner 1999). These analyses suggest a central role of Rad51 and Dmc1 (and its meiosis-specific cofactors) to bias DNA strand invasion towards the homolog, but the biochemical basis for this bias remains to be determined. As an additional mechanism that was not discussed here but is pertinent, a meiosis-specific barrier to intersister recombination is envisioned that involves the meiosis-specific proteins Red1 and Hop1 that control the meiosis-specific checkpoint kinase Mek1 (see also Niu et al. 2005; Wan et al. 2004). The roles of Red1 and Hop1 as well as the potential targets of Mek1 that could be involved in establishing the intersister barrier remain to be determined (for a detailed discussion see Hunter 2007). It appears that a combination of meiosis-specific aspects of the recombination machinery and as yet poorly understood structural aspects of meiotic chromosomes will be involved.
Beyond the biochemical mechanisms that guarantee DNA strand exchange between homologs in the meiotic bivalent, many questions remain for how the resulting DNA joint molecules are processed to ensure a CO exchange. Most of these questions concern how apparently undifferentiated precursors are processed for dissimilar fates. This question of precursor fate appears at the earliest stages of recombination at a given locus – how is a single chromatid chosen for the Spo11-catalyzed break and what ensures that only one chromatid of four receives this fate? Almost immediately, a question of fate choice resurfaces for the development of DSBs to CO or NCO pathways. Genetic evidence supports the operation of at least two CO pathways in budding yeast, one defined by the consecutive actions of Mer3, Msh4-Msh5, and Mlh1-Mlh3, and another defined by Mus81-Mms4/Eme1. How is a DSB selected for maturation along one of these pathways? At what point do the different pathways diverge, and what are the deciding factors and contexts that determine their fates? How is CO distribution and interference achieved in the Msh4-Msh5 pathway? What is the in vivo substrate and mechanism for CO in the Mus81-Mms4/Eme1 and Mei9 pathways? What is the identity of the resolvase expected to process the dHJ intermediates of the Msh4-Msh5 pathway? An explanation for the taxonomic distribution of these CO pathways is needed, as is an understanding of whether the different pathways apply to special circumstances of chromosome structure. Finally, the specialized contributions of MMR proteins in meiotic recombination are poorly understood. Some possibilities for biochemical query include hDNA quality detection, the designation of strand exchange intermediates to NCO or CO resolution pathways, and the direction of strand incisions to catalyze CO at dHJs. Models for meiotic recombination have been updated with the understanding that several pathways process Spo11-induced breaks, and that NCO and CO designations occur far earlier than once anticipated. Now challenges remain to further characterize the newly recognized pathway variations, their underlying DNA structural intermediates, and their biochemical processing.
Acknowledgments
The support of our research by the National Institutes of Health, the Susan G. Komen Breast Cancer Foundation, and University of California BioStar program is gratefully acknowledged. KTE enjoyed the support of an NIH predoctoral training grant. We thank Neil Hunter for continued discussions and critical comments on the manuscript. We appreciate the helpful comments by Shannon Ceballos, Clare Fasching, Ryan Janke, Xuan Li, Jie Liu, Eugene Nadezhdin, Erin Schwartz, Jessica Sneeden, William Wright, and Xiao-Ping Zhang. We offer our sincere apologies for omitting all the references and great work that could not be discussed here due to space constraints. WDH dedicates this review to the memory of his Ph.D. mentor Urs Leupold, the founder of fission yeast genetics, who passed away on 9 October, 2006 after touching the lives of many scientists.
Abbreviations
- ChIP
chromatin immunoprecipitation
- CO
crossover
- dHJ
double Holliday junction
- DSB
double-stranded DNA break
- DSBR
double-stranded DNA break repair model
- dsDNA
double-stranded DNA
- hDNA
heteroduplex DNA
- HJ
holliday junction
- HR
homologous recombination
- MMR
mismatch repair
- MRX
Mre11-Rad50-Xrs2
- MRN
MRE11-RAD50-NBS1
- MRX/N
Mre11-Rad50-Xrs2 or Mre11-Rad50-NBS1
- NCO
non-crossover
- NHEJ
nonhomologous end-joining
- SDSA
synthesis-dependent strand annealing model
- SEI
single-end invasion
- SSA
single-strand annealing
- ssDNA
single-stranded DNA
References
- Aboussekhra A, Chanet R, Adjiri A, Fabre F. Semi-dominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA protein. Mol Cell Biol. 1992;12:3224–3234. doi: 10.1128/mcb.12.7.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, McVey M, Sekelsky JJ. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265–267. doi: 10.1126/science.1077198. [DOI] [PubMed] [Google Scholar]
- Akamatsu Y, Dziadkowiec D, Ikeguchi M, Shinagawa H, Iwasaki H. Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc Natl Acad Sci USA. 2003;100:15770–15775. doi: 10.1073/pnas.2632890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y, et al. Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J. 2007;26:1352–1362. doi: 10.1038/sj.emboj.7601582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggest an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- Alexeev A, Mazin A, Kowalczykowski SC. Rad54 protein possesses chromatin-remodeling activity stimulated by a Rad51-ssDNA nucleoprotein filament. Nature Struct Biol. 2003;10:182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- Alexiadis V, Kadonaga JT. Strand pairing by Rad54 and Rad51 is enhanced by chromatin. Genes Dev. 2003;16:2767–2771. doi: 10.1101/gad.1032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Amitani I, Baskin RJ, Kowalczykowski SC. Visualization of Rad54, a chromatin remodeling protein, translocating on single DNA molecules. Mol Cell. 2006;23:143–148. doi: 10.1016/j.molcel.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Arbel A, Zenvirth D, Simchen G. Sister chromatid-based DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J. 1999;18:2648–2658. doi: 10.1093/emboj/18.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora C, Kee K, Maleki S, Keeney S. Antiviral protein Ski8 is a direct partner of Spo11 in meiotic DNA break formation, independent of its cytoplasmic role in RNA metabolism. Mol Cell. 2004;13:549–559. doi: 10.1016/s1097-2765(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Bachrati CZ, Borts RH, Hickson ID. Mobile D-loops are a preferred substrate for the Bloom’s syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Symington LS. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. doi: 10.1101/gad.10.16.2025. [DOI] [PubMed] [Google Scholar]
- Baker SM, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nature Gen. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Bastin-Shanower SA, Fricke WM, Mullen JR, Brill SJ. The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10. Mol Cell Biol. 2003;23:3487–3496. doi: 10.1128/MCB.23.10.3487-3496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beernink HTH, Morrical SW. RMPs: Recombination/replication mediator proteins. Trends Biochem Sci. 1999;24:385–389. doi: 10.1016/s0968-0004(99)01451-6. [DOI] [PubMed] [Google Scholar]
- Bergerat A, deMassy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- Bhaskara V, et al. Rad50 adenylate kinase activity regulates DNA tethering by Mre11/Rad50 complexes. Mol Cell. 2007;25:647–661. doi: 10.1016/j.molcel.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco PR, Tracy RB, Kowalczykowski SC. DNA strand exchange proteins: A biochemical and physical comparison. Front Biosci. 1998;3:570–603. doi: 10.2741/a304. [DOI] [PubMed] [Google Scholar]
- Bishop DK. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994;79:1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Bishop DK, Park D, Xu L, Kleckner N. DMC1: A meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Blais V, et al. RNA interference inhibition of Mus81 reduces mitotic recombination in human cells. Mol Biol Cell. 2004;15:552–562. doi: 10.1091/mbc.E03-08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Gaillard P-HL, McDonald WH, Shanahan P, Yates JR, Russell P. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Lopez-Girona A, Shanahan P, Interthal H, Heyer WD, Russell P. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol Cell Biol. 2000;20:8758–8766. doi: 10.1128/mcb.20.23.8758-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CS, Kvaratskhelia M, Richard D, White MF, Hunter WN. Structure of Hjc, a Holliday junction resolvase, from Sulfolobus solfataricus. Proc Natl Acad Sci USA. 2001;98:5509–5514. doi: 10.1073/pnas.091613398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synap-tonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- Borts RH, Lichten M, Haber JE. Analysis of meiosis-defective mutations in yeast by physical monitoring of recombination. Genetics. 1986;113:551–567. doi: 10.1093/genetics/113.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugreev DV, Golub EI, Stasiak AZ, Stasiak A, Mazin AV. Activation of human meiosis-specific recombinase Dmc1 by Ca2+ J Biol Chem. 2005;280:26886–26895. doi: 10.1074/jbc.M502248200. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Hanaoka F, Mazin AV. Rad54 dissociates homologous recombination intermediates by branch migration. Nature Struct Mol Biol. 2007a;14:746–753. doi: 10.1038/nsmb1268. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Mazina OM, Mazin AV. Rad54 protein promotes branch migration of Holliday junctions. Nature. 2006;442:590–593. doi: 10.1038/nature04889. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Yu X, Egelman EH, Mazin AV. Novel pro- and anti-recombination activities of the Bloom’s syndrome helicase. Genes Dev. 2007b;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartagena-Lirola H, Guerini I, Viscardi V, Lucchini G, Longhese MP. Budding yeast Sae2 is an in vivo target of the Mec1 and Tel1 checkpoint kinases during meiosis. Cell Cycle. 2006;5:1549–1559. doi: 10.4161/cc.5.14.2916. [DOI] [PubMed] [Google Scholar]
- Cha RS, Weiner BM, Keeney S, Dekker J, Kleckner N. Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev. 2000;14:493–503. [PMC free article] [PubMed] [Google Scholar]
- Chang M, et al. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J. 2005;24:2024–2033. doi: 10.1038/sj.emboj.7600684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-F, Brill SJ. Binding and activation of DNA topoisomerase III by the Rmi1 subunit. J Biol Chem. 2007;282:28971–28979. doi: 10.1074/jbc.M705427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-B, et al. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell. 2001;8:1117–1127. doi: 10.1016/s1097-2765(01)00375-6. [DOI] [PubMed] [Google Scholar]
- Chi P, et al. Yeast recombination factor Rdh54 functionally interacts with the Rad51 recombinase and catalyzes Rad51 removal from DNA. J Biol Chem. 2006;281:26268–26279. doi: 10.1074/jbc.M602983200. [DOI] [PubMed] [Google Scholar]
- Chi P, San Filippo J, Sehorn MG, Petukhova GV, Sung P. Bipartite stimulatory action of the Hop2-Mnd1 complex on the Rad51 recombinase. Genes Dev. 2007;21:1747–1757. doi: 10.1101/gad.1563007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clever B, Interthal H, Schmuckli-Maurer J, King J, Sigrist M, Heyer WD. Recombi-national repair in yeast: Functional interactions between Rad51 and Rad54 proteins. EMBO J. 1997;16:2535–2544. doi: 10.1093/emboj/16.9.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clever B, Schmuckli-Maurer J, Sigrist M, Glassner B, Heyer W-D. Specific negative effects resulting from elevated levels of the recombinational repair protein Rad54p in Saccharomyces cerevisiae. Yeast. 1999;15:721–740. doi: 10.1002/(SICI)1097-0061(19990630)15:9<721::AID-YEA414>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Constantinou A, Chen XB, McGowan CH, West SC. Holliday junction resolution in human cells: two junction endonucleases with distinct substrate specificities. EMBO J. 2002;21:5577–5585. doi: 10.1093/emboj/cdf554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A, Davies AA, West SC. Branch migration and Holliday junction resolution catalyzed by activities from mammalian cells. Cell. 2001;104:259–268. doi: 10.1016/s0092-8674(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Conway AB, et al. Crystal structure of a Rad51 filament. Nature Struct Mol Biol. 2004;11:791–796. doi: 10.1038/nsmb795. [DOI] [PubMed] [Google Scholar]
- Cromie GA, Hyppa RW, Taylor AF, Zakharyevich K, Hunter N, Smith GR. Single Holliday junctions are intermediates of meiotic recombination. Cell. 2006;127:1167–1178. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie GA, Smith GR. Branching out: Meiotic recombination and its regulation. Trends Cell Biol. 2007;17:448–455. doi: 10.1016/j.tcb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- D’Amours D, Jackson SP. The MRE11 complex: At the crossroads of DNA repair and checkpoint signalling. Nature Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- Davis AP, Symington LS. The yeast recombinational repair protein Rad59 interacts with Rad52 and stimulates single-strand annealing. Genetics. 2001;159:515–525. doi: 10.1093/genetics/159.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Symington LS. The Rad52-Rad59 complex interacts with Rad51 and replication protein A. DNA Repair. 2003;2:1127–1134. doi: 10.1016/s1568-7864(03)00121-6. [DOI] [PubMed] [Google Scholar]
- De Jager M, Dronkert ML, Modesti M, Beerens CE, Kanaar R, van Gent DC. DNA-binding and strand-annealing activities of human Mre11: Implications for its roles in DNA double-strand break repair pathways. Nucleic Acids Res. 2001a;29:1317–1325. doi: 10.1093/nar/29.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager M, van Noort J, van Gent DC, Dekker C, Kanaar R, Wyman C. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol Cell. 2001b;8:1129–1135. doi: 10.1016/s1097-2765(01)00381-1. [DOI] [PubMed] [Google Scholar]
- De los Santos T, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics. 2003;164:81–94. doi: 10.1093/genetics/164.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De los Santos T, Loidl J, Larkin B, Hollingsworth N. A role for MMS4 in the processing of recombination intermediates during meiosis in Saccharomyces cerevisiae. Genetics. 2001;159:1511–1525. doi: 10.1093/genetics/159.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CL, Ahn JS, Dixon J, Whitby MC. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- Dray E, Siaud N, Dubois E, Doutriaux MP. Interaction between Arabidopsis Brca2 and its partners Rad51, Dmc1, and Dss1. Plant Physiol. 2006;140:1059–1069. doi: 10.1104/pp.105.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser ME, et al. DMC1 functions in a Saccharomyces cerevisiae meiotic pathway that is largely independent of the RAD51 pathway. Genetics. 1997;147:533–544. doi: 10.1093/genetics/147.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman EH. A tale of two polymers: New insights into helical filaments. Nature Rev Mol Cell Biol. 2003;4:621–630. doi: 10.1038/nrm1176. [DOI] [PubMed] [Google Scholar]
- Eggler AL, Inman RB, Cox MM. The Rad51-dependent pairing of long DNA substrates is stabilized by replication protein A. J Biol Chem. 2002;277:39280–39288. doi: 10.1074/jbc.M204328200. [DOI] [PubMed] [Google Scholar]
- Ehmsen KT, Heyer WD. Saccharomyces cerevisiae Mus81-Mms4 is a catalytic structure-selective endonuclease. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm1152. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C, Schmidt H, Smith GR. Swi5 acts in meiotic DNA joint molecule formation in Schizosaccharomyces pombe. Genetics. 2004;168:1891–1898. doi: 10.1534/genetics.104.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht JA, Voelkel-Meiman K, Roeder GS. Meiosis-specific RNA splicing in yeast. Cell. 1991;66:1257–1268. doi: 10.1016/0092-8674(91)90047-3. [DOI] [PubMed] [Google Scholar]
- Enomoto R, Kinebuchi T, Sato M, Yagi H, Kurumizaka H, Yokoyama S. Stimulation of DNA strand exchange by the human TBPIP/Hop2-Mnd1 complex. J Biol Chem. 2006;281:5575–5581. doi: 10.1074/jbc.M506506200. [DOI] [PubMed] [Google Scholar]
- Fabisiewicz A, Worth L., Jr Escherichia coli MutS, L modulate RuvAB-dependent branch migration between diverged DNA. J Biol Chem. 2001;276:9413–9420. doi: 10.1074/jbc.M005176200. [DOI] [PubMed] [Google Scholar]
- Fiorentini P, Huang KN, Tishkoff DX, Kolodner RD, Symington LS. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol Cell Biol. 1997;17:2764–2773. doi: 10.1128/mcb.17.5.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmenich AA, Eliasarnanz M, Berg P. A novel allele of Saccharomyces cerevisiae RFA1 that is deficient in recombination and repair and suppressible by RAD52. Mol Cell Biol. 1995;15:1620–1631. doi: 10.1128/mcb.15.3.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaus A, Owen-Hughes T. Mechanisms for ATP-dependent chromatin remodeling: Farewell to the tuna-can octamer? Curr Op Gen Dev. 2004;14:165–173. doi: 10.1016/j.gde.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Fleck O, Lehmann E, Schar P, Kohli J. Involvement of nucleotide-excision repair in msh2 pms1-independent mismatch repair. Nature Genet. 1999;21:314–317. doi: 10.1038/6838. [DOI] [PubMed] [Google Scholar]
- Formosa T, Alberts BM. DNA synthesis dependent on genetic recombination: Characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986;47:793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Fricke WM, Bastin-Shanower SA, Brill SJ. Substrate specificity of the Saccharomyces cerevisiae Mus81-Mms4 endonuclease. DNA Repair. 2005;4:243–251. doi: 10.1016/j.dnarep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 1998;17:6412–6425. doi: 10.1093/emboj/17.21.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P-H, Noguchi E, Shanahan P, Russell P. The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell. 2003;12:747–759. doi: 10.1016/s1097-2765(03)00342-3. [DOI] [PubMed] [Google Scholar]
- Gangloff S, de Massy B, Arthur L, Rothstein R, Fabre F. The essential role of yeast topoisomerase III in meiosis depends on recombination. EMBO J. 1999;18:1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior SL, Olivares H, Ear U, Hari DM, Weichselbaum R, Bishop DK. Assembly of RecA-like recombinases: Distinct roles for mediator proteins in mitosis and meiosis. Proc Natl Acad Sci USA. 2001;98:8411–8418. doi: 10.1073/pnas.121046198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior SL, Wong AK, Kora Y, Shinohara A, Bishop DK. Rad52 associates with RPA and functions with Rad55 and Rad57 to assemble meiotic recombination complexes. Genes Dev. 1998;12:2208–2221. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell LJ, Osman F, Gilbert RJ, Whitby MC. Mus81 cleavage of Holliday junctions: A failsafe for processing meiotic recombination intermediates? EMBO J. 2007;26:1891–1901. doi: 10.1038/sj.emboj.7601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton JL, DeRisi JL. Mnd1p: An evolutionarily conserved protein required for meiotic recombination. Proc Natl Acad Sci USA. 2002;99:6895–6900. doi: 10.1073/pnas.102167899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton JL, Hawley RS. Homologous chromosome interactions in meiosis: Diversity amidst conservation. Nat Rev Genet. 2005;6:477–487. doi: 10.1038/nrg1614. [DOI] [PubMed] [Google Scholar]
- Haber JE, Heyer WD. The fuss about Mus81. Cell. 2001;107:551–554. doi: 10.1016/s0092-8674(01)00593-1. [DOI] [PubMed] [Google Scholar]
- Habraken Y, Sung P, Prakash L, Prakash S. Holiday junction cleavage by yeast Rad1 protein. Nature. 1994;371:531–534. doi: 10.1038/371531a0. [DOI] [PubMed] [Google Scholar]
- Haruta N, et al. The Swi5-Sfr1 complex stimulates Rhp51/Rad51- and Dmc1-mediated DNA strand exchange in vitro. Nature Struct Mol Biol. 2006;13:823–830. doi: 10.1038/nsmb1136. [DOI] [PubMed] [Google Scholar]
- Hayase A, Takagi M, Miyazaki T, Oshiumi H, Shinohara M, Shinohara A. A protein complex containing Mei5 and Sae3 promotes the assembly of the meiosis-specific RecA homolog Dmc1. Cell. 2004;119:927–940. doi: 10.1016/j.cell.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Hays SL, Firmenich AA, Massey P, Banerjee R, Berg P. Studies of the interaction between Rad52 protein and the yeast single-stranded DNA binding protein RPA. Mol Cell Biol. 1998;18:4400–4406. doi: 10.1128/mcb.18.7.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JM, et al. Mnd1/Hop2 facilitates Dmc1-dependent interhomolog crossover formation in meiosis of budding yeast. Mol Cell Biol. 2006;26:2913–2923. doi: 10.1128/MCB.26.8.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer WD. Biochemistry of eukaryotic homologous recombination. In: Aguilera A, Rothstein R, editors. Molecular genetics of recombination. Springer; Berlin Heidelberg New York: 2007. pp. 95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer WD, Ehmsen KT, Solinger JA. Holliday junctions in the eukaryotic nucleus: Resolution in sight? Trends Biochem Sci. 2003;10:548–557. doi: 10.1016/j.tibs.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Heyer WD, Li X, Rolfsmeier M, Zhang XP. Rad54: The Swiss Army knife of homologous recombination? Nucleic Acids Res. 2006;34:4115–4125. doi: 10.1093/nar/gkl481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwagen A, Amon A. Checking your breaks: surveillance mechanisms of meiotic recombination. Curr Biol. 2006;16:R217–228. doi: 10.1016/j.cub.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann ER, Borts RH. Meiotic recombination intermediates and mismatch repair proteins. Cytogenet Genome Res. 2004;107:232–248. doi: 10.1159/000080601. [DOI] [PubMed] [Google Scholar]
- Holliday R. A mechanism for gene conversion in fungi. Genet Res. 1964;5:282–304. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- Hollingsworth NM, Brill SJ. The Mus81 solution to resolution: Generating meiotic crossovers without Holliday junctions. Genes Dev. 2004;18:117–125. doi: 10.1101/gad.1165904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth NM, Ponte L, Halsey C. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 1995;9:1728–1739. doi: 10.1101/gad.9.14.1728. [DOI] [PubMed] [Google Scholar]
- Holzen TM, Shah PP, Olivares HA, Bishop DK. Tid1/Rdh54 promotes dissociation of Dmc1 from nonrecombinogenic sites on meiotic chromatin. Genes Dev. 2006;20:2593–2604. doi: 10.1101/gad.1447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ERL, Shinohara A, Bishop DK. Saccharomyces cerevisiae Dmc1 protein promotes renaturation of single-strand DNA (ssDNA) and assimilation of ssDNA into homologous super-coiled duplex DNA. J Biol Chem. 2001;276:41906–41912. doi: 10.1074/jbc.M105563200. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Tainer JA. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr Opin Struct Biol. 2003;13:249–255. doi: 10.1016/s0959-440x(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. Meiotic recombination. In: Aguilera A, Rothstein R, editors. Homologous recombination. Springer; Berlin Heidelberg New York: 2007. pp. 381–441. [Google Scholar]
- Hunter N, Borts RH. Mlh1 is unique among mismatch repair proteins in its ability to promote crossing-over during meiosis. Genes Dev. 1997;11:1573–1582. doi: 10.1101/gad.11.12.1573. [DOI] [PubMed] [Google Scholar]
- Hunter N, Chambers SR, Louis EJ, Borts RH. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 1996;15:1726–1733. [PMC free article] [PubMed] [Google Scholar]
- Hunter N, Kleckner N. The single-end invasion: An asymmetric intermediate at the double strand break to double Holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Hyde H, Davies AA, Benson FE, West SC. Resolution of recombination intermediates by a mammalian activity functionally analogous to Escherichia coli RuvC resolvase. J Biol Chem. 1994;269:5202–5209. [PubMed] [Google Scholar]
- Interthal H, Heyer WD. MUS81 encodes a novel helix-hairpin-helix protein involved in the response to UV- and methylation-induced DNA damage in Saccharomyces cerevisiae. Mol Gen Genet. 2000;263:812–827. doi: 10.1007/s004380000241. [DOI] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Van Komen S, Krebs JE, Sung P, Peterson CL. Rad54p is a chromatin remodeling enzyme required for heteroduplex joint formation with chromatin. J Biol Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- Jensen LE, Jauert PA, Kirkpatrick DT. The large loop repair and mismatch repair pathways of Saccharomyces cerevisiae act on distinct substrates during meiosis. Genetics. 2005;170:1033–1043. doi: 10.1534/genetics.104.033670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop L, Rockmill B, Roeder GS, Lichten M. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of Sgs1. Plos Genetics. 2006;2:1402–1412. doi: 10.1371/journal.pgen.0020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, et al. Direct association between the yeast Rad51 and Rad54 recombination proteins. J Biol Chem. 1996;271:33181–33186. doi: 10.1074/jbc.271.52.33181. [DOI] [PubMed] [Google Scholar]
- Jiricny J. The multifaceted mismatch-repair system. Nature Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Kagawa W, et al. Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. Mol Cell. 2002;10:359–371. doi: 10.1016/s1097-2765(02)00587-7. [DOI] [PubMed] [Google Scholar]
- Kaliraman V, Mullen JR, Fricke WM, Bastin-Shanower SA, Brill SJ. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev. 2001;15:2730–2740. doi: 10.1101/gad.932201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantake N, Madiraju M, Sugiyama T, Kowalczykowski C. Escherichia coli RecO protein anneals ssDNA complexed with its cognate ssDNA-binding protein: A common step in genetic recombination. Proc Natl Acad Sci USA. 2002;99:15327–15332. doi: 10.1073/pnas.252633399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow JK, Constantinou A, Li JL, West SC, Hickson ID. The Bloom’s syndrome gene product promotes branch migration of Holliday junctions. Proc Natl Acad Sci USA. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, et al. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Kearney HM, Kirkpatrick DT, Gerton JL, Petes TD. Meiotic recombination involving heterozygous large insertions in Saccharomyces cerevisiae: Formation and repair of large, unpaired DNA loops. Genetics. 2001;158:1457–1476. doi: 10.1093/genetics/158.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Protacio RU, Arora C, Keeney S. Spatial organization and dynamics of the association of Rec102 and Rec104 with meiotic chromosomes. EMBO J. 2004;23:1815–1824. doi: 10.1038/sj.emboj.7600184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Khazanehdari KA, Borts RH. EXO1 and MSH4 differentially affect crossing-over and segregation. Chromosoma. 2000;109:94–102. doi: 10.1007/s004120050416. [DOI] [PubMed] [Google Scholar]
- Kiianitsa K, Solinger JA, Heyer WD. Rad54 protein exerts diverse modes of ATPase activity on duplex DNA partially and fully covered with Rad51 protein. J Biol Chem. 2002;277:46205–46215. doi: 10.1074/jbc.M207967200. [DOI] [PubMed] [Google Scholar]
- Kiianitsa K, Solinger JA, Heyer WD. Terminal association of Rad54 protein with the Rad51-dsDNA filament. Proc Natl Acad Sci USA. 2006;103:9767–9772. doi: 10.1073/pnas.0604240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PM, Paffett KS, Solinger JA, Heyer WD, Nickoloff JA. Spontaneous and double-strand break-induced recombination, and gene conversion tract lengths, are differentially affected by overexpression of wild-type or ATPase-defective yeast Rad54. Nucleic Acids Res. 2002;30:2727–2735. doi: 10.1093/nar/gkf413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DT, Petes TD. Repair of DNA loops involves DNA-mismatch and nucleotide-excision repair proteins. Nature. 1997;387:929–931. doi: 10.1038/43225. [DOI] [PubMed] [Google Scholar]
- Klein HL. RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics. 1997;147:1533–1543. doi: 10.1093/genetics/147.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci L, et al. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature. 2003;423:305–309. doi: 10.1038/nature01577. [DOI] [PubMed] [Google Scholar]
- Krejci L, Song BW, Bussen W, Rothstein R, Mortensen UH, Sung P. Interaction with Rad51 is indispensable for recombination mediator function of Rad52. J Biol Chem. 2002;277:40132–40141. doi: 10.1074/jbc.M206511200. [DOI] [PubMed] [Google Scholar]
- Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separationat metaphase II in females. J Cell Biol. 2007;176:581–592. doi: 10.1083/jcb.200608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao JP, Oh SD, Shinohara A, Hunter N. Rad52 promotes post-invasion steps of meiotic double-strand break repair. Mol Cell. 2008;29:517–524. doi: 10.1016/j.molcel.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, et al. Calcium ion promotes yeast Dmc1 activity via formation of long and fine helical filaments with single-stranded DNA. J Biol Chem. 2005;280:40980–40984. doi: 10.1074/jbc.M505896200. [DOI] [PubMed] [Google Scholar]
- Leem SH, Ropp PA, Sugino A. The yeast Saccharomyces cerevisiae DNA polymerase IV: Possible involvement in double-strand break DNA repair. Nucleic Acids Res. 1994;22:3011–3017. doi: 10.1093/nar/22.15.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol Cell. 2007;28:638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JY, Chua PR, Roeder GS. The meiosis-specific hop2 protein of S. cerevisiae ensures synapsis between homologous chromosomes. Cell. 1998;94:375–386. doi: 10.1016/s0092-8674(00)81480-4. [DOI] [PubMed] [Google Scholar]
- Li J, Hooker GW, Roeder GS. Saccharomyces cerevisiae Mer2, Mei4 and Rec114 form a complex required for meiotic double-strand break formation. Genetics. 2006;173:1969–1981. doi: 10.1534/genetics.106.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Rad51 and Rad54 ATPase activities are both required to modulate Rad51-dsDNA filament dynamics. Nucleic Acids Res. 2007;35:4124–4140. doi: 10.1093/nar/gkm412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZF, Golub EI, Gupta R, Radding CM. Recombination activities of HsDmc1 protein, the meiotic human homolog of RecA protein. Proc Natl Acad Sci USA. 1997;94:11221–11226. doi: 10.1073/pnas.94.21.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: Spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Liu YL, Masson JY, Shah R, O’Regan P, West SC. RAD51C is required for Holliday junction processing in mammalian cells. Science. 2004;303:243–246. doi: 10.1126/science.1093037. [DOI] [PubMed] [Google Scholar]
- Liu YL, Tarsounas M, O’Regan P, West SC. Role of RAD51C and XRCC3 in genetic recombination and DNA repair. J Biol Chem. 2007;282:1973–1979. doi: 10.1074/jbc.M609066200. [DOI] [PubMed] [Google Scholar]
- Lydall D, Nikolsky Y, Bishop DK, Weinert T. A Meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature. 1996;383:840–843. doi: 10.1038/383840a0. [DOI] [PubMed] [Google Scholar]
- Madrona AY, Wilson DK. The structure of Ski8p, a protein regulating mRNA degradation: Implications for WD protein structure. Protein Sci. 2004;13:1557–1565. doi: 10.1110/ps.04704704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Ross L, Dawson D, Hoekstra MF, Haber JE. Meiotic recombination initiated by a double-strand break in rad50Δ yeast cells otherwise unable to initiate meiotic recombination. Genetics. 1996;143:741–754. doi: 10.1093/genetics/143.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloisel L, Bhargava J, Roeder GS. A role for DNA polymerase in gene conversion and crossing over during meiosis in Saccharomyces cerevisiae. Genetics. 2004;167:1133–1142. doi: 10.1534/genetics.104.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri HW, Craig TJ, Morgan A. SGS1 is a multicopy suppressor of srs2: Functional overlap between DNA helicases. Nucleic Acids Res. 2002;30:1103–1113. doi: 10.1093/nar/30.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V, et al. Sws1 is a conserved regulator of homologous recombination in eukaryotic cells. EMBO J. 2006;25:2564–2574. doi: 10.1038/sj.emboj.7601141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazin AV, Alexeev AA, Kowalczykowski SC. A novel function of Rad54 protein –Stabilization of the Rad51 nucleoprotein filament. J Biol Chem. 2003;278:14029–14036. doi: 10.1074/jbc.M212779200. [DOI] [PubMed] [Google Scholar]
- Mazin AV, Bornarth CJ, Solinger JA, Heyer W-D, Kowalczykowski SC. Rad54 protein is targeted to pairing loci by the Rad51 nucleoprotein filament. Mol Cell. 2000a;6:583–592. doi: 10.1016/s1097-2765(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Mazin AV, Zaitseva E, Sung P, Kowalczykowski SC. Tailed duplex DNA is the preferred substrate for Rad51 protein-mediated homologous pairing. EMBO J. 2000b;19:1148–1156. doi: 10.1093/emboj/19.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazina OM, Mazin AV, Nakagawa T, Kolodner RD, Kowalczykowski SC. Saccharomyces cerevisiae MER3 helicase stimulates 3′-5′ Heteroduplex extension by Rad51: Implications for crossover control in meiotic recombination. Cell. 2004;117:47–56. doi: 10.1016/s0092-8674(04)00294-6. [DOI] [PubMed] [Google Scholar]
- McIlwraith MJ, Vaisman A, Liu YL, Fanning E, Woodgate R, West SC. Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- McKee AHZ, Kleckner N. A general method for identifying recessive diploid-specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics. 1997a;146:797–816. doi: 10.1093/genetics/146.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AHZ, Kleckner N. Mutations in Saccharomyces cerevisiae that block meiotic prophase chromosome metabolism and confer cell cycle arrest at pachytene identify two new meiosis-specific genes SAE1 and SAE3. Genetics. 1997b;146:817–834. doi: 10.1093/genetics/146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M, Adams M, Staeva-Vieira E, Sekelsky JJ. Evidence for multiple cycles of strand invasion during repair of double-strand gaps in Drosophila. Genetics. 2004a;167:699–705. doi: 10.1534/genetics.103.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M, LaRocque JR, Adams MD, Sekelsky JJ. Formation of deletions during double-strand break repair in Drosophila DmBlm mutants occurs after strand invasion. Proc Natl Acad Sci USA. 2004b;101:15694–15699. doi: 10.1073/pnas.0406157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281:30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Ferguson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Herrero F, de Jager M, Dekker NH, Kanaar R, Wyman C, Dekker C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature. 2005;437:440–443. doi: 10.1038/nature03927. [DOI] [PubMed] [Google Scholar]
- Morimatsu K, Kowalczykowski SC. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: A universal step of recombinational repair. Mol Cell. 2003;11:1337–1347. doi: 10.1016/s1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Mortensen UH, Bendixen C, Sunjevaric I, Rothstein R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc Natl Acad Sci USA. 1996;93:10729–10734. doi: 10.1073/pnas.93.20.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. The mechanism of crossing over. Am Nat. 1916;50:193–221. [Google Scholar]
- Muller HJ. The mechanism of crossing over. Am Nat. 1916;50:284–305. [Google Scholar]
- Muller HJ. The mechanism of crossing over. Am Nat. 1916;50:350–366. [Google Scholar]
- Muller HJ. The mechanism of crossing over. Am Nat. 1916;50:421–434. [Google Scholar]
- Munz P. An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics. 1994;137:701–707. doi: 10.1093/genetics/137.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K, Datta A, Chen C, Kolodner RD. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat Genet. 2001;27:113–116. doi: 10.1038/83673. [DOI] [PubMed] [Google Scholar]
- Nairz K, Klein F. mre11S - A yeast mutation that blocks double-strand break processing and permits nonhomologous synapsis in meiosis. Genes Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Flores-Rozas H, Kolodner RD. The MER3 helicase involved in meiotic crossing over is stimulated by single-stranded DNA-binding proteins and unwinds DNA in the 3′ to 5′ direction. J Biol Chem. 2001;276:31487–31493. doi: 10.1074/jbc.M104003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kolodner RD. Saccharomyces cerevisiae Mer3 is a DNA helicase involved in meiotic crossing over. Mol Cell Biol. 2002a;22:3281–3291. doi: 10.1128/MCB.22.10.3281-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kolodner RD. The MER3 DNA helicase catalyzes the unwinding of Holliday junctions. J Biol Chem. 2002b;277:28019–28024. doi: 10.1074/jbc.M204165200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ogawa H. Involvement of the MRE2 gene of yeast in formation of meiosis-specific double-strand breaks and crossover recombination through RNA splicing. Genes Cells. 1997;2:65–79. doi: 10.1046/j.1365-2443.1997.d01-283.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ogawa H. The Saccharomyces cerevisiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis. EMBO J. 1999;18:5714–5723. doi: 10.1093/emboj/18.20.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namsaraev EA, Berg P. Branch migration during Rad51-promoted strand exchange proceeds in either direction. Proc Natl Acad Sci USA. 1998;95:10477–10481. doi: 10.1073/pnas.95.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth KA. Molecular genetics of yeast mating type. Annu Rev Genet. 1982;16:439–500. doi: 10.1146/annurev.ge.16.120182.002255. [DOI] [PubMed] [Google Scholar]
- Nassif N, Penney J, Pal S, Engels WR, Gloor GB. Efficient copying of nonhomolgous sequences from ectopic sites via P-element-induced gap repair. Mol Cell Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Keeney S. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature. 2006;442:153–158. doi: 10.1038/nature04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Ramachandran M, Trelles-Sticken E, Scherthan H, Goldman ASH. Wild-type levels of Spo11-induced DSBs are required for normal single-strand resection during meiosis. Mol Cell. 2002;9:835–846. doi: 10.1016/s1097-2765(02)00498-7. [DOI] [PubMed] [Google Scholar]
- New JH, Sugiyama T, Zaitseva E, Kowalczykowski SC. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, et al. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Amitani I, Baskin RJ, Kowalczykowski SC. Single-molecule imaging of Tid1/Rdh54, a Rad54 homolog that translocates on duplex DNA and can disrupt joint molecules. J Biol Chem. 2007;282:30776–30784. doi: 10.1074/jbc.M704767200. [DOI] [PubMed] [Google Scholar]
- Nishino T, Komori K, Ishino Y, Morikawa K. X-ray and biochemical anatomy of an archaeal XPF/Rad1/Mus81 family nuclease: Similarity between its endonuclease domain and restriction enzymes. Structure. 2003;11:445–457. doi: 10.1016/s0969-2126(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Niu HY, Wan L, Baumgartner B, Schaefer D, Loidl J, Hollingsworth NM. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol Cell Biol. 2005;16:5804–5818. doi: 10.1091/mbc.E05-05-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Yu X, Shinohara A, Egelman EH. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science. 1993;259:1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- Oh SD, Lao JP, Hwang PYH, Taylor AF, Smith GR, Hunter N. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell. 2007;130:259–272. doi: 10.1016/j.cell.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T, Szostak J, Rothstein R. Yeast transformation: A model system for the study of recombination. Proc Natl Acad Sci USA. 1981;78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver TL, Szostak JW. Fungal recombination. Microbiol Rev. 1985;49:33–85. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orren DK, Theodore S, Machwe A. The Werner syndrome helicase/exonuclease (WRN) disrupts and degrades D-loops in vitro. Biochemistry. 2002;41:13483–13488. doi: 10.1021/bi0266986. [DOI] [PubMed] [Google Scholar]
- Osman F, Dixon J, Doe CL, Whitby MC. Generating crossovers by resolution of nicked Holliday junctions: A role of Mus81-Eme1 in meiosis. Mol Cell. 2003;12:761–774. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin MJ, Kadonaga JT. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell. 1997;88:737–740. doi: 10.1016/s0092-8674(00)81918-2. [DOI] [PubMed] [Google Scholar]
- Penkner A, et al. A conserved function for a Caenorhabditis elegans Com1/Sae2/CtIP protein homolog in meiotic recombination. Embo J. 2007;26:5071–5082. doi: 10.1038/sj.emboj.7601916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova G, Stratton S, Sung P. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature. 1998;393:91–94. doi: 10.1038/30037. [DOI] [PubMed] [Google Scholar]
- Petukhova G, Stratton SA, Sung P. Single strand DNA binding and annealing activities in the yeast recombination factor Rad59. J Biol Chem. 1999a;274:33839–33842. doi: 10.1074/jbc.274.48.33839. [DOI] [PubMed] [Google Scholar]
- Petukhova G, Sung P, Klein H. Promotion of Rad51-dependent D-loop formation by yeast recombination factor Rdh54/Tid1. Genes Dev. 2000;14:2206–2215. doi: 10.1101/gad.826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova G, Van Komen S, Vergano S, Klein H, Sung P. Yeast Rad54 promotes Rad51-dependent homologous DNA pairing via ATP hydrolysis-driven change in DNA double helix conformation. J Biol Chem. 1999b;274:29453–29462. doi: 10.1074/jbc.274.41.29453. [DOI] [PubMed] [Google Scholar]
- Petukhova GV, Pezza RJ, Vanevski F, Ploquin M, Masson JY, Camerini-Otero RD. The Hop2 and Mnd1 proteins act in concert with Rad51 and Dmc1 in meiotic recombination. Nature Struct Mol Biol. 2005;12:449–453. doi: 10.1038/nsmb923. [DOI] [PubMed] [Google Scholar]
- Pezza RJ, Petukhova GV, Ghirlando R, Camerini-Otero RD. Molecular activities of meiosis-specific proteins Hop2, Mnd1, and the Hop2-Mnd1 complex. J Biol Chem. 2006;281:18426–18434. doi: 10.1074/jbc.M601073200. [DOI] [PubMed] [Google Scholar]
- Pezza RJ, Voloshin ON, Vanevski F, Camerini-Otero RD. Hop2/Mnd1 acts on two critical steps in Dmc1-promoted homologous pairing. Genes Dev. 2007;21:1758–1766. doi: 10.1101/gad.1562907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank JL, Wu JH, Hsieh TS. Topoisomerase III alpha and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Natl Acad Sci USA. 2006;103:11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploquin M, et al. Stimulation of fission yeast and mouse Hop2-Mnd1 of the Dmc1 and Rad51 recombinases. Nucleic Acids Res. 2007;35:2719–2733. doi: 10.1093/nar/gkm174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Robertson RB, Visnapuu ML, Chi P, Sung P, Greene EC. A DNA-translocating Snf2 molecular motor: Saccharomyces cerevisiae Rdh54 displays proces-sive translocation and extrudes DNA loops. J Mol Biol. 2007;369:940–953. doi: 10.1016/j.jmb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz S, Amon A, Klein F. Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics. 1997;146:781–795. doi: 10.1093/genetics/146.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puizina J, Siroky J, Mokros P, Schweizer D, Riha K. Mre11 deficiency in Arabidop-sis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell. 2004;16:1968–1978. doi: 10.1105/tpc.104.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford SJ, Goley E, Baxter K, McMahan S, Sekelsky J. Drosophila ERCC1 is required for a subset of MEI-9-dependent meiotic crossovers. Genetics. 2005;170:1737–1745. doi: 10.1534/genetics.104.036178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford SJ, McMahan S, Blanton HL, Sekelsky J. Heteroduplex DNA in meiotic recombination in Drosophila mei-9 mutants. Genetics. 2007;176:63–72. doi: 10.1534/genetics.107.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray AJ, Strathern JN. Error-prone DNA polymerases: When making a mistake is the only way to get ahead. Annu Rev Genet. 2003;37:31–66. doi: 10.1146/annurev.genet.37.042203.132748. [DOI] [PubMed] [Google Scholar]
- Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J Biol Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- Resnick MA. The repair of double-strand breaks in DNA: A model involving recombination. J Theoret Biol. 1976;59:97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Resnick MA, Nitiss J, Edwards C, Malone RE. Meiosis can induce recombination in rad52 mutants of Saccharomyces cerevisiae. Genetics. 1986;113:531–550. doi: 10.1093/genetics/113.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkers T, et al. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol Cell Biol. 1998;18:6423–6429. doi: 10.1128/mcb.18.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B, Fung JC, Branda SS, Roeder GS. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol. 2003;13:1954–1962. doi: 10.1016/j.cub.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Rockmill B, Roeder GS. The yeast med1 mutant undergoes both meiotic homolog nondisjunction and precocious separation of sister chromatids. Genetics. 1994;136:65–74. doi: 10.1093/genetics/136.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B, Sym M, Scherthan H, Roeder GS. Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes Dev. 1995;9:2684–2695. doi: 10.1101/gad.9.21.2684. [DOI] [PubMed] [Google Scholar]
- Roeder GS. Meiotic chromosomes: It takes two to tango. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- Ross-Macdonald P, Roeder GS. Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell. 1994;79:1069–1080. doi: 10.1016/0092-8674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Sartori AA, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau S, Stasiak AZ, Banville I, Ploquin M, Stasiak A, Masson JY. Fission yeast Rad51 and Dmc1, two efficient DNA recombinases forming helical nucleoprotein filaments. Mol Cell Biol. 2005;25:4377–4387. doi: 10.1128/MCB.25.11.4377-4387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield MJ, Hsieh P. DNA mismatch repair: Molecular mechanisms and biological function. Annu Rev Micro. 2003;57:579–608. doi: 10.1146/annurev.micro.57.030502.090847. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: Meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Seet BT, Dikic I, Zhou MM, Pawson T. Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol. 2006;7:473–483. doi: 10.1038/nrm1960. [DOI] [PubMed] [Google Scholar]
- Sehorn MG, Sigurdsson S, Bussen W, Unger VM, Sung P. Human meiotic recombinase Dmc1 promotes ATP-dependent homologous DNA strand exchange. Nature. 2004;429:433–437. doi: 10.1038/nature02563. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Brodsky MH, Burtis KC. DNA repair in Drosophila: Insights from the Drosophila genome sequence. J Cell Biol. 2000a;150:F31–F36. doi: 10.1083/jcb.150.2.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky JJ, Hollis KJ, Eimerl AI, Burtis KC, Hawley RS. Nucleotide excision repair endonuclease genes in Drosophila melanogaster. Mutat Res. 2000b;459:219–228. doi: 10.1016/s0921-8777(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Mckim KS, Chin GM, Hawley RS. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics. 1995;141:619–627. doi: 10.1093/genetics/141.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Suzuki M, Shinohara M. Isolation and characterization of novel xrs2 mutations in Saccharomyces cerevisiae. Genetics. 2005;170:71–85. doi: 10.1534/genetics.104.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, et al. Purification and characterization of a new DNA polymerase from budding yeast Saccharomyces cerevisiae – A probable homolog of mammalian DNA polymerase beta. J Biol Chem. 1993;268:27148–27153. [PubMed] [Google Scholar]
- Shinohara A, Gasior S, Ogawa T, Kleckner N, Bishop DK. Saccharomyces cerevisiae recA homologues RAD51 and DMC1 have both distinct and overlapping roles in meiotic recombination. Genes Cells. 1997a;2:615–629. doi: 10.1046/j.1365-2443.1997.1480347.x. [DOI] [PubMed] [Google Scholar]
- Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Shinohara A, Ogawa T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature. 1998;391:404–407. doi: 10.1038/34943. [DOI] [PubMed] [Google Scholar]
- Shinohara A, Shinohara M, Ohta T, Matsuda S, Ogawa T. Rad52 forms ring structures and co-operates with RPA in single-strand annealing. Genes Cell. 1998;3:145–156. doi: 10.1046/j.1365-2443.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Gasior SL, Bishop DK, Shinohara A. Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc Natl Acad Sci USA. 2000;97:10814–10819. doi: 10.1073/pnas.97.20.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Sakai K, Shinohara A, Bishop DY. Crossover interference in Saccharomyces cerevisiae requires a TID1/RDH54- and DMC1-dependent pathway. Genetics. 2003;163:1273–1286. doi: 10.1093/genetics/163.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Shita-Yamaguchi E, Buerstedde JM, Shinagawa H, Ogawa H, Shinohara A. Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics. 1997b;147:1545–1556. doi: 10.1093/genetics/147.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E, Weinstein J, Rothstein R. Genetic screen for top3 suppressors in Saccharomyces cerevisiae identifies SHU1, SHU2, PSY3 and CSM2: Four genes involved in error-free DNA repair. Genetics. 2005;169:1275–1289. doi: 10.1534/genetics.104.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaud N, Dray E, Gy I, Gerard E, Takvorian N, Doutriaux MP. Brca2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with Dmc1. EMBO J. 2004;23:1392–1401. doi: 10.1038/sj.emboj.7600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Van Komen S, Petukhova G, Sung P. Homologous DNA pairing by human recombination factors Rad51 and Rad54. J Biol Chem. 2002;277:42790–42794. doi: 10.1074/jbc.M208004200. [DOI] [PubMed] [Google Scholar]
- Singleton MR, Wentzell LM, Liu YL, West SC, Wigley DB. Structure of the single-strand annealing domain of human RAD52 protein. Proc Natl Acad Sci USA. 2002;99:13492–13497. doi: 10.1073/pnas.212449899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GR, Boddy MN, Shanahan P, Russell P. Fission yeast Mus81Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics. 2003;165:2289–2293. doi: 10.1093/genetics/165.4.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden T, Acharya S, Butz C, Berardini M, Fishel R. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell. 2004;15:437–451. doi: 10.1016/j.molcel.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Solinger JA, Heyer W-D. Rad54 protein stimulates the postsynaptic phase of Rad51 protein-mediated DNA strand exchange. Proc Natl Acad Sci USA. 2001;98:8447–8453. doi: 10.1073/pnas.121009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Kiianitsa K, Heyer W-D. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol Cell. 2002;10:1175–1188. doi: 10.1016/s1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- Solinger JA, Lutz G, Sugiyama T, Kowalczykowski SC, Heyer W-D. Rad54 protein stimulates heteroduplex DNA formation in the synaptic phase of DNA strand exchange via specific interactions with the presynaptic Rad51 nucleoprotein filament. J Mol Biol. 2001;307:1207–1221. doi: 10.1006/jmbi.2001.4555. [DOI] [PubMed] [Google Scholar]
- Sprouse RO, Brenowitz M, Auble DT. Snf2/Swi2-related ATPase Mot1 drives displacement of TATA-binding protein by gripping DNA. EMBO J. 2006;25:1492–1504. doi: 10.1038/sj.emboj.7601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl FW, Foss HM, Young LS, Borts RH, Abdullah MFF, Copenhaver GP. Does crossover interference count in Saccharomyces cerevisiae? Genetics. 2004;168:35–48. doi: 10.1534/genetics.104.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak AZ, et al. The human Rad52 protein exists as a heptameric ring. Curr Biol. 2000;10:337–340. doi: 10.1016/s0960-9822(00)00385-7. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Theunissen JWF, Morales M, Petrini JHJ. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair. 2004;3:845–854. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH. The behavior of the chromosomes as studied through linkage. Z Indukt Abstammungs Vererbungsl. 1915;13:234–287. [Google Scholar]
- Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc Natl Acad Sci USA. 2004;101:9315–9320. doi: 10.1073/pnas.0305749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Wang X, Haber JE. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol Cell. 2003;12:209–219. doi: 10.1016/s1097-2765(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kantake N, Wu Y, Kowalczykowski SC. Rad52-mediated DNA annealing after Rad51-mediated DNA strand exchange promotes second ssDNA capture. EMBO J. 2006;25:5539–5548. doi: 10.1038/sj.emboj.7601412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kowalczykowski SC. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J Biol Chem. 2002;277:31663–31672. doi: 10.1074/jbc.M203494200. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, New JH, Kowalczykowski SC. DNA annealing by Rad52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc Natl Acad Sci USA. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Zaitseva EM, Kowalczykowski SC. A single-stranded DNA-binding protein is needed for efficient presynaptic complex formation by the Saccharomyces cerevisiae Rad51 protein. J Biol Chem. 1997;272:7940–7945. doi: 10.1074/jbc.272.12.7940. [DOI] [PubMed] [Google Scholar]
- Sun H, Treco D, Szostak JW. Extensive 3′-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994;265:1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J Biol Chem. 1997a;272:28194–28197. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- Sung P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 1997b;11:1111–1121. doi: 10.1101/gad.11.9.1111. [DOI] [PubMed] [Google Scholar]
- Symington LS, Heyer WD. Some disassembly required: Role of DNA translocases in the disruption of recombination intermediates and dead-end complexes. Genes Dev. 2006;20:2479–2486. doi: 10.1101/gad.1477106. [DOI] [PubMed] [Google Scholar]
- Szankasi P, Smith GR. A DNA exonuclease induced during meiosis of Schizosaccharomyces pombe. J Biol Chem. 1992;267:3014–3023. [PubMed] [Google Scholar]
- Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Tan TLR, Kanaar R, Wyman C. Rad54, a Jack of all trades in homologous recombination. DNA Repair. 2003;2:787–794. doi: 10.1016/s1568-7864(03)00070-3. [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Davies D, West SC. BRCA2-dependent and independent formation of RAD51 nuclear foci. Oncogene. 2003;22:1115–1123. doi: 10.1038/sj.onc.1206263. [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Morita T, Pearlman RE, Moens PB. RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol. 1999;147:207–219. doi: 10.1083/jcb.147.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa M, et al. Meiotic recombination-related DNA synthesis and its implications for cross-over and non-cross-over recombinant formation. Proc Natl Acad Sci USA. 2007;104:5965–5970. doi: 10.1073/pnas.0611490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund T, Esashi F, West SC. Interactions between human Brca2 protein and the meiosis-specific recombinase Dmc1. EMBO J. 2007;26:2915–2922. doi: 10.1038/sj.emboj.7601739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Erdeniz N, Symington LS, Liskay RM. EX01 - A multi-tasking eukaryotic nuclease. DNA Repair. 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Trowbridge K, McKim K, Brill SJ, Sekelsky J. Synthetic lethality of Drosophila in the absence of the MUS81 endonuclease and the DmBlm helicase is associated with elevated apoptosis. Genetics. 2007;176:1993–2001. doi: 10.1534/genetics.106.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KM, Yuan SSF, Lee E, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- Tsodikov OV, Enzlin JH, Scharer OD, Ellenberger T. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc Natl Acad Sci USA. 2005;102:11236–11241. doi: 10.1073/pnas.0504341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Ogawa H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol Cell Biol. 1998;18:260–268. doi: 10.1128/mcb.18.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Ogawa H. Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:2221–2233. doi: 10.1091/mbc.11.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Roeder GS. The Mndl protein forms a complex with Hop2 to promote homologous chromosome pairing and meiotic double-strand break. Mol Cell Biol. 2002;22:3078–3088. doi: 10.1128/MCB.22.9.3078-3088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Roeder GS. The importance of genetic recombination for fidelity of chromosome pairing in meiosis. Dev Cell. 2003;5:915–925. doi: 10.1016/s1534-5807(03)00357-5. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H, Roeder GS. The budding yeast mei5 and sae3 proteins act together with dmc1 during meiotic recombination. Genetics. 2004;168:1219–1230. doi: 10.1534/genetics.103.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Roeder GS. Budding yeast Hed1 down-regulates the mitotic recombination machinery when meiotic recombination is impaired. Genes Dev. 2006;20:1766–1775. doi: 10.1101/gad.1422506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uanschou C, et al. A novel plant gene essential for meiosis is related to the human CtIP and the yeast COM1/SAE2 gene. Embo J. 2007;26:5061–5070. doi: 10.1038/sj.emboj.7601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Ohta T, Oshiumi H, Tomizawa J-I, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- Van Brabant AJ, Ye T, Sanz M, German JL, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- Van Komen S, Petukhova G, Sigurdsson S, Stratton S, Sung P. Superhelicity-driven homologous DNA pairing by yeast recombination factors Rad51 and Rad54. Mol Cell. 2000;6:563–572. doi: 10.1016/s1097-2765(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- Villeneuve AM, Hillers KJ. Whence meiosis? Cell. 2001;106:647–650. doi: 10.1016/s0092-8674(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Wan LH, de los Santos T, Zhang C, Shokat K, Hollingsworth NM. Mek1 kinase activity functions downstream of RED1 in the regulation of meiotic double strand break repair in budding yeast. Mol Biol Cell. 2004;15:11–23. doi: 10.1091/mbc.E03-07-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TF, Kung WM. Supercomplex formation between Mlh1-Mlh3 and Sgs1-Top3 heterocomplexes in meiotic yeast cells. Biochem Biophys Res Commun. 2002;296:949–953. doi: 10.1016/s0006-291x(02)02034-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yokobayashi S, Yamamoto M, Nurse P. Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature. 2001;409:359–363. doi: 10.1038/35053103. [DOI] [PubMed] [Google Scholar]
- Wei KC, et al. Inactivation of exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SC. Holliday junctions cleaved by Rad1? Nature. 1995;373:27–28. doi: 10.1038/373027a0. [DOI] [PubMed] [Google Scholar]
- West SC. Processing of recombination intermediates by the RuvABC proteins. Annu Rev Genet. 1997;31:213–244. doi: 10.1146/annurev.genet.31.1.213. [DOI] [PubMed] [Google Scholar]
- Whitby MC, Osman F, Dixon J. Cleavage of model replication forks by fission yeast Mus81-Eme1 and budding yeast Mus81-Mms4. J Biol Chem. 2003;278:6928–6935. doi: 10.1074/jbc.M210006200. [DOI] [PubMed] [Google Scholar]
- Wiltzius JJW, Hohl M, Fleming JC, Petrini JHJ. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nature Struct Mol Biol. 2005;12:403–407. doi: 10.1038/nsmb928. [DOI] [PubMed] [Google Scholar]
- Wold MS. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- Wolner B, Peterson CL. ATP-dependent and ATP-independent roles for the Rad54 chromatin remodeling enzyme during recombinational repair of a DNA double strand break. J Biol Chem. 2005;280:10855–10860. doi: 10.1074/jbc.M414388200. [DOI] [PubMed] [Google Scholar]
- Wolner B, van Komen S, Sung P, Peterson CL. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol Cell. 2003;12:221–232. doi: 10.1016/s1097-2765(03)00242-9. [DOI] [PubMed] [Google Scholar]
- Worth L, Clark S, Radman M, Modrich P. Mismatch repair proteins MutS and MutL inhibit RecA-catalyzed strand transfer between diverged DNAs. Proc Natl Acad Sci USA. 1994;91:3238–3241. doi: 10.1073/pnas.91.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc Natl Acad Sci USA. 2006a;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Hickson ID. The Bloom’s syndrome helicase suppresses crossing-over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sugiyama T, Kowalczykowski SC. DNA annealing mediated by Rad52 and Rad59 proteins. J Biol Chem. 2006b;281:15441–15449. doi: 10.1074/jbc.M601827200. [DOI] [PubMed] [Google Scholar]
- Xu L, Marians KJ. A dynamic RecA filament permits DNA polymerase-catalyzed extension of the invading strand in recombination intermediates. J Biol Chem. 2002;277:14321–14328. doi: 10.1074/jbc.M112418200. [DOI] [PubMed] [Google Scholar]
- Yang HJ, et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002;297:1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Li QB, Fan J, Holloman WK, Pavletich NP. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA–ssDNA junction. Nature. 2005;433:653–657. doi: 10.1038/nature03234. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Kearney H, Kramer BC, Sekelsky JJ. Mutational analysis of the Drosophila DNA repair and recombination gene mei-9. Genetics. 2004;167:263–273. doi: 10.1534/genetics.167.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O, Majumder S, Kramer B, Sekelsky JJ. Drosophila MUS312 interacts with the nucleotide excision repair endonuclease MEI-9 to generate meiotic crossovers. Mol Cell. 2002;10:1503–1509. doi: 10.1016/s1097-2765(02)00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Hyppa RW, Smith GR. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics. 2004;167:593–605. doi: 10.1534/genetics.103.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Schreckhise RW, Steiner WW, Smith GR. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol Cell. 2002;9:253–263. doi: 10.1016/s1097-2765(02)00452-5. [DOI] [PubMed] [Google Scholar]
- Yu X, Jacobs SA, West SC, Ogawa T, Egelman EH. Domain structure and dynamics in the helical filaments formed by RecA and Rad51 on DNA. Proc Natl Acad Sci USA. 2001;98:8419–8425. doi: 10.1073/pnas.111005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva EM, Zaitsev EN, Kowalczykowski SC. The DNA binding properties of Saccharomyces cerevisiae Rad51 protein. J Biol Chem. 1999;274:2907–2915. doi: 10.1074/jbc.274.5.2907. [DOI] [PubMed] [Google Scholar]
- Zalevsky J, MacQueen AJ, Duffy JB, Kemphues KJ, Villeneuve AM. Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics. 1999;153:1271–1283. doi: 10.1093/genetics/153.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fan HY, Goldman JA, Kingston RE. Homology-driven chromatin remodeling by human RAD54. Nat Struct Mol Biol. 2007;14:397–405. doi: 10.1038/nsmb1223. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. Meiotic chromosomes: Integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- Zierhut C, Berlinger M, Rupp C, Shinohara A, Klein F. Mnd1 is required for meiotic interhomolog repair. Curr Biol. 2004;14:752–762. doi: 10.1016/j.cub.2004.04.030. [DOI] [PubMed] [Google Scholar]