Abstract
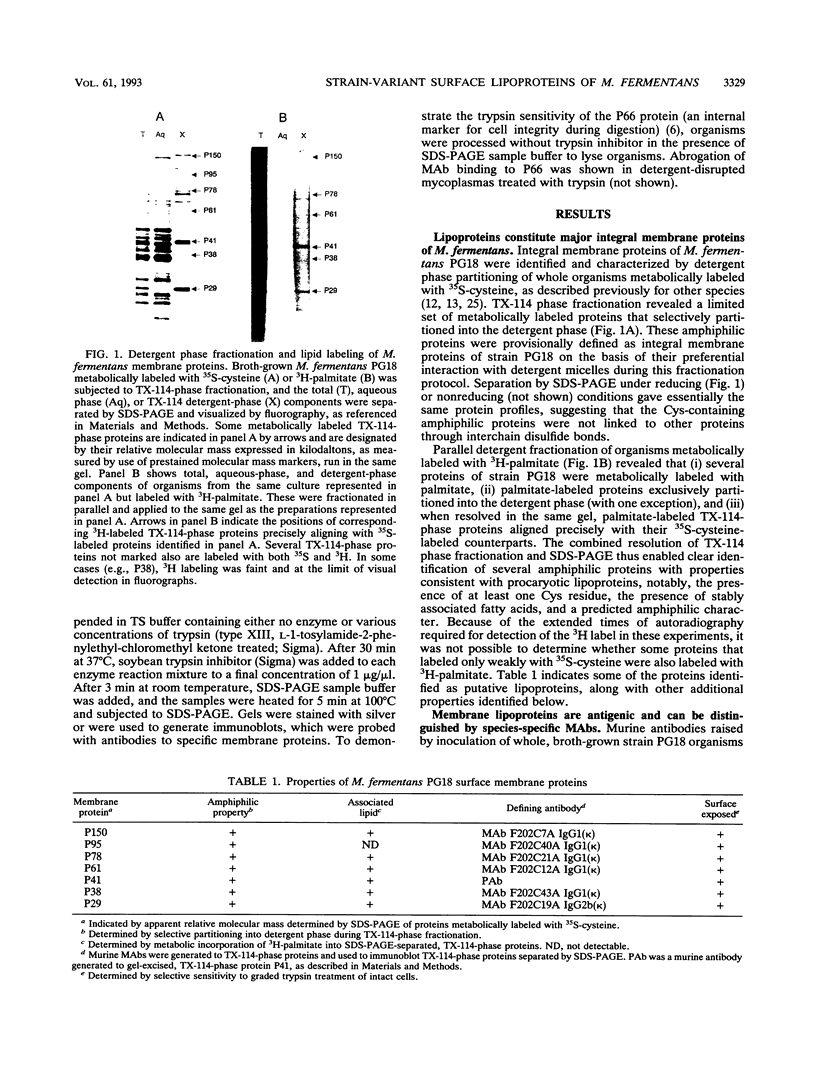
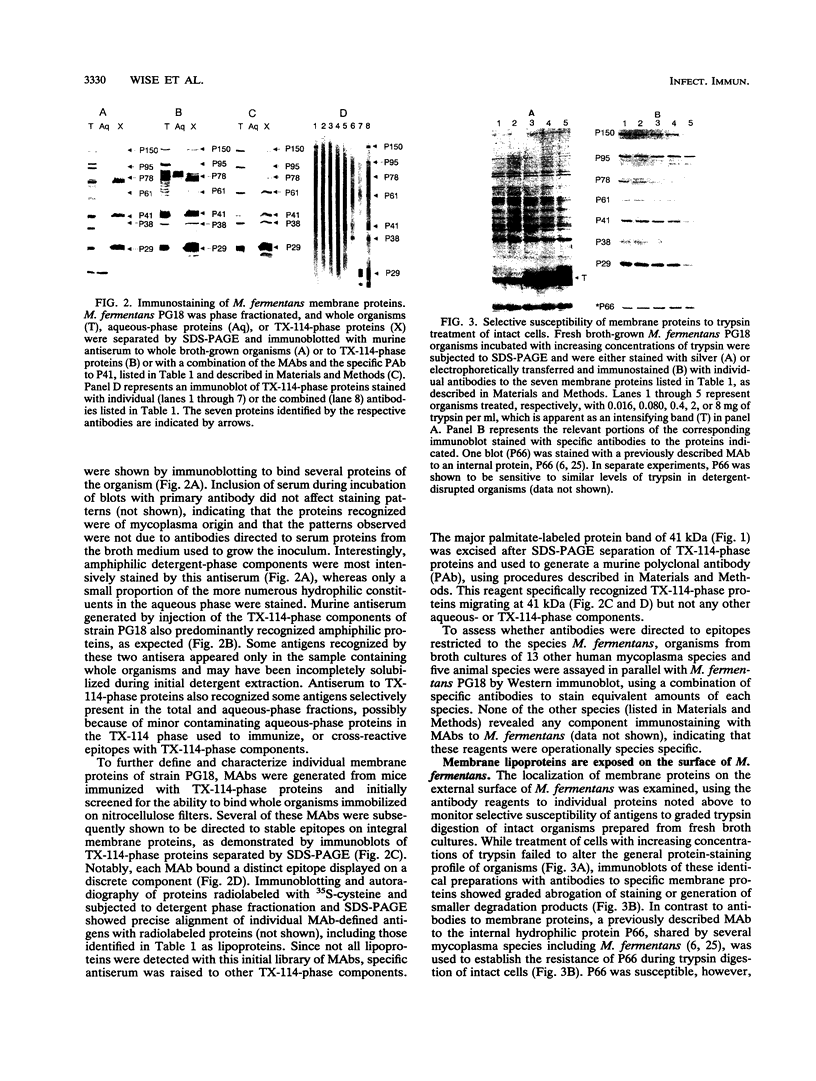
The wall-less procaryote Mycoplasma fermentans is currently being examined as an agent potentially associated with human disease, including infectious processes affecting immunocompromised individuals. To delineate and understand the interactions of M. fermentans with its host, specific membrane surface components were characterized as markers for detecting the organism and for assessing heterogeneity in antigenic surface architecture within this mycoplasma species. Detergent phase fractionation of metabolically labeled organisms of type strain PG18 identified a family of prominent integral membrane proteins; several of these labeled with 35S-cysteine and 3H-palmitate, which are characteristics of procaryotic lipoproteins. Specific monoclonal and polyclonal antibodies raised to strain PG18 components further distinguished seven of these membrane proteins, which were localized on the organism's surface by monitoring their selective susceptibility during trypsin treatment of intact cells. With these antibodies, Western immunoblot profiles of surface membrane antigens expressed on strain PG18 were compared with those expressed on the recently identified Incognitus strain of M. fermentans, as well as with several other human and animal mycoplasma species. While the antibodies were specific for M. fermentans, marked differences were observed between the strains in the size of one surface lipoprotein and in the apparent levels of several antigens expressed in the cultured populations analyzed. Some monoclonal antibodies to strain PG18 and a previously described monoclonal antibody to strain Incognitus showed apparent selectivity for the strain used for immunization. Monoclonal antibodies developed here recognize stable epitopes defining a family of surface lipoproteins and provide critical tools to determine the basis of surface variation in this mycoplasma species and to assess the location and antigenic phenotypes of organisms in the human host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Boyer M. J., Keith J., Watson-McKown R., Wise K. S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988 Feb;56(2):295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. S., Hayes M. M., Wang R. Y., Armstrong D., Kundsin R. B., Lo S. C. Detection and isolation of Mycoplasma fermentans from urine of human immunodeficiency virus type 1-infected patients. Arch Pathol Lab Med. 1993 May;117(5):511–514. [PubMed] [Google Scholar]
- EDWARD D. G., FREUNDT E. A. The classification and nomenclature of organisms of the pleuropneumonia group. J Gen Microbiol. 1956 Feb;14(1):197–207. doi: 10.1099/00221287-14-1-197. [DOI] [PubMed] [Google Scholar]
- Hakkarainen K., Jansson E., Ranki A., Valle S. L., Krohn K. J. Serological responses to mycoplasmas in HIV-infected and non-infected individuals. AIDS. 1992 Nov;6(11):1287–1292. doi: 10.1097/00002030-199211000-00008. [DOI] [PubMed] [Google Scholar]
- Kim M. F., Heidari M. B., Stull S. J., McIntosh M. A., Wise K. S. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae p65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect Immun. 1990 Aug;58(8):2637–2643. doi: 10.1128/iai.58.8.2637-2643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. C., Shih J. W., Newton P. B., 3rd, Wong D. M., Hayes M. M., Benish J. R., Wear D. J., Wang R. Y. Virus-like infectious agent (VLIA) is a novel pathogenic mycoplasma: Mycoplasma incognitus. Am J Trop Med Hyg. 1989 Nov;41(5):586–600. doi: 10.4269/ajtmh.1989.41.586. [DOI] [PubMed] [Google Scholar]
- Markham P. F., Glew M. D., Whithear K. G., Walker I. D. Molecular cloning of a member of the gene family that encodes pMGA, a hemagglutinin of Mycoplasma gallisepticum. Infect Immun. 1993 Mar;61(3):903–909. doi: 10.1128/iai.61.3.903-909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOL C. S., EDWARD D. G. Role of organisms of the pleuropneumonia group in human genital infections. Br J Vener Dis. 1953 Sep;29(3):141–150. doi: 10.1136/sti.29.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUITER M., WENTHOLT H. M. Isolation of a pleuropneumonia-like organism from a skin lesion associated with a fusospirochetal flora. J Invest Dermatol. 1955 Jan;24(1):31–34. doi: 10.1038/jid.1955.5. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillard C., Carle P., Bové J. M., Bébéar C., Lo S. C., Shih J. W., Wang R. Y., Rose D. L., Tully J. G. Genetic and serologic relatedness between Mycoplasma fermentans strains and a mycoplasma recently identified in tissues of AIDS and non-AIDS patients. Res Virol. 1990 May-Jun;141(3):385–395. doi: 10.1016/0923-2516(90)90010-g. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Sasaki Y., Kita M., Suzuki K., Watanabe H., Honda M. Evidence that Lo's mycoplasma (Mycoplasma fermentans incognitus) is not a unique strain among Mycoplasma fermentans strains. J Clin Microbiol. 1992 Sep;30(9):2435–2440. doi: 10.1128/jcm.30.9.2435-2440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Städtlander C. T., Zuhua C., Watson H. L., Cassell G. H. Protein and antigen heterogeneity among strains of Mycoplasma fermentans. Infect Immun. 1991 Sep;59(9):3319–3322. doi: 10.1128/iai.59.9.3319-3322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Shih J. W., Grandinetti T., Pierce P. F., Hayes M. M., Wear D. J., Alter H. J., Lo S. C. High frequency of antibodies to Mycoplasma penetrans in HIV-infected patients. Lancet. 1992 Nov 28;340(8831):1312–1316. doi: 10.1016/0140-6736(92)92493-y. [DOI] [PubMed] [Google Scholar]
- Wise K. S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993 May;1(2):59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yogev D., Rosengarten R., Watson-McKown R., Wise K. S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5' regulatory sequences. EMBO J. 1991 Dec;10(13):4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






