Abstract
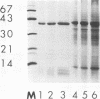
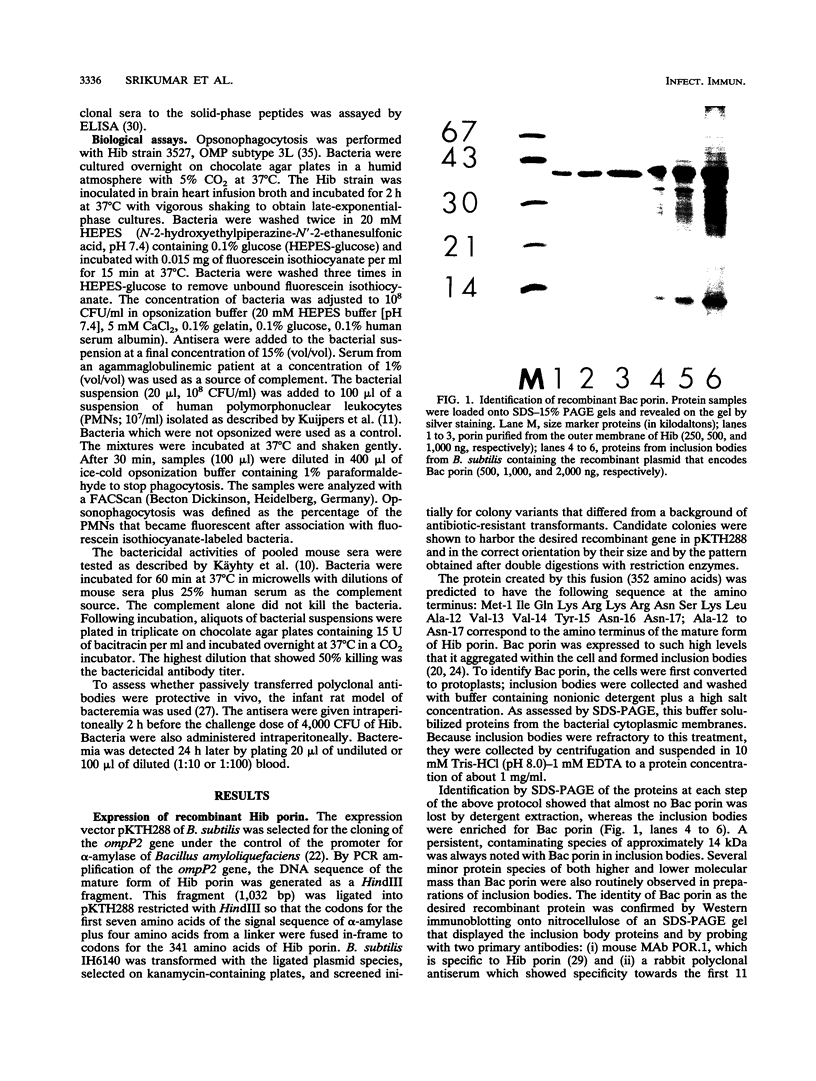
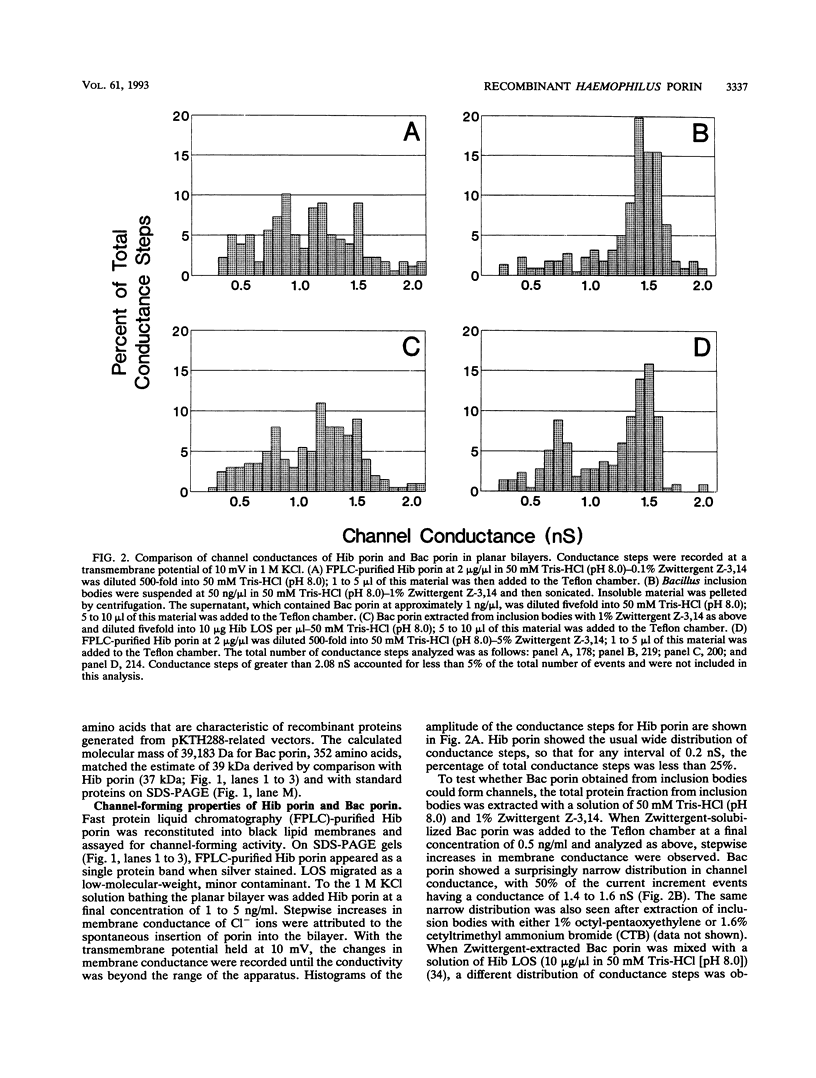
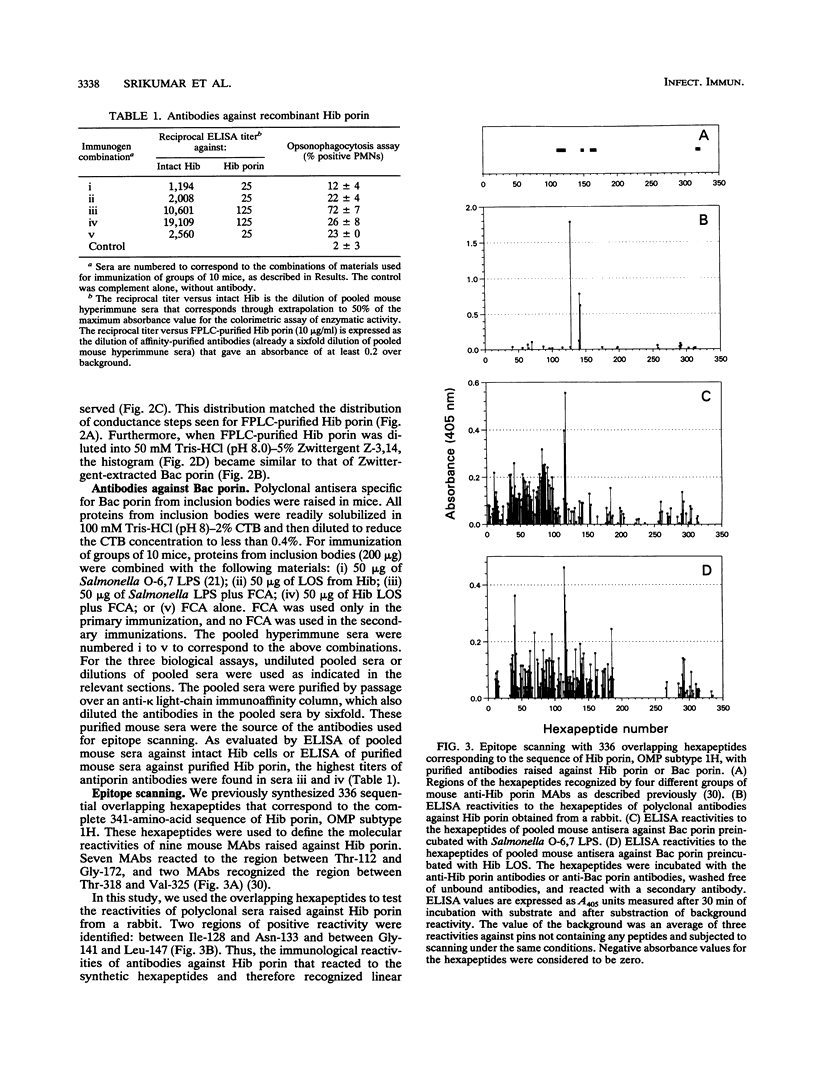
The major surface-located, channel-forming protein in the outer membrane of Haemophilus influenzae type b (Hib) is porin (341 amino acids; M(r), 37,782). In order to generate Hib porin that is devoid of lipooligosaccharides and capsular polysaccharide, the Hib porin gene ompP2 was subcloned into a plasmid vector and recombinant Hib porin was expressed in Bacillus subtilis. Recombinant porin was produced in large quantities in B. subtilis and formed intracellular inclusion bodies. Recombinant porin was extracted from inclusion bodies and shown to be active in forming pores in synthetic black lipid membranes. However, these pores demonstrated different pore characteristics than wild-type Hib porin. Mouse hyperimmune sera against recombinant porin were generated and subjected to epitope scanning with a library of 336 overlapping synthetic hexapeptides that corresponded to the entire sequence of Hib porin. The epitope specificities of the anti-recombinant porin antibodies were similar to those of antibodies against Hib porin: selected regions near the amino terminus which include a buried loop in the native structure of Hib porin were more immunogenic than regions at the carboxy terminus. Although some mouse anti-recombinant porin antibodies mediated complement-dependent binding to Hib by polymorphonuclear leucocytes in opsonophagocytosis assays, the antibodies were not bactericidal, nor did they abrogate bacteremia in the infant rat model of infection. It was concluded that the native state of Hib porin is required for the generation of a protective immune response against the bacterium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Chin A. C., Vachon V. Recombinant porin of Haemophilus influenzae type b. J Infect Dis. 1992 Jun;165 (Suppl 1):S188–S191. doi: 10.1093/infdis/165-supplement_1-s188. [DOI] [PubMed] [Google Scholar]
- Dick W. E., Jr, Beurret M. Glycoconjugates of bacterial carbohydrate antigens. A survey and consideration of design and preparation factors. Contrib Microbiol Immunol. 1989;10:48–114. [PubMed] [Google Scholar]
- Eisele J. L., Rosenbusch J. P. In vitro folding and oligomerization of a membrane protein. Transition of bacterial porin from random coil to native conformation. J Biol Chem. 1990 Jun 25;265(18):10217–10220. [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Hasemann C., Clausell A., Capra J. D., Orth K., Moomaw C. R., Slaughter C. A., Latimer J. L., Miller E. E. Primary structure of the porin protein of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989 Apr;57(4):1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers T. W., Eckmann C. M., Weening R. S., Roos D. A rapid turbidimetric assay of phagocytosis and serum opsonizing capacity. J Immunol Methods. 1989 Nov 13;124(1):85–94. doi: 10.1016/0022-1759(89)90189-0. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Mäkelä O., Eskola J., Saarinen L., Seppälä I. Isotype distribution and bactericidal activity of antibodies after immunization with Haemophilus influenzae type b vaccines at 18-24 months of age. J Infect Dis. 1988 Nov;158(5):973–982. doi: 10.1093/infdis/158.5.973. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992 Feb;6(4):435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Butcher S., Idänpän-Heikkilä I., Wahlström E., Muttilainen S., Runeberg-Nyman K., Sarvas M., Mäkelä P. H. The class 1 outer membrane protein of Neisseria meningitidis produced in Bacillus subtilis can give rise to protective immunity. Mol Microbiol. 1992 Sep;6(17):2499–2506. doi: 10.1111/j.1365-2958.1992.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Olander R. M. The role of the O antigen in adjuvant activity of lipopolysaccharide. FEMS Microbiol Lett. 1991 Sep 15;67(1):51–54. doi: 10.1016/0378-1097(91)90442-d. [DOI] [PubMed] [Google Scholar]
- Palva I., Pettersson R. F., Kalkkinen N., Lehtovaara P., Sarvas M., Söderlund H., Takkinen K., Käriäinen L. Nucleotide sequence of the promoter and NH2-terminal signal peptide region of the alpha-amylase gene from Bacillus amyloliquefaciens. Gene. 1981 Oct;15(1):43–51. doi: 10.1016/0378-1119(81)90103-7. [DOI] [PubMed] [Google Scholar]
- Palva I., Sarvas M., Lehtovaara P., Sibakov M., Käriäinen L. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5582–5586. doi: 10.1073/pnas.79.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puohiniemi R., Simonen M., Muttilainen S., Himanen J. P., Sarvas M. Secretion of the Escherichia coli outer membrane proteins OmpA and OmpF in Bacillus subtilis is blocked at an early intracellular step. Mol Microbiol. 1992 Apr;6(8):981–990. doi: 10.1111/j.1365-2958.1992.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Sikkema D. J., Murphy T. F. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1992 Dec;60(12):5204–5211. doi: 10.1128/iai.60.12.5204-5211.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srikumar R., Chin A. C., Vachon V., Richardson C. D., Ratcliffe M. J., Saarinen L., Käyhty H., Mäkelä P. H., Coulton J. W. Monoclonal antibodies specific to porin of Haemophilus influenzae type b: localization of their cognate epitopes and tests of their biological activities. Mol Microbiol. 1992 Mar;6(5):665–676. doi: 10.1111/j.1365-2958.1992.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Srikumar R., Dahan D., Gras M. F., Ratcliffe M. J., van Alphen L., Coulton J. W. Antigenic sites on porin of Haemophilus influenzae type b: mapping with synthetic peptides and evaluation of structure predictions. J Bacteriol. 1992 Jun;174(12):4007–4016. doi: 10.1128/jb.174.12.4007-4016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Burke C. J., Shi C., Friedman A., Donnelly J. J., Liu M. A. Pore formation and mitogenicity in blood cells by the class 2 protein of Neisseria meningitidis. J Biol Chem. 1992 Sep 25;267(27):19266–19271. [PubMed] [Google Scholar]
- Vachon V., Kristjanson D. N., Coulton J. W. Outer membrane porin protein of Haemophilus influenzae type b: pore size and subunit structure. Can J Microbiol. 1988 Feb;34(2):134–140. doi: 10.1139/m88-027. [DOI] [PubMed] [Google Scholar]
- Vachon V., Laprade R., Coulton J. W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986 Sep 25;861(1):74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]