Abstract
The zebrafish has rapidly become a favored model vertebrate organism, well suited for studies of developmental processes using large-scale genetic screens. In particular, zebrafish morphological and behavioral genetic screens have led to the identification of genes important for development of the retinal photoreceptors. This may help clarify the genetic mechanisms underlying human photoreceptor development and dysfunction in retinal diseases. In this review, we present the advantages of zebrafish as a vertebrate model organism, summarize retinal and photoreceptor cell development in zebrafish, with emphasis on the rod photoreceptors, and describe zebrafish visual behaviors that can be used for genetic screens. We then describe some of the photoreceptor cell mutants that have been isolated in morphological and behavioral screens and discuss the limitations of current screening methods for uncovering mutations that specifically affect rod function. Finally, we present some alternative strategies to target the rod developmental pathway in zebrafish.
Keywords: Zebrafish, Retinal development, Genetics
1. Introduction
It has been almost 25 years since the pioneering work of George Streisinger established the zebrafish (Danio rerio) as a suitable model vertebrate organism for genetic studies of development [1]. Since then, large-scale genetic screens conducted by several laboratories have identified thousands of mutations affecting almost every aspect of visible developmental processes [2–4]. In particular, genetic screens using morphological and behavioral criteria have uncovered numerous mutations affecting development of the visual system, especially the rod and cone photoreceptors [5–9].
The identification of genes important for rod photoreceptor cell development could increase our understanding of the mechanisms of rod photoreceptor cell dysfunction and degeneration in human diseases such as retinitis pigmentosa (RP). Retinitis pigmentosa is the most common form of human inherited photoreceptor degeneration and is an important cause of visual impairment and blindness [10,11]. Patients with RP initially experience night blindness and a loss of peripheral vision in adolescence or early adulthood, due to degeneration of the rod photoreceptors. Degeneration of the cone photoreceptors follows, and patients may become completely blind by 30–60 years of age.
To date, 40 genetic loci that are associated with RP have been identified, and the genes for 33 of these loci have been cloned (for a comprehensive list, see RetNet, http://www.sph.uth.tmc.edu/RetNet/). Some of the mutations are in genes of obvious importance, such as those involved in the rod phototransduction pathway and in retinoid metabolism. But there are also several genes of unknown function, and genes, such as those that code for pre-mRNA splicing factors, whose role in causing RP is not clear. Furthermore, it is not well understood how mutations in genes expressed solely in rods lead to the death of cones as well. Finally, many of the genes causing RP, perhaps as much as 50% of all cases [11], remain to be discovered.
Because the anatomical organization and development of the retina, including the photoreceptors, are remarkably conserved in virtually all vertebrate species, zebrafish are a good model in which to study photoreceptor development and disease. This review will focus on the major findings of the morphological and behavioral screens for zebrafish visual system mutants with regard to photoreceptor cell development. The advantages and limitations of these studies for investigating rod development and function will be considered, and alternative screening strategies to target rod photoreceptor development will be presented.
2. Zebrafish as a model vertebrate organism
The zebrafish is a small, freshwater teleost that is commonly found in pet stores. Adult zebrafish are easily reared in the laboratory, reaching roughly 3–5 cm in length. One obvious advantage of using zebrafish for genetic studies is its robust reproduction: zebrafish reach sexual maturity 2–3 months after hatching, and a single breeding pair can produce well over 200 fertilized eggs per weekly mating. Another unique benefit of the zebrafish is that the embryo develops externally and both embryos and newly hatched larvae are optically transparent. Therefore, developmental processes and gene expression can easily be observed and studied under the light microscope. Furthermore, development is extremely rapid; by 5 days post-fertilization (dpf) larvae have hatched from their chorions and are actively swimming and searching for food. Thanks to its ease of maintenance, large brood size and optical clarity the zebrafish is one of a select few vertebrate model organisms in which large-scale genetic screens are feasible.
The underlying principal of a forward genetic screen is the isolation of loci which, when mutated, disrupt a specific developmental pathway and result in an identifiable phenotype (Fig. 1). Because the ultimate goal of a genetic screen is to isolate the genes responsible for mutations of interest, genomic resources must be available to facilitate the mapping and cloning process. Considerable progress has been made in the area of zebrafish genomics. The zebrafish genome sequencing project is nearing completion. Construction of fine resolution genetic linkage maps and improved mapping techniques [12–15] have made the positional cloning of genes a more straightforward process. More recently, a large-scale screen using a retrovirus as an insertional mutagen has led to the rapid cloning of 315 genes essential for embryonic and early larval development [4]. Technological advances, such as TILLING [16] and morpholino gene knockdown methods [17], have facilitated reverse genetic approaches to studying the roles of specific gene products in development. These advances have greatly enhanced the power of zebrafish as a genetic model organism.
Fig. 1.
Traditional screen to uncover recessive mutations in F3 larvae. Male fish are mutagenized with ENU and mated to females to produce an F1 population. The F1 fish are mated to generate F2 families. Sibling mating of F2 families uncovers recessive mutations in 1/4 of the progeny in 1/4 of the crosses.
3. Zebrafish retinal development
The structure destined to become the zebrafish retina begins as an evagination from the anterior neural keel (a solid rod of cells analogous to the mammalian neural tube) at around 11 h post-fertilization (hpf) [18]. This optic primordium subsequently invaginates to form a two-layered optic cup. The outer layer gives rise to the retinal pigmented epithelium and the inner layer gives rise to the neural retina. At 24 hpf the presumptive retina consists of a single, pseudostratified epithelial layer which undergoes rapid cell proliferation over the next 12 h [19]. Beginning at around 28 hpf, the first cells to exit mitosis and differentiate are the retinal ganglion cells [20]. These are the output neurons of the retina, which send their axons via the optic nerve to the visual centers of the brain. Two additional periods of neurogenesis follow, producing the cells of the inner nuclear layer (the amacrine cells, bipolar cells, Muller glia, and horizontal cells) and finally the cells of the outer retina, the rod and cone photoreceptors [21]. In addition to the ordered temporal sequence (from inner to outer retina) mentioned above, neurogenesis in the retina also occurs in a defined spatial sequence. Differentiation of retinal neurons occurs first in a ventronasal region (termed the “ventral patch”) and then proceeds in a counterclockwise direction to the ventrotemporal region [22,23]. By 60 hpf, the retina has become fully laminated (Fig. 2), with three distinct nuclear layers separated by two plexiform layers [19]. Most retinal cell types can be distinguished by morphological or cytochemical criteria by 72–96 hpf.
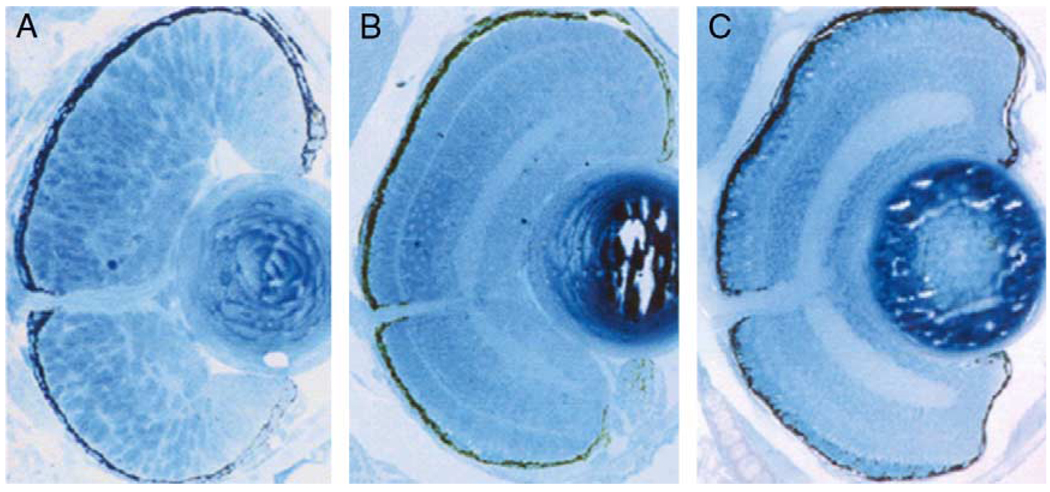
Fig. 2.
Rapid development of the zebrafish eye. (A) 36 hpf. (B) 54 hpf. (C) 120 hpf. Note the progressive lamination of the retina.
4. Zebrafish photoreceptors
Zebrafish possess one type of rod photoreceptor and four distinct classes of cones sensitive to UV, blue, red, and green light. As in most teleosts, each cone type is distinguishable by its morphology and spectral sensitivity [24,25]; the short single cones are UV sensitive, the long single cones are sensitive to blue light, and the red and green sensitive cones form a pair in a double-cone structure (with the red cone being the principal or longer member of the pair). Within the photoreceptor cell layer, the rod nuclei are located vitread (i.e., closer to the inner retina) to the cone nuclei, but the rod outer segments project beyond the outer segments of the cones and interdigitate with the retinal pigmented epithelium (RPE).
In the photoreceptor cell layer the cones are arranged in a crystalline mosaic pattern. The mosaic consists of alternating rows of double cones and single cones, arranged such that the green cones in a double cone pair always flank an UV cone, and the red cones are always next to the blue cones in the adjacent row. It was formerly thought that the rods were inserted into the cone mosaic with a random distribution, but recent work from our lab has shown that rod photoreceptors form a regular mosaic as well, positioning themselves in a square surrounding each UV cone [26]. This mosaic arrangement is apparent by about 10 dpf.
Development of photoreceptors, as for the other classes of neurons in the retina, has been shown to be regulated by both intrinsic factors and by environmental cues (for review, see Ref. [27].) Retinal progenitor cells are hypothesized to pass through a series of competence states, during which they are able to give rise to a subset of all possible retinal cell types in response to environmental signals. Some of the extrinsic factors that have been implicated in promoting rod photoreceptor specification and differentiation in vertebrates are retinoic acid [28], taurine [29], fibroblast growth factors (FGFs) [30], ciliary neurotrophic factor (CNTF) [31] and sonic hedgehog [32]. Several transcription factors are also proposed to regulate rod photoreceptor development, including Crx [33], Nrl [34], STAT3 [35], and NeuroD [36]. While progress has been made in identifying factors involved in rod development, the genetic mechanisms underlying this process are still largely unknown.
One interesting aspect of photoreceptor cell development is that the rods follow a developmental program that is distinct from the cones. In the ventral patch, rhodopsin expression precedes cone opsin expression, but in the dorsal and central retina rod differentiation occurs subsequent to the generation of cones [19,23]. Rods first become detectable in the ventral patch at 50 hpf, and differentiation is mostly confined to this area for several hours. Thereafter, scattered rod development is observed throughout the rest of the retina, but rod density and maturation remain greatest in the ventral patch even at 84 hpf. At 5 dpf, when many of the genetic screens for visual mutants have been conducted, the number of rods in the zebrafish retina and the length of the rod outer segments have not reached mature levels. Rather, rods are not considered to be anatomically mature until about 20 dpf [37].
With regard to functional circuitry in the retina, photoreceptor synaptic terminals become distinguishable by light and electron microscopy beginning at about 62 hpf [19]. It is difficult to distinguish the terminals of rods from cones in larval zebrafish by electron microscopy. However, our lab has shown, using a transgenic line of zebrafish expressing GFP in the rods, that rod terminals are visible as early as 5 dpf [26].
Zebrafish visual responses can be measured physiologically by electroretinogram (ERG). The ERG records the summed field potential in the retina in response to light. ERG responses can be measured in zebrafish beginning at 72 hpf [38], with a clear response at about 5 dpf [39]. However, rod signaling cannot be detected by ERG until about 15 dpf, and is not really robust until about 21 dpf [40,41]. Furthermore, behavioral assays based on the optomotor response (see below) suggest that the rods are not functional until 14 dpf [40].
The physiological and behavioral data suggest that rod photoreceptors do not become functional until almost 2 weeks after the first rods are detectable by immunolabeling and in situ hybridization. It is curious that it would take so long for the rods to become functionally mature, whereas cone function is detectable 1–2 days after cones first appear. An alternative explanation is that current assays for measuring rod function are not sensitive enough to detect the first rod signals. Strategies to address this question will be discussed later in the review.
5. Early zebrafish behaviors
Larval zebrafish display various behaviors that can be used to great effect in genetic screens. One of the earliest testable behaviors is the touch response, which begins at about 2–3 dpf. A stimulus applied to the tail with a probe will cause the animal to swim rapidly away from the stimulus source. A behavioral screen employing the touch response yielded mutants defective for a wide variety of processes, including muscle formation and motor neuron axonal pathfinding [42].
Zebrafish are highly visual animals, and must be able to search for food and avoid predators soon after hatching. Therefore, it is not surprising that three different visual behaviors can be measured in zebrafish larvae by 6 dpf. These include the startle, optokinetic and optomotor responses.
The visual startle response can be detected as early as 68 hpf [43], which corresponds with the first appearance of functional synapses in the outer and inner plexiform layers of the retina [19]. The visual startle response occurs when a sudden decrease in illumination provokes an abrupt body movement. By 79 hpf nearly 100% of normal zebrafish will respond to the stimulus, but around this time zebrafish larvae are also making spontaneous swimming movements, therefore the startle response is not useful for genetic screens. It also only assesses the ability to distinguish light from dark, and does not measure development of form vision. This can be better studied by evaluating tracking eye movements, or the optokinetic response (OKR).
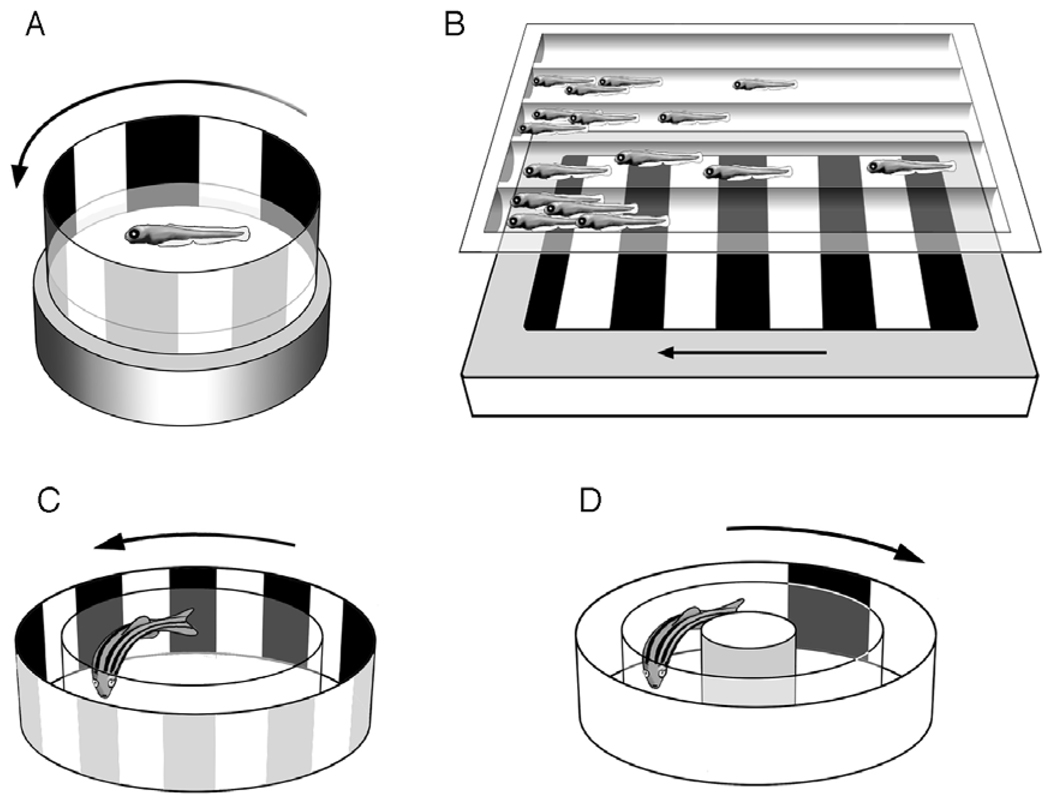
The OKR can be measured by placing a zebrafish larva (immobilized in a petri dish) inside a rotating circular chamber containing an alternating pattern of light and dark stripes on the interior (Fig. 3A). The zebrafish will respond to movement of the stripes with a visual tracking behavior that is composed of two movements—a smooth pursuit movement followed by a quick saccade, which resets the position of the eyes after the stimulus has left the visual field. Fine resolution of the response can be achieved by altering the intensity or wavelength of illumination, or the velocity of drum rotation. The OKR can first be detected at 73 hpf, and is fully developed by 80 hpf [43]. The OKR is very robust (nearly 100% response by 5 dpf) and has been used successfully in two genetic screens [7,9].
Fig. 3.
Zebrafish visual behavior assays. (A) Optokinetic response (OKR). (B) Population screening for larval optomotor response (OMR). (C) Adult OMR. (D) Escape response. This figure was graciously provided by Stephan Neuhauss.
The optomotor response (OMR) is similar to the OKR, except that swimming, rather than eye movement, is the behavior being measured. By about 6 dpf, zebrafish larvae will swim to follow moving visual stimuli (Fig. 3B, C). One advantage of using the OMR to evaluate visual function is that, by using a computer monitor displaying the moving stripes beneath a rectangular chamber, entire clutches of larvae can be tested at the same time. Fish with normal vision will follow the moving stripes and accumulate at one end of the chamber; 90% will respond to the stimulus within 60 s [9].
Adult zebrafish can be screened for visual system deficits by measuring the escape response, which is elicited when the fish encounter what they perceive as a threatening object (Fig. 3D). The fish is placed in a round transparent chamber with a pole in the middle. In response to appearance of a black segment on a rotating drum surrounding the chamber, the fish will hide behind the pole. This assay has been used to test the timecourse of dark adaptation in adult fish, and to isolate zebrafish mutants with dominantly inherited retinal degenerations [8].
6. Photoreceptor mutants isolated in zebrafish genetic screens
The first large-scale genetic screens for zebrafish developmental mutants were carried out by two groups, one in Boston [2] and one in Tubingen [3]. Collectively, these chemical mutagenesis screens isolated over 2000 mutations affecting the first 5 days of zebrafish development. Researchers in the Boston group used morphological criteria, such as reduction in eye size or altered eye shape, to identify 49 mutations causing altered retinal development [5]. Of these, several displayed photoreceptor degeneration phenotypes. For example, in the mutants mikre oko (mok) and niezerka (nie) photoreceptor loss begins in the peripheral retina, but almost all photoreceptors are absent across the entire retina by 5 dpf. The mok mutant displays one of the earliest defects in photoreceptor cell development, with abnormal photoreceptor morphology apparent at 3 dpf. Photoreceptor outer segments fail to form properly in mok retinas, and further analysis has shown that this results from a defect in cell–cell interactions within the photoreceptor cell layer [44]. Therefore, studies of this mutant may help to shed light on the role of inter-photoreceptor interactions in the development of rods and cones.
The mutants brudas (bru) and elipsa (eli), display a different pattern of photoreceptor cell loss. By 5 dpf, the majority of the photoreceptors in the central retina of these mutants is gone, but photoreceptors in the peripheral retina remain intact (the retina of teleost fishes, unlike mammals, continues to grow throughout the life of the animal, with addition of new cells to all retinal layers occurring from a proliferative zone located at the retinal margin). The oval (ovl) mutant, isolated in a simultaneous screen for zebrafish with altered body shape [45], shares this pattern of photoreceptor degeneration in the central retina, although photoreceptor loss is delayed relative to bru and eli. [46]. In contrast to mok, the defects in bru, eli and ovl are photoreceptor-autonomous, rather than involving cell–cell interactions.
Recently, the ovl locus was shown to encode IFT88, a component of the ciliary transport mechanism [47]. Loss of IFT88 results in defective assembly of the thin cilium connecting the photoreceptor inner and outer segments. The authors hypothesize that photoreceptor cell death in this mutant is caused by ectopic accumulation of activated opsin molecules in the inner segments of the photoreceptors [48].
A genetic screen for mutations affecting eye morphology was also conducted by John Dowling’s group at Harvard University [6]. This screen yielded 27 mutations, of which four were found to specifically affect development of the photoreceptors. For example, the photoreceptors absent (pca) mutant, like the eli, bru and ovl mutants above, loses photoreceptors in the central retina by 5 dpf but retains photoreceptor differentiation at the retinal margin.
The pca mutant, like the other photoreceptor mutants mentioned so far, displays loss of both rod and cone photoreceptors. Furthermore, many of the mutants described above have additional phenotypes outside of the retina. For example, eli has a curled body axis and pronephric cysts, and bru displays a reduced touch response.
To isolate mutants with more subtle and/or specific defects in retinal development, behavioral genetic screens are very useful. Behavioral screens for zebrafish with visual defects have uncovered several mutations causing photoreceptor cell loss or alterations in photoreceptor function. Brockerhoff et al. used the OKR as a primary screen to identify mutants with poor vision [7]. Electroretinograms (ERGs) were then recorded to identify which of these mutants had defects in the outer retina. This process yielded four mutants with morphologically normal eyes but with abnormal OKRs and ERGs [7,49]. One mutant, no optokinetic response a (noa), was recently shown to have a mutation in a gene that encodes a component of the pyruvate dehydrogenase (PDH) complex [50]. This mutant has a phenotype similar to human patients with PDH deficiency. Presumably, photoreceptors are particularly sensitive to alterations in mitochondrial function because of their high energy requirements. Interestingly, addition of ketogenic substrates (medium- to long-chain fatty acids) to the fish water resulted in improved vision and near wild-type feeding and swimming behaviors. This mutant may therefore be a good model for the human disease and a suitable organism in which to test potential therapeutic compounds.
Another interesting mutant isolated from the behavioral screen is no optokinetic response c (nrc) [7]. Histological analysis of nrc revealed that the photoreceptor terminals were abnormal, containing synaptic ribbons that were not anchored to the presynaptic membrane, as in wild-type terminals. Curiously, the ribbon synapses of the second order bipolar neurons appeared unaffected. Positional cloning revealed that the gene mutated in nrc is synaptojanin (Synj1) [51]. Synj1 is involved in the regulation of clathrin-mediated endocytosis and the actin cytoskeleton at conventional synapses. However, its role in regulation of cone ribbon synapses was heretofore uncharacterized, demonstrating the power of zebrafish genetic screens to identify novel functions for previously described genes.
Another behavioral screen based on the OKR was conducted by Neuhauss et al. [9]. This was an “off-the-shelf” behavioral screen using the stock of zebrafish mutants generated by the Tubingen group [3]. These mutants, which were isolated using morphological and anatomical criteria other than eye size and shape, were re-examined for visual system deficit using the OKR and OMR assays. Twenty-five mutants were identified that showed specific visual system defects. Four of these displayed photoreceptor degeneration.
In an interesting modification of the OKR behavioral screen, Brockerhoff et al. isolated a zebrafish mutant that displays a normal OKR in white light, but has no OKR in red light [52]. In this mutant, partial optokinetic response b (pob), the red cone photoreceptors degenerate between 3.5 and 5 dpf, but other cone photoreceptors are unaffected. Interestingly, the mutation in pob is not in the red opsin gene, suggesting the existence of a novel molecule responsible for the establishment of red cones. To date, pob is the only color-blind zebrafish mutant described and is also the first retinal mutant reported in which only a single type of cone photoreceptor is affected. This mutant will therefore be very useful in studying the genetic mechanisms underlying differentiation of specific photoreceptor classes.
Behavioral screening has also been used to great effect to isolate dominant mutations that cause visual dysfunction in adult zebrafish. Li and colleagues used the escape response, which is testable in zebrafish at 2 to 3 months of age, to measure the visual threshold of F1 fish derived from ENU-mutagenized males [8,53–55]. Three mutants, night blindness a (nba), night blindness c (nbc), and night blindness d (nbd) displayed reduced escape responses in dim light as a result of photoreceptor dysfunction. In the nba mutant, rod photoreceptors begin to degenerate in the central retina after 2 months of age [8]. Rod degeneration spreads as the animals age, and eventually the cones degenerate as well. In the nbc and nbd mutants, the escape response does not begin to deteriorate until 1 year and 2 years of age, respectively, and the rod degeneration phenotype is more variable [54,55]. Both nbc and nbd display a normal ERG, perhaps due to continued photoreceptor neurogenesis in the peripheral retina. The late onset of phenotype, dominant mode of inheritance (approximately 40% of human RP cases are due to dominant mutations), and the involvement primarily of rod photoreceptors make these mutants good candidates for zebrafish models of RP. Interestingly, however, nba, nbc, and nbd all cause early lethality in homozygous animals, suggesting that the genes involved are not photoreceptor-specific.
All of the mutants described above were isolated from genetic screens using the chemical mutagen ethyl nitrosourea (ENU). While ENU treatment is extremely efficient at inducing point mutations, subsequent isolation of mutated genes requires a positional cloning approach, which is fairly labor intensive. It is not surprising, therefore, that only a handful of the genes causing photoreceptor degenerations in zebrafish have been cloned. Recently, Nancy Hopkins’ group at MIT carried out an insertional mutagenesis screen using a pseudotyped retrovirus as the mutagenic agent [56–58]. Over 500 mutants affecting embryonic and early larval development were isolated, and over 300 genes have already been cloned [4,59].
One insertional mutant causing degeneration of the photoreceptors, not really finished (nrf), has been characterized [60]. The nrf phenotype is similar to that of mok and nie, in that most of the photoreceptors are absent from the entire retina by 5 dpf. However, some photoreceptors in the central retina do escape cell death, and those that survive have a recordable ERG. The gene mutated in nrf is a homologue of human Nuclear Respiratory Factor-1 (NRF-1), a transcription factor involved in the regulation of many genes, including cytochrome C and histones. Further studies are needed to identify the NRF-1 target genes that are required specifically for photoreceptor cell survival.
7. Alternative strategies to study rod development
While all of the screens conducted thus far have yielded interesting mutants with photoreceptor phenotypes, they have been limited in targeting defects in rod photoreceptor development. The mutants isolated in the large-scale morphological screens affect both rod and cone photoreceptors and result in early, non-specific degeneration. These mutants also tend to have other phenotypes outside of the eye and are lethal, making them poor models for human retinal disease. The larval behavioral mutants, with the exception of pob, also affect both rods and cones. Furthermore, the OKR and OMR behavioral assays are carried out on 5 dpf larvae, an age when rod function is probably not well developed. And while the adult behavioral screens have yielded mutants with greater impairment of rod function, it can take up to 2 years before a phenotype is detectable. Therefore, an alternative, less time-consuming approach to identifying rod-specific mutants would be desirable.
One promising approach that is being employed in our laboratory is to use antibodies to immunolabel the developing rod photoreceptors of 5 dpf larvae and to screen for abnormal labeling patterns by fluorescence microscopy (Fig. 4). This technique can reveal defects, not only in formation of the rods, but also in development of proper rod morphology and patterning of the rod mosaic. Using this approach, we have identified mutants with altered rod development, some of which show no other obvious abnormalities. For example, in one mutant we observed intense labeling of existing rods, however some of the rods showed an overly elongated, “stringy” morphology (Fig. 4C). In another mutant, immunolabeling revealed well-organized rods in the photoreceptor cell layer, but also ectopic rod photoreceptors clustered in the inner retina (Fig. 4E, F). And a third mutant displayed an uneven distribution of rods and incomplete lamination in the photoreceptor cell layer (Fig. 4D). Further screening and analysis using the rod-specific antibodies should help to uncover some of the genes important for rod development and for rod mosaic formation.
Fig. 4.
Immunofluorescence labeling identifies alterations in rod photoreceptor patterning and morphology. (A, B) Two views of rod labeling in wild-type 5 dpf larvae. (C) The rods in this mutant display an altered, “stringy” morphology. (D) In this mutant, the pattern and spacing of the rods is not normal. (E, F) In this mutant, the rods look normal in the outer nuclear layer, but ectopic rods are also visible in the inner retina, and sometimes the choroid fissure fails to close (F).
Another potential approach to isolating mutants with defective rod photoreceptor development is a rod-targeted behavioral screen. However, as discussed above, the current behavioral assays do not effectively asses rod input in larval or juvenile fish. Before an assay of this type can be developed, more work must be done to determine precisely at what time the rods become functional in zebrafish.
While anatomical and immunohistochemical studies have shown that rods begin appearing in the zebrafish retina as early as 50–60 hpf [19,23], electrophysiological studies suggest that rods do not make a significant contribution to the zebrafish ERG until 14–15 dpf [41]. Our laboratory has shown, using a transgenic line of fish expressing GFP in the rods, that rod terminals become distinguishable as early as 5 dpf. It is possible, therefore, that rod synaptic transmission is occurring earlier than 14 dpf but is not significant enough to be detected by ERG.
An alternative method to investigate development of rod function is to evaluate synaptic transmission in larval and juvenile zebrafish photoreceptors with synaptic vesicle markers. Rea et al. recently showed that the fluorescent styryl dye FM1-43 could be specifically internalized by cone photoreceptor synaptic terminals in the anole lizard [61]. We are currently investigating using this dye to label rod photoreceptor terminals as well. Dye uptake by rods in larval or juvenile fish would indicate that they are capable of releasing neurotransmitter, and therefore may be functional at this age.
Finally, the transgenic lines of zebrafish, created by our laboratory and by others [26,62,63] which express GFP in the rods could themselves be used in a chemical mutagenesis screen. Mutants could be isolated by monitoring F3 fish for changes in GFP fluorescence. Because GFP expression in the rods can be observed in living embryos, this approach would combine the ease and rapidity of a morphological screen with the selectivity of an immunolabeling screen.
8. Conclusions
Zebrafish have proven to be a valuable model organism for studies of retinal and photoreceptor development. Genetic screens have led to the isolation of many mutants displaying various patterns of photoreceptor degeneration, and some of the genes underlying these defects have been cloned. Further analysis of these mutants will no doubt shed light on the mechanisms of photoreceptor development. However, current screening methods are limited in their ability to identify unique players in the developmental program that underlies rod photoreceptor generation. New screens based on immunolabeling and transgenic technologies, coupled with more in-depth studies of rod development in wild-type fish, should lead to the identification of important rod-specific genes. This, in turn, may contribute to our understanding of rod dysfunction in human retinal disease.
Acknowledgements
We would like to thank Mary Mullins, Michael Granato and their laboratory members for providing us with the embryos used in our pilot screen for rod photoreceptor mutants. This work was supported by NIH Grant EY13020.
Footnotes
Note added in proof
Subsequent to the submission of the manuscript, Gross and colleagues [64] reported the characterization of 40 zebrafish mutants, originally isolated in the Hopkins lab retroviral screen, which display defects in eye development and/or visual system function. Six of these mutants (including NRF-1) display defects in formation or maintenance of retinal photoreceptors.
References
- 1.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291(5813):293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 2.Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101(35):12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malicki J, Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL, Stainier DY, et al. Mutations affecting development of the zebrafish retina. Development. 1996;123:263–273. doi: 10.1242/dev.123.1.263. [DOI] [PubMed] [Google Scholar]
- 6.Fadool JM, Brockerhoff SE, Hyatt GA, Dowling JE. Mutations affecting eye morphology in the developing zebrafish (Danio rerio) Dev Genet. 1997;20(3):288–295. doi: 10.1002/(SICI)1520-6408(1997)20:3<288::AID-DVG11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci U S A. 1995;92(23):10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Dowling JE. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc Natl Acad Sci U S A. 1997;94(21):11645–11650. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhauss SC, Biehlmaier O, Seeliger MW, Das T, Kohler K, Harris WA, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19(19):8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 11.Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11(10):1219–1227. doi: 10.1093/hmg/11.10.1219. [DOI] [PubMed] [Google Scholar]
- 12.Stickney HL, Schmutz J, Woods IG, Holtzer CC, Dickson MC, Kelly PD, et al. Rapid mapping of zebrafish mutations with SNPs and oligonucleotide microarrays. Genome Res. 2002;12(12):1929–1934. doi: 10.1101/gr.777302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods IG, Kelly PD, Chu F, Ngo-Hazelett P, Yan YL, Huang H, et al. A comparative map of the zebrafish genome. Genome Res. 2000;10(12):1903–1914. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hukriede NA, Joly L, Tsang M, Miles J, Tellis P, Epstein JA, et al. Radiation hybrid mapping of the zebrafish genome. Proc Natl Acad Sci U S A. 1999;96(17):9745–9750. doi: 10.1073/pnas.96.17.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimoda N, Knapik EW, Ziniti J, Sim C, Yamada E, Kaplan S, et al. Zebrafish genetic map with 2000 microsatellite markers. Genomics. 1999;58(3):219–232. doi: 10.1006/geno.1999.5824. [DOI] [PubMed] [Google Scholar]
- 16.Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RH, Cuppen E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003;13(12):2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243(2):209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol. 1994;344(4):532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404(4):515–536. [PubMed] [Google Scholar]
- 20.Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207(2):309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- 21.Nawrocki LW. Ph.D. Thesis. University of Oregon; 1985. Development of the neural retina in the zebrafish, Brachydanio rerio. [Google Scholar]
- 22.Schmitt EA, Dowling JE. Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. J Comp Neurol. 1996;371(2):222–234. doi: 10.1002/(SICI)1096-9861(19960722)371:2<222::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Raymond PA, Barthel LK, Curran GA. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J Comp Neurol. 1995;359(4):537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- 24.Nawrocki L, Bremiller R, Streisinger G, Kaplan M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vision Res. 1985;25(11):1569–1576. doi: 10.1016/0042-6989(85)90127-0. [DOI] [PubMed] [Google Scholar]
- 25.Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A. 1993;90(13):6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258(2):277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 27.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2(2):109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 28.Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc Natl Acad Sci U S A. 1996;93(23):13298–13303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41(6):867–879. doi: 10.1016/s0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- 30.McFarlane S, Zuber ME, Holt CE. A role for the fibroblast growth factor receptor in cell fate decisions in the developing vertebrate retina. Development. 1998;125(20):3967–3975. doi: 10.1242/dev.125.20.3967. [DOI] [PubMed] [Google Scholar]
- 31.Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124(5):1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 32.Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for hedgehog genes in zebrafish retinal development. Dev Biol. 2000;220(2):238–252. doi: 10.1006/dbio.2000.9629. [DOI] [PubMed] [Google Scholar]
- 33.Shen YC, Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev Biol. 2004;269(1):237–251. doi: 10.1016/j.ydbio.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29(4):447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa Y, Nakao K, Shimazaki T, Takeda J, Akira S, Ishihara K, et al. Downregulation of STAT3 activation is required for presumptive rod photoreceptor cells to differentiate in the postnatal retina. Mol Cell Neurosci. 2004;26(2):258–270. doi: 10.1016/j.mcn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Hitchcock P, Kakuk-Atkins L. The basic helix–loop–helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J Comp Neurol. 2004;477(1):108–117. doi: 10.1002/cne.20244. [DOI] [PubMed] [Google Scholar]
- 37.Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio: I. Structure. J Comp Neurol. 1984;224(1):107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- 38.Branchek T. The development of photoreceptors in the zebrafish, Brachydanio rerio: II. Function. J Comp Neurol. 1984;224(1):116–122. doi: 10.1002/cne.902240110. [DOI] [PubMed] [Google Scholar]
- 39.Saszik S, Bilotta J, Givin CM. ERG assessment of zebrafish retinal development. Vis Neurosci. 1999;16(5):881–888. doi: 10.1017/s0952523899165076. [DOI] [PubMed] [Google Scholar]
- 40.Clark DT. Ph.D. Thesis. University of Oregon; 1981. Visual responses in developing zebrafish. [Google Scholar]
- 41.Bilotta J, Saszik S, Sutherland SE. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev Dyn. 2001;222(4):564–570. doi: 10.1002/dvdy.1188. [DOI] [PubMed] [Google Scholar]
- 42.Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- 43.Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180(2):646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- 44.Doerre G, Malicki J. A mutation of early photoreceptor development, Structure. mikre oko, reveals cell-cell interactions involved in the survival and differentiation of zebrafish photoreceptors. J Neurosci. 2001;21(17):6745–6757. doi: 10.1523/JNEUROSCI.21-17-06745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- 46.Doerre G, Malicki J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech Dev. 2002;110(1–2):125–138. doi: 10.1016/s0925-4773(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 47.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42(5):703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 48.Alfinito PD, Townes-Anderson E. Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc Natl Acad Sci U S A. 2002;99(8):5655–5660. doi: 10.1073/pnas.072557799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brockerhoff SE, Dowling JE, Hurley JB. Zebrafish retinal mutants. Vision Res. 1998;38(10):1335–1339. doi: 10.1016/s0042-6989(97)00227-7. [DOI] [PubMed] [Google Scholar]
- 50.Taylor MR, Hurley JB, Van Epps HA, Brockerhoff SE. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc Natl Acad Sci U S A. 2004;101(13):4584–4589. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, et al. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24(40):8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brockerhoff SE, Hurley JB, Niemi GA, Dowling JE. A new form of inherited red-blindness identified in zebrafish. J Neurosci. 1997;17(11):4236–4242. doi: 10.1523/JNEUROSCI.17-11-04236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J Neurosci. 2000;20(5):1883–1892. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maaswinkel H, Ren JQ, Li L. Slow-progressing photoreceptor cell degeneration in night blindness c mutant zebrafish. J Neurocytol. 2003;32(9):1107–1116. doi: 10.1023/B:NEUR.0000021905.33091.f1. [DOI] [PubMed] [Google Scholar]
- 55.Maaswinkel H, Mason B, Li L. ENU-induced late-onset night blindness associated with rod photoreceptor cell degeneration in zebrafish. Mech Ageing Dev. 2003;124(10–12):1065–1071. doi: 10.1016/j.mad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Gaiano N, Allende M, Amsterdam A, Kawakami K, Hopkins N. Highly efficient germ-line transmission of proviral insertions in zebrafish. Proc Natl Acad Sci U S A. 1996;93(15):7777–7782. doi: 10.1073/pnas.93.15.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaiano N, Amsterdam A, Kawakami K, Allende M, Becker T, Hopkins N. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature. 1996;383(6603):829–832. doi: 10.1038/383829a0. [DOI] [PubMed] [Google Scholar]
- 58.Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, et al. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13(20):2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- 60.Becker TS, Burgess SM, Amsterdam AH, Allende ML, Hopkins N. not really finished is crucial for development of the zebrafish outer retina and encodes a transcription factor highly homologous to human nuclear respiratory factor-1 and avian initiation binding repressor. Development. 1998;125(22):4369–4378. doi: 10.1242/dev.125.22.4369. [DOI] [PubMed] [Google Scholar]
- 61.Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41(5):755–766. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 62.Perkins BD, Kainz PM, O’Malley DM, Dowling JE. Transgenic expression of a GFP-rhodopsin COOH-terminal fusion protein in zebrafish rod photoreceptors. Vis Neurosci. 2002;19(3):257–264. doi: 10.1017/s0952523802192030. [DOI] [PubMed] [Google Scholar]
- 63.Hamaoka T, Takechi M, Chinen A, Nishiwaki Y, Kawamura S. Visualization of rod photoreceptor development using GFP-transgenic zebrafish. Genesis. 2002;34(3):215–220. doi: 10.1002/gene.10155. [DOI] [PubMed] [Google Scholar]
- 64.Gross JM, Perkins BD, Amsterdam A, Egana A, Darland T, Matsui JI, et al. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics. 2005;170(1):245–261. doi: 10.1534/genetics.104.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]