Summary
We reported earlier on the oncogenic properties of Grm1 by demonstrating that stable Grm1-mouse-melanocytic clones proliferate in the absence of growth supplement and anchorage in vitro. In addition these clones also exhibit aggressive tumorigenic phenotypes in vivo with short latency in tumor formation in both immunodeficient and syngeneic mice. We also detected strong activation of AKT in allograft tumors specifically AKT2 as the predominant isoform involved. In parallel we assessed several human melanoma biopsy samples and found again that AKT2 was the predominantly activated AKT in these human melanoma biopsies. In cultured stable Grm1-mouse melanocytic clones, as well as an mGlu1 expressing human melanoma cell line, C8161, stimulation of mGlu1 by its agonist led to the activation of AKT, while pre-incubation with mGlu1-antagonist abolished mGlu1-agonist-induced AKT activation. In addition, a reduction in tumor volume of Grm1-mouse-melanocytic-allografts was detected in the presence of small interfering AKT2 RNA (siAKT2). Taken together, these results showed that, in addition to the MAPK pathway previously reported being a downstream target of stimulated mGlu1, AKT2 is another downstream target in Grm1 mediated melanocyte transformation.
Keywords: Grm1, mGlu1, melanoma, AKT2, Bcl-2, inducible siRNA
INTRODUCTION
Melanoma is the most aggressive form of skin cancer and is characterized by the uncontrolled growth of melanocytes. Although the transformation of melanocytes has been studied extensively by many research groups, the mechanisms leading to neoplastic transformations remain a challenging task due to the complexity and diversity of the disease. Previously, we have reported that spontaneous melanoma in a transgenic mouse line (TG-3) was due to the aberrant expression of metabotropic glutamate receptor 1 (mGlu1) in melanocytes. This notion was verified with a subsequent transgenic mouse line, TG(Grm1)Epv (E), that displayed a predisposition to melanoma where mGlu1 expression was regulated by a melanocyte-specific promoter, Dct (dopachrome tautomerase) (Pollock et al., 2003). Based on these results, we have isolated several stable Grm1-clones derived from two immortalized murine melanocytes, melan-a and B10.BR. We showed that these stable Grm1-melanocytic clones displayed fully transformed phenotypes as evident by formation of tumors in allografts in both immunodeficient nude and syngeneic mice (Shin et al., 2008b).
mGlu1 is normally activated by its natural ligand, L-glutamate, a predominant neurotransmitter in the mammalian nervous system. The glutamate signaling pathway, originally thought to be restricted to neuronal cells, is now known to be involved in proliferation, migration, and differentiation in a variety of non-neuronal tissues such as bone, testis, pancreas, lung, heart, and the skin (Hinoi et al., 2004, Skerry and Genever, 2001, Shin et al., 2008a). The stimulation of mGlu1 by glutamate triggers the activation of the extracellular signal-regulated kinase (ERK) via the mitogen activated protein (MAP) kinase cascade (RAS-RAF-MEK-ERK) in neuronal and non-neuronal cells (Thandi et al., 2002, Karim et al., 2001, Gerber et al., 2007). Previously, we verified that ERK is one of the downstream targets involved in mGlu1 signaling in melanoma cell lines derived from TG-3 tumors as well as mGlu1-expressing human melanoma cell lines and stable Grm1-melanocytic cell lines (MASS/B10SS) (Marin et al., 2006, Namkoong et al., 2007, Shin et al., 2008b). The specificity of mGlu1-induced ERK activation has been demonstrated by the absence of activated ERK when cells are preincubated with mGlu1 specific antagonists followed by stimulation with known agonists (Namkoong et al., 2007, Shin et al., 2008b).
We showed that, in addition to the activation of ERK, mGlu1 also triggers the activation of protein kinase B (AKT) in stable Grm1-mouse melanocytes (Shin et al., 2008b). Several groups have previously reported the involvement of AKT in mGlu1 mediated cell signaling. Hou and Klann demonstrated that activation of mGlu1 led to PI3K-AKT-mTOR signaling cascade, which is required for protein synthesis in the brain (Hou and Klann, 2004). In addition, Banko and co-workers demonstrated that stimulation of mGlu1 in the brain resulted in activation of both PI3K-AKT and MEK-ERK signaling cascades (Banko et al., 2006).
Initially, AKT was identified as a component of a fusion product of the retroviral oncogene, v-akt, that can induce leukemia in mice (Bellacosa et al., 1991). Since then, three isoforms of AKT (AKT1, 2, and 3) have been identified (Robertson, 2005). The oncological significance of amplified Akt/PKB gene and its expression has been well documented in various human cancers including gastric carcinoma, ovarian, pancreatic, and breast tumors (Staal, 1987, Bellacosa et al., 1995, Cheng et al., 1996). In melanoma, the AKT signaling pathway, especially via the AKT3 isoform, has been reported to play a critical role in inhibiting apoptosis (Stahl et al., 2004, Dhawan et al., 2002). AKT mediates its activity by phosphorylating the transcription factor cyclic AMP response element-binding protein (CREB) as well as IkB kinase, which further activates nuclear factor-kB (NF-kB) (Du and Montminy, 1998). Both CREB and NF-kB regulate the expression of many survival genes including Bcl-2 (Skorski et al., 1997, Pugazhenthi et al., 2000). In this report, we present evidences that AKT2 isoform is a major downstream target of mGlu1-mediated cellular transformation and attains anti-apoptotic properties by elevating the intracellular levels of Bcl-2.
RESULTS
Basal levels of activated AKT are detected in allografts of stable Grm1-melan-a (MASS) clones
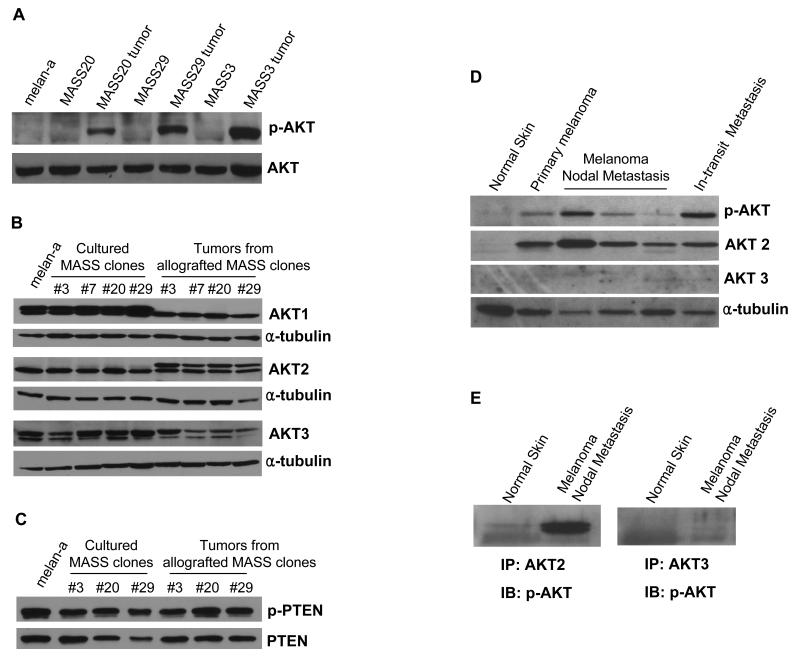
Several investigators have reported that, in parallel with the MAPK pathways, the AKT signaling pathway is required during tumor formation to induce angiogenesis (Trisciuoglio et al., 2005, Govindarajan et al., 2007). Previously we observed strong angiogenic activities in allografts from MASS clones (Shin et al., 2008b). Thus we hypothesized that AKT signaling may also be involved in the tumorigenic process of these MASS clones. Excised MASS allografts from immunodeficient mice were then used to assess AKT activation by Western immunoblots. Strong basal levels of phosphorylated AKT were detected in all excised tumor samples compared to the parental MASS clones in culture and were not detected in the non-tumorigenic parent cell line, melan-a (Figure 1A). In order to assess which isoform(s) of AKT (AKT1, 2, and 3) were specifically expressed in these tumor samples, we performed Western immunoblots using antibodies directed against each AKT isoform. Elevated levels of doublet AKT1 were detected in cultured melan-a and MASS clones whereas allograft tumor samples showed reduced expression of AKT1 with a single band (Figure 1B, top panel). Unlike AKT1, expression of AKT2 isoform showed a slower migrating band, presumably the phosphorylated form of AKT2, only in allograft tumor samples but not in the corresponding cultured cell lines (Figure 1B, middle panel). Expression of AKT3 was found at similar levels in both cultured and excised tumor samples (Figure 1B, bottom panel).
Figure 1. Differential patterns of activated AKT and AKT isoforms in cultured MASS clones and excised allograft tumors of MASS clones.
(A) Basal levels of phosphorylated AKT in melan-a cells, cultured MASS clones, and corresponding excised allografts were examined by Western immunoblots. Each lane contains 25μg of protein lysates and the transferred membrane was probed with antibody for phosphorylated AKT (Ser473). Membrane was stripped and reprobed with α-tubulin to show equal loadings. Elevated levels of phosphorylated AKT were detected only in allograft tumor samples, much less in cultured MASS cell lines and none in melan-a. (B) Expression patterns of AKT isoforms in cultured MASS clones and respective excised allografted tumors of MASS clones were investigated by Western immunoblots. Among AKT isoforms, only AKT2 showed differential bands in allograft tumor samples compared to corresponding cultured MASS clones. (C) Western immunoblots of phosphorylated and non-phosphorylated PTEN proteins in immortalized melanocytes, melan-a, MASS clones, and tumors from allografted corresponding MASS clones. (D) Activation of AKT (pAKT) and expression profiles of AKT2 and AKT3 in normal human skin and human melanoma biopsies. A total of 25 μg of protein lysates from each sample was loaded in each lane. α-tubulin was used as a loading control. (E) Immunoprecipitation of protein lysates from a nodal metastatic melanoma biopsy and normal skin samples with mouse anti-human AKT2 and mouse anti-human AKT3 using protein-L-agarose beads. Western immunoblots were performed with rabbit anti-human p-AKT.
PTEN (phosphatase and tensin homolog deleted on chromosome ten) is a negative regulator of the AKT signaling pathways (Wu et al., 2003, Cantley and Neel, 1999, Stambolic et al., 1998). We were interested to know if PTEN was involved in the activity of AKT2 in MASS clones or their corresponding allografted tumor samples. Western immunoblots of these samples were performed using antibodies against the phosphorylated-PTEN and total PTEN; no significant difference in either p-PTEN or PTEN levels among MASS clones and their corresponding allografted tumor samples. This suggests that AKT2 activation in these MASS allografts is likely independent of PTEN (Figure 1C).
We reported previously that the ectopic expression of mGlu1 was detected in 40-60% of human melanoma cell lines and biopsies but not in normal skin or benign nevi (Namkoong et al., 2007, Pollock et al., 2003). Currently we show that activated AKT (pAKT) is also detected in human melanoma biopsies positive for mGlu1 expression but not normal skin. This led us to further identify which AKT isoform is responsible for the activation of AKT in these human biopsies. In contrast to previous findings by other laboratories, AKT2 was the predominant isoform detected in these mGlu1 expressing melanoma biopsy samples while very little if any of the AKT3 isoform was detected (Figure 1D). To further confirm that AKT2 was indeed the predominant isoform leading to AKT activation, we performed IP-Westerns. Protein extracts from human melanoma nodal metastasis or normal skin were treated first with antibodies to AKT2 and AKT3 followed by immunoblots using p-AKT antibody. Only AKT2 isoform was detected in human melanoma nodal metastasis not in normal skin, while very similar, low levels of AKT3 were detected in both normal skin and melanoma nodal metastasis samples (Figure 1E).
AKT is one of the downstream targets of mGlu1
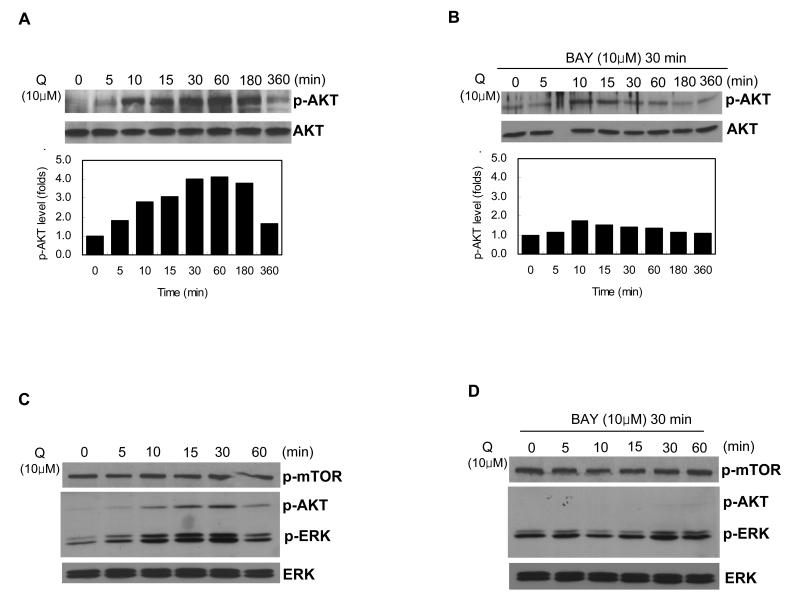
Excised allograft tumor samples showed differentially activated AKT (Figure 1A), which was identified as AKT2. We were interested to know if activation of AKT in MASS clones was mediated via mGlu1. We treated MASS clones with a known agonist of mGlu1, L-Quisqualate (Q), which resulted in strong activation of AKT after 10 min. Activated AKT was sustained for 3 hours with a maximum level of activation after 1hour (Figure 2A). In order to determine whether the activation of AKT was a cause or consequence of mGlu1 activation, cells were pre-incubated with the mGlu1-antagonist BAY 36-7620 (BAY) for 30 min followed by stimulation with Q. Pre-incubation of these cells rendered these cells non-responsive to mGlu1-agonist, Q, and resulted in the absence of AKT activation (Figure 2B). We also tested AKT activity in mGlu1-expressing C8161 human melanoma cell line under the same conditions. As we observed with MASS clones, p-AKT in C8161 was upregulated by Q treatment with the maximum pAKT level at 30min (Figure 2C). Similarly, Q activated ERK with the maximum pERK levels at 30min (Figure 2C). Pretreatment of C8161 cells with Bay followed by stimulation with Q resulted in the absence of any activation of AKT (Figure 2D). In addition, there was also a lack of modulation in levels of activated ERK (Figure 2D). These results suggest that stimulation of mGlu1 led to activation of dual signaling pathways (ERK and AKT) in mGlu1 expressing human melanoma cells. We next evaluate if mTOR is the effector of AKT activation in C8161. Activation of mTOR (p-mTOR) was not detected by the same treatment (Figure 2C and 2D). These results imply that mTOR was not the effector in mGlu1-AKT signaling cascade.
Figure 2. Modulation of AKT in MASS clones by mGlu1-agonist/antagonist.
(A) Western immunoblots showed that stimulation of mGlu1 by its agonist, L-Quisqualate (Q; 10 μM) led to the activation of AKT in MASS20 cells. Cells were grown in the glutamate/glutamine free RPMI medium supplemented with Glutamax™ to reduce free glutamate, the natural ligand for mGlu1. For induction experiment, cells were incubated with Q for the given time points and protein lysates were prepared thereafter. (B) Pre-treatment of MASS20 cells with mGlu1 specific antagonist, BAY 43-7620 (BAY; 10 μM), for 30 min prior to the stimulation with Q for the given time points resulted in the absence of AKT activation. Same membranes were stripped and reprobed with α-tubulin to show equal loadings. (C) A mGlu1-positive human melanoma cell line, C8161, was treated the same way as described in (A), Stimulation of C8161 cells by Q resulted in the activation of both AKT and ERK but not mTOR. (D) C8161 cells were treated the same way as described in (B). Lack of activation or modulation in AKT, ERK or mTOR was observed.
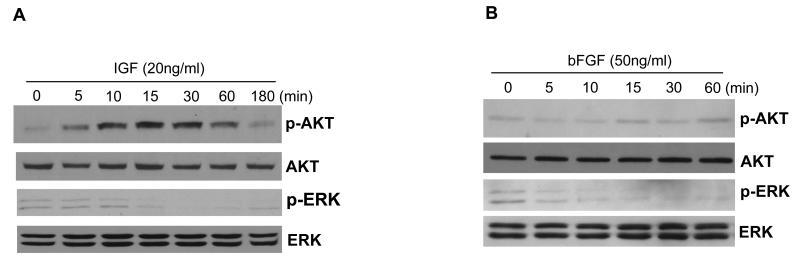
We next tested if AKT is activated in MASS clones by growth factors such as basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and insulin-like growth factor 1 (IGF-1). Only IGF-1 treatment in MASS clones resulted in strong activation of AKT after 5 min and lasted up to 1 hour (Figure 3A). In contrast, treatment of MASS cells with bFGF, EGF or PDGF did not stimulate AKT, an example of treatment with bFGF is shown (Figure 3B). Interestingly, ERK was not activated by either of these growth factors in these MASS cells (Figures 3a and 3B).
Figure 3. Activation of AKT by IGF in MASS20.
MASS20 cells were stimulated with supplement of IGF (20ng/ml) or bFGF (50ng/ml) for the time points indicated. Cell lysates were prepared at each time point and used in Western immunoblots. (A) Activation of AKT was detected in MASS20 cells after 5 min in the presence of IGF with the maximal levels of induction at 15 min. AKT activation was sustained up to 1 hour and decreased to the basal level after 3 hours. However, activation of ERK was not detected under similar conditions. (B) Supplement of bFGF was not able to induce activation of either AKT or ERK. Membranes were stripped and reprobed with total ERK antibody to show equal loading.
AKT activation in MASS clones is mediated via AKT2
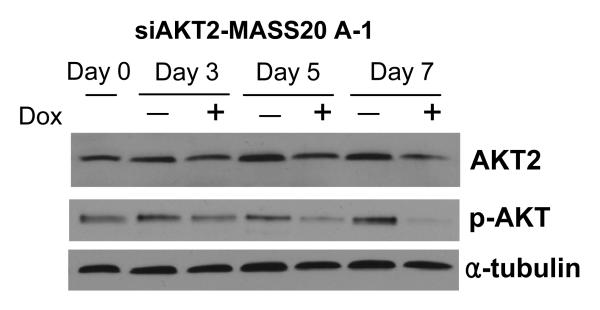
In order to verify that AKT2 is the major isoform participating in mGlu1-agonist stimulated cell signaling cascade, we generated stable inducible siAKT2 clones using a tetracycline-inducible siRNA system that we previously had utilized for siGrm1 experiments (Shin et al., 2008b). TetO-siAKT2 recombinant DNA was transfected into TetR-MASS clones and several stable siAKT2 clones were selected by their resistance to hygromycin. Suppression of AKT2 expression by siAKT2 induced by doxycycline (an analog of tetracycline) was assessed by Western immunoblots (Figure 4). Addition of doxycycline to stable siAKT2 clones induced suppression of AKT2 protein expression after 3 days and lasted to at least 7 days post-treatment (Figure 4 top panel). Inhibition of AKT2 expression was correlated with a decrease in levels of phosphorylated AKT after 3 days, which further demonstrated that AKT2 is the predominant isoform for activation of AKT in MASS clones (Figure 4 middle panel).
Figure 4. Suppression of p-AKT by inducible siRNA for AKT2 (siAKT2).
Cells were grown in the presence or absence of the inducer of siRNA, doxycycline, for the indicated time points up to 7 days. Cell lysates were prepared at each time point and used in Western immunoblots. Expression of AKT2 was suppressed by siAKT2 in the presence of doxycycline (2μg/ml). Activation of AKT (phosphorylated AKT) was also suppressed in accordance with the decreased level of AKT2. Membrane was stripped and reprobed with α-tubulin to show equal loadings.
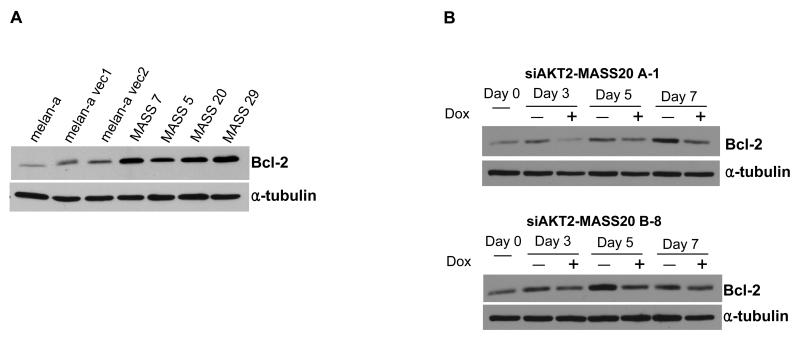
Bcl-2 is one of the downstream targets of AKT2
We reported previously that the growth properties of MASS clones were characteristic of transformed cells (Shin et al., 2008b). We were interested in knowing if one of the functions of AKT2 is to promote anti-apoptotic responses in these MASS clones by increasing expression of anti-apoptotic proteins such as Bcl-2. We detected elevated levels of Bcl-2 only in MASS clones but not in either melan-a or vector control clones (Figure 5A). Furthermore, we observed that Bcl-2 were modulated in siAKT2 clones in the presence of the inducer, doxycycline. Levels of Bcl-2 were reduced after 3 days in the presence of doxycycline suggesting that Bcl-2 is a downstream target of AKT signaling pathway in MASS clones (Figure 5B). Interestingly, induction of siAKT2 led to de-differentiation of these cells, which was evident by an obvious reduction in the pigmentation of doxycycline-treated cells only (data not shown). Taken together, these results suggest that the AKT signaling cascade, which acts in synergy with the MAPK pathway, is another effector of mGlu1 in the initiation and maintenance of Grm1-mediated transformation in MASS clones.
Figure 5. Expression of Bcl-2 in MASS clones and its modulation by siAKT2.
(A) Over-expression of an anti-apoptotic protein, Bcl-2, was detected by Western immunoblots in all MASS clones tested. In contrast, the parental cells, melan-a or vector clones showed relatively low levels of Bcl-2 expression. (B) Inducible siRNA for AKT2 was transfected into MASS20-TetR. Several stable siAKT2-MASS20 clones were selected. These clones were grown in the presence and absence of doxycycline (2μg/ml) for the indicated time points up to 7 days. Western immunoblots showed that expression of Bcl-2 was suppressed in the presence of doxycycline (2μg/ml) in the growth medium. Suppression of Bcl-2 expression was detected after 3 days of doxycycline treatment.
Inhibition of AKT2 in MASS clones led to suppression of cell invasion in vitro and reduction of in vivo tumorigenicity
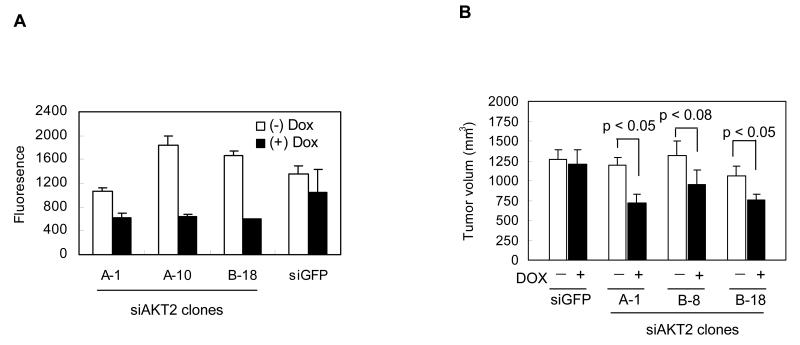
Previously, we reported that MASS clones displayed fully transformed phenotypes in cultured conditions and enhanced angiogenesis and invasiveness to organs in MASS-allografts in immunodeficient and syngeneic mice (Shin et al., 2008b). To investigate if AKT2 is required to maintain the invasiveness of these clones, we carried out invasion assays with three different stable, inducible siAKT2-MASS20 clones in the presence or absence of doxycycline (Figure 6A). In the absence of doxycycline, siAKT2-MASS20 clones penetrated and passed through the basement membrane matrix after 48 hrs. However, in the presence of doxycycline, the invasiveness of siAKT2-MASS clones was suppressed by more than 40% (Figure 6A). In contrast, control siGFP-MASS20 cells penetrated into the basement membrane matrix regardless if doxycycline was present (Figure 6A). Taken together, these results demonstrate that AKT2 contributes to the invasiveness of MASS clones.
Figure 6. Invasion and tumorigenicity assays of stable siAKT2-MASS20 clones.
(A) Effect of siRNA for AKT2 on invasiveness of MASS20 cells was assessed by the penetrance of cells into a basement membrane matrix coated chamber. Several siAKT2-MASS clones were plated at 2×104 cells in the upper chamber containing serum free RPMI medium in the presence or absence of doxycycline (2μg/ml). Induction of siAKT2 suppressed invasiveness of MASS cells but not siGFP control clone. (B) The effects of siAKT2 on the tumorigenicity of MASS clones were assessed by inoculating three different inducible siAKT2 clones (106cells/site) into immunodeficient nude mice. Supplement of the inducer of siAKT2, doxycycline, to the drinking water suppressed tumor formation in inducible siAKT2 clones (siAKT2 A-1, B-8, and B-18) but not siGFP control clone. Approximately, 30% of tumor volumes were suppressed in siAKT2 clones. Tumor volumes supplemented with doxycycline were compared with tumors in the absence of doxycycline. p; P-values (t-test), Bar; SD.
Subsequently, we examined the tumorigenic potential of these inducible siAKT2-MASS clones in vivo. Three inducible siAKT2-MASS clones and one control siGFP-MASS clone were inoculated subcutaneously into immunodeficient, athymic nude mice. Doxycycline (0.1 %, w/v) was added to the drinking water when the tumor volumes of inoculated cells reached 6mm3. Tumor volumes in the doxycycline-treatment groups (siAKT2) were decreased approximately 30% in comparison to the control group (no doxycycline) at day 19 (Figure 6B) while the siGFP control group showed no change in tumor volumes regardless of whether doxycycline was present (Figure 6B). These results show that AKT signaling participates in mGlu1-induced tumorigenesis in vivo.
DISCUSSION
The malignant transformation of cells, including melanocytes, often involves activation of at least two different signaling pathways. The possibility of another signaling cascade, in addition to ERK, being activated by stimulation of mGlu1 is partly predicted by previous studies by others. Activation of the cellular homologue of the viral oncogene v-Akt (AKT/PKB), together with the constitutively stimulated MAPK pathway induced by activating mutation of RAS, led to more aggressive phenotypes as demonstrated by a shorter latency of tumorigenesis in comparison to activation of MAPK alone in D6-mel-RAS xenografts (Whitwam et al., 2007). In Grm1 transformed mouse melanocytes, activated forms of AKT were only detected in MASS-allografts. In addition, AKT became stimulated in corresponding cultured MASS cells when the cells were stimulated by mGlu1-agonist. These results strongly suggest that in vivo the MASS cells are constitutively stimulated in the “tumor microenvironment” possibly through an autocrine loop due to the presence of excess released glutamate, the natural ligand of mGlu and/or other “factors” (Shin et al., 2008b). While in cultured conditions, the growth media supplies all the necessary nutrients for cell proliferation; constitutively activated AKT is not required. However, in assays assessing the functionality of mGlu1 by its agonist or antagonist in the stable Grm1 mouse melanocytic clones, the cells were growing in growth media without glutamate/glutamine but supplemented with GlutaMax™ which does not break down into free glutamate. In these experiments, AKT was activated only in the presence of mGlu1-agonist, L-Quisqualate. In addition, we also showed that activation of AKT was independent of PTEN. We hypothesize that the activation of mGlu1 may induce strong enough signals to overcome PTEN regulation considering that activated AKT was sustained up to 6hrs in mGlu1-agonist treated MASS cells. Similar results were observed by others that in the presence of growth factors in PTEN transfected cells, AKT became stimulated via PI3K-dependent pathways (Myers et al., 1998, Stambolic et al., 1998). We also provided evidences that AKT2 is the predominant AKT isoform that is stimulated in human melanoma biopsies. Recently, Stahl and co-workers reported that dysregulation of AKT3 with PTEN mutation promoted human melanoma xenograft tumor progression (Stahl et al., 2004). The discrepancy between our results and theirs may be attributed to several factors including assessment method (IHC vs Western immunoblots), PTEN expression status (no loss of PTEN expression in our system), and antibody used.
We reported earlier that stable Grm1-mouse-melanocytic clones displayed transformed phenotypes including acquisition of anchorage independent growth. In vivo allografts of these clones were very tumorigenic with a short latency, strong angiogenic activities and invasiveness (Shin et al., 2008b). We hypothesized that the in vivo microenvironment including neighboring keratinocytes, fibroblasts, and endothelial cells may contribute in part to the tumorigenic phenotypes, perhaps by the release of growth factors to stimulate proliferation of Grm1-melanocytes. We co-cultured MASS clones with keratinocytes and/or fibroblasts and performed MTT growth assays with the “conditioned” media collected from the co-cultures. However, no growth advantages for these MASS cells were observed in “conditioned” media (data not shown). These results suggest a possible existence of another source of stimulators for the proliferation of these cells only in vivo and not under normal cultured cell conditions.
We then tested if stable Grm1 clones respond to the stimuli from exogenous growth factors such as bFGF, EGF, PDGF, and IGF-1. Interestingly, only IGF-1 was sufficient to activate AKT when the phosphorylated forms of ERK or AKT were used as readouts, whereas ERK was not activated by any growth factor tested. These results suggest that AKT activation may be triggered by IGF-1 (or similar growth factors) in part, and is probably an event occurring in vivo rather than in cultured conditions. Several cellular sources of IGF-1 in the skin have been identified including basal keratinocytes, endothelial cells, and dendritic epidermal T-cells (Tavakkol et al., 1999, Rudman et al., 1997, Sharp et al., 2005). It is likely that keratinocytes release IGF-1 only in an in vivo microenvironment since IGF-1 expression was never detected in cultured keratinocytes (Rudman et al., 1997). This may explain, at least in part, why the co-culture experiments of MASS cells with keratinocytes did not confer any growth advantages in MASS cells. Taken together, mGlu1 in MASS clones and growth factors such as IGF-1 from surrounding tissues may confer synergic effects toward the activation of AKT in vivo.
Unlike normal cells, transformed cells show resistance toward apoptotic signals. We detected high levels of one of the anti-apoptotic proteins, Bcl-2, in MASS clones. In addition, the inhibition of AKT signaling by siRNA for AKT2 led to the decrease of Bcl-2 expression suggesting the AKT signaling cascade is linked to anti-apoptotic signals via Bcl-2. It is possible that suppression of AKT2 by siRNA in in vivo allografts is partly mediated through a reduction in expression of anti-apoptotic proteins such as Bcl-2. This notion may explain why siRNA for AKT2 suppresses tumor growth in allografts of siAKT2-MASS clones.
Taken together, we have identified that mGlu1 activates dual signaling cascades of MAPK (ERK) and AKT2. Exogenously introduced Grm1 into melan-a cells induced over-expression of Bcl-2, which was identified as a downstream target of AKT2. Blockade of mGlu1 function/activity would provide novel therapeutic options for melanoma patients.
MATERIALS AND METHODS
Antibodies and Reagents
Anti-phosphorylated AKT (Ser473), anti-AKT1 (2H10), anti-AKT2 (5B5), anti-AKT3, anti-phosphorylated and non-phosphorylated PTEN, anti-phosphorylated ERK 1/2 (Thr202/Tyr204), and anti-ERK 1/2 antibody were purchased from Cell Signaling (Danvers, MA); Anti-Bcl-2 antibody and mouse anti-human AKT2 for IP were purchased from Invitrogen (Carlsbad, CA); monoclonal anti-α-tubulin antibody was obtained from Sigma (St. Louis, MO). Mouse anti-human AKT3 was purchased from Abcam Inc. (Cambridge, MA). L-Quisqualate [(L)-(+)-α-amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic acid] was purchased from Tocris (Ellisville, MO). BAY 36-7620 [(3aS,6aS)-6a-naphtalen-2-ylmethyl-5-methyliden-hexahydro-cyclopental [c]-furan-1-on] was a gift from Bayer (West Haven, CT).
Cell culture conditions
Spontaneously immortalized, normal murine melanocytes, melan-a cells, were provided by Dr. Dorothy Bennett (St. George’s, University of London, UK). Melan-a cells were maintained in RPMI (GibcoBRL Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO), 100 U/ml penicillin/streptomycin (GibcoBRL Laboratories, Grand Island, NY) and 200 nM TPA (Sigma, St. Louis, MO). Stable Grm1-melanocytic transfectants (MASS clones) were produced as reported previously (Shin et al., 2008b). MASS clones were grown in the same media as melan-a but with the selective marker, G418 (400μg/ml) and in the absence of TPA. Human melanoma cell line, C8161 was maintained in RPMI (GibcoBRL Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO), and 100 U/ml penicillin/streptomycin (GibcoBRL Laboratories, Grand Island, NY). For induction experiments, the conditions were the same as reported (Shin et al., 2008b). Briefly, cells were grown in glutamate/glutamine free RPMI plus 10 % dialyzed fetal bovine serum (GibcoBRL Laboratories, Grand Island, NY) with the supplement of GlutaMax™ (GibcoBRL Laboratories, Grand Island, NY) to minimize the effect of glutamate, the natural ligand of mGlu1 for 3 days before the cells were plated out in glu/gln free RPMI medium for induction experiments.
Western immunoblots
Protein lysates were prepared as described previously (Cohen-Solal et al., 2002). Briefly, media was removed and cells were washed once with ice-cold phosphate buffered saline (PBS). After removal of PBS, extraction buffer was added directly to the plates and cells were collected with a cell scraper. Cell extracts were incubated on ice for 20 minutes. Cell debris was removed by centrifugation at 10,000 × g at 4°C for 20 minutes and supernatant was collected to measure protein concentration and for Western immunoblot analysis. For the quantification of protein expression, the densitometry values of phosphorylated proteins were normalized to the values of the corresponding total protein bands using Adobe Photoshop software (Adobe systems Inc. San Jose, California).
Immunoprecipitation
For immunoprecipitation, protein-L-agarose beads were used (Santa Cruz Biotechnology, Santa Cruz, CA). Procedures were performed as per the manufacturer’s instructions. Briefly, 500μg of tissue biopsy extracts were incubated with mouse anti-human AKT2 (Invitrogen, Carlsbad, CA) or mouse anti-human AKT3 (Abcam Inc., Cambridge, MA) for 1 hour at 4°C. Then 20 μl of protein-L-agarose beads was added and incubated at 4°C overnight. After collect the pellets by centrifugation at 2500 rpm for 5min at 4°C, the pellets were washed 4 times with 1 × PBS. Washed pellets were then mixed with 40 μl of 1x electrophoresis buffer and boiled for 2-3 min. Samples were centrifuged and loaded for SDS gel electrophoresis.
Inducible siRNA for AKT2
The siRNA sequences for AKT2 (siAKT2) were as follows: sense strand 5′-GATCCGATAGCCCGCATCCACTCTTCCCTCTCATTCATGAGAGGGAAGAGTGCTTGCGGGCTATCTTTTTTGGAAA-3′ and antisense strand 5′AGCTTTTCCAAAAAAGATAGCCCGCAAGCACTCTTCCCTCTCATGAATGAGAGGGAAGAGTGGATGCGGGCTATCG-3′. These sequences were cloned into pRNATin-H1.2/Hygro vector with tetracycline operator (siAKT2-TetO) (GenScript, Piscataway, NJ) and used for further experiments. A plasmid encoding tetracycline repressor (TetR) was obtained from Dr. Daiya Takai (University of Southern California, Los Angeles, CA). A total of 2.5 μg of TetR plasmid DNA and 0.5 μg of puromycin plasmid DNA were co-transfected into one of the stable Grm1-melanocytic clones, MASS20. Stable MASS20-TetR clones were selected with 0.8 μg/ml of puromycin (Sigma, St. Louis, MO). Expression of TetR was confirmed by RT-PCR and Western immunoblots with rabbit polyclonal anti-TetR (Abcam Inc. Cambridge, MA). Subsequently, MASS20-TetR clones were transfected with the siAKT2-TetO plasmid (siAKT2-MASS clones). Selection of siAKT2-MASS stable clones was performed with 100 μg/ml of hygromycin.
Allograft assay
All animal studies were approved by the Institutional Review Board for the Animal Care and Facilities Committee of Rutgers University. Nude mice were purchased from Taconic (Hudson, NY). To assess the effect of siAKT2 on Grm1-induced tumorigenesis of MASS clones, 106 cells from each stable siAKT2-MASS clone were injected subcutaneously into the dorsal flanks of six-week old male immunodeficient nude mice (Taconic, Hudson, NY). Appearance of tumor was monitored daily and measured twice a week. When the tumor volumes reach 6mm3, mice were divided randomly into experimental and control groups with 10-12 mice per group. Mice in the experimental group were fed with doxycycline (0.1% w/v) containing drinking water and the control group was fed with regular drinking water. Drinking water was changed every 3 days. Tumor volumes were measured twice a week with a vernier caliper and calculated by the formula (d2xD/2) as described (Stepulak et al., 2005).
Invasion assay
Invasiveness of inducible siAKT2-MASS clones was assessed using InnoCyte™ cell invasion assay kit from EMD chemicals Inc. (San Diago, CA). Briefly, 2×104 cells were plated in the inner chamber with serum free RPMI medium in the presence (2μg/ml) or absence of doxycycline. After 48hrs, cells that passed through the basement matrix membrane were dissolved with the Cell Staining Solution. The fluorescence was measured at an excitation of 485nm and an emission of 520nm using GENios plate reader (Tecan, Durham, NC).
Significance.
Metastatic melanoma is among the most difficult diseases to treat. Over the last several years, numerous novel mouse models have emerged to facilitate and enhance our knowledge towards the contribution of genetic and epigenetic modifications in melanomagenesis. Our group previously described an unknown component of melanoma pathogenesis. We have demonstrated that the ectopic expression of mGlu1 in melanocytes was sufficient to induce spontaneous melanoma development in vivo. Subsequently, we also showed that 40-60% of human melanoma cell lines and biopsies expressed mGlu1. Introduction of Grm1 into mouse melanocytes resulted in cell transformation in vitro and tumorigenesis in vivo. Now we show that allograft tumors display activated AKT and have provided evidences identifying AKT2 as the dominant isoform contributing to these tumorigenic phenotypes, in addition to MAPK, in Grm1 mediated melanocyte transformation.
ACKNOWLEDGEMENT
This work was supported by the following grants: National Cancer Institute grants R01CA108720 and RO1CA124975, and New Jersey Commission on Cancer Research Development Award. We wish to thank Dr. Dorothy Bennett for melan-a cells and Dr. Daiya Takai for providing inducible TetR plasmid. We also thank Jeffrey Martino for his kind help in the preparation of the manuscript.
Footnotes
There is no conflicting interest in this research among authors.
REFERENCES
- BANKO JL, HOU L, POULIN F, SONENBERG N, KLANN E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–73. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLACOSA A, DE FEO D, GODWIN AK, BELL DW, CHENG JQ, ALTOMARE DA, WAN M, DUBEAU L, SCAMBIA G, MASCIULLO V, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- BELLACOSA A, TESTA JR, STAAL SP, TSICHLIS PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- CANTLEY LC, NEEL BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG JQ, RUGGERI B, KLEIN WM, SONODA G, ALTOMARE DA, WATSON DK, TESTA JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-SOLAL KA, CRESPO-CARBONE SM, NAMKOONG J, MACKASON KR, ROBERTS KG, REUHL KR, CHEN S. Progressive appearance of pigmentation in amelanotic melanoma lesions. Pigment Cell Res. 2002;15:282–9. doi: 10.1034/j.1600-0749.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- DHAWAN P, SINGH AB, ELLIS DL, RICHMOND A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–42. [PubMed] [Google Scholar]
- DU K, MONTMINY M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–9. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- GERBER U, GEE CE, BENQUET P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- GOVINDARAJAN B, SLIGH JE, VINCENT BJ, LI M, CANTER JA, NICKOLOFF BJ, RODENBURG RJ, SMEITINK JA, OBERLEY L, ZHANG Y, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINOI E, TAKARADA T, UESHIMA T, TSUCHIHASHI Y, YONEDA Y. Glutamate signaling in peripheral tissues. Eur J Biochem. 2004;271:1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- HOU L, KLANN E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–61. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARIM F, WANG CC, GEREAU RWT. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–9. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIN YE, NAMKOONG J, COHEN-SOLAL K, SHIN SS, MARTINO JJ, OKA M, CHEN S. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–86. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- MYERS MP, PASS I, BATTY IH, VAN DER KAAY J, STOLAROV JP, HEMMINGS BA, WIGLER MH, DOWNES CP, TONKS NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAMKOONG J, SHIN SS, LEE HJ, MARIN YE, WALL BA, GOYDOS JS, CHEN S. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- POLLOCK PM, COHEN-SOLAL K, SOOD R, NAMKOONG J, MARTINO JJ, KOGANTI A, ZHU H, ROBBINS C, MAKALOWSKA I, SHIN SS, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- PUGAZHENTHI S, NESTEROVA A, SABLE C, HEIDENREICH KA, BOXER LM, HEASLEY LE, REUSCH JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- ROBERTSON GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–85. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- RUDMAN SM, PHILPOTT MP, THOMAS GA, KEALEY T. The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol. 1997;109:770–7. doi: 10.1111/1523-1747.ep12340934. [DOI] [PubMed] [Google Scholar]
- SHARP LL, JAMESON JM, CAUVI G, HAVRAN WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–9. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- SHIN SS, MARTINO JJ, CHEN S. Metabotropic glutamate receptors (mGlus) and cellular transformation. Neuropharmacology. 2008a;55:396–402. doi: 10.1016/j.neuropharm.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIN SS, NAMKOONG J, WALL BA, GLEASON R, LEE HJ, CHEN S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008b;21:368–78. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKERRY TM, GENEVER PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol. Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- SKORSKI T, BELLACOSA A, NIEBOROWSKA-SKORSKA M, MAJEWSKI M, MARTINEZ R, CHOI JK, TROTTA R, WLODARSKI P, PERROTTI D, CHAN TO, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. Embo J. 1997;16:6151–61. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAAL SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHL JM, SHARMA A, CHEUNG M, ZIMMERMAN M, CHENG JQ, BOSENBERG MW, KESTER M, SANDIRASEGARANE L, ROBERTSON GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- STAMBOLIC V, SUZUKI A, DE LA POMPA JL, BROTHERS GM, MIRTSOS C, SASAKI T, RULAND J, PENNINGER JM, SIDEROVSKI DP, MAK TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- STEPULAK A, SIFRINGER M, RZESKI W, ENDESFELDER S, GRATOPP A, POHL EE, BITTIGAU P, FELDERHOFF-MUESER U, KAINDL AM, BUHRER C, et al. NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc Natl Acad Sci U S A. 2005;102:15605–10. doi: 10.1073/pnas.0507679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVAKKOL A, VARANI J, ELDER JT, ZOUBOULIS CC. Maintenance of human skin in organ culture: role for insulin-like growth factor-1 receptor and epidermal growth factor receptor. Arch Dermatol Res. 1999;291:643–51. doi: 10.1007/s004030050469. [DOI] [PubMed] [Google Scholar]
- THANDI S, BLANK JL, CHALLISS RA. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem. 2002;83:1139–53. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- TRISCIUOGLIO D, IERVOLINO A, ZUPI G, DEL BUFALO D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–62. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITWAM T, VANBROCKLIN MW, RUSSO ME, HAAK PT, BILGILI D, RESAU JH, KOO HM, HOLMEN SL. Differential oncogenic potential of activated RAS isoforms in melanocytes. Oncogene. 2007;26:4563–70. doi: 10.1038/sj.onc.1210239. [DOI] [PubMed] [Google Scholar]
- WU H, GOEL V, HALUSKA FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–22. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]