Abstract
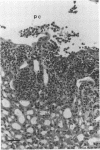
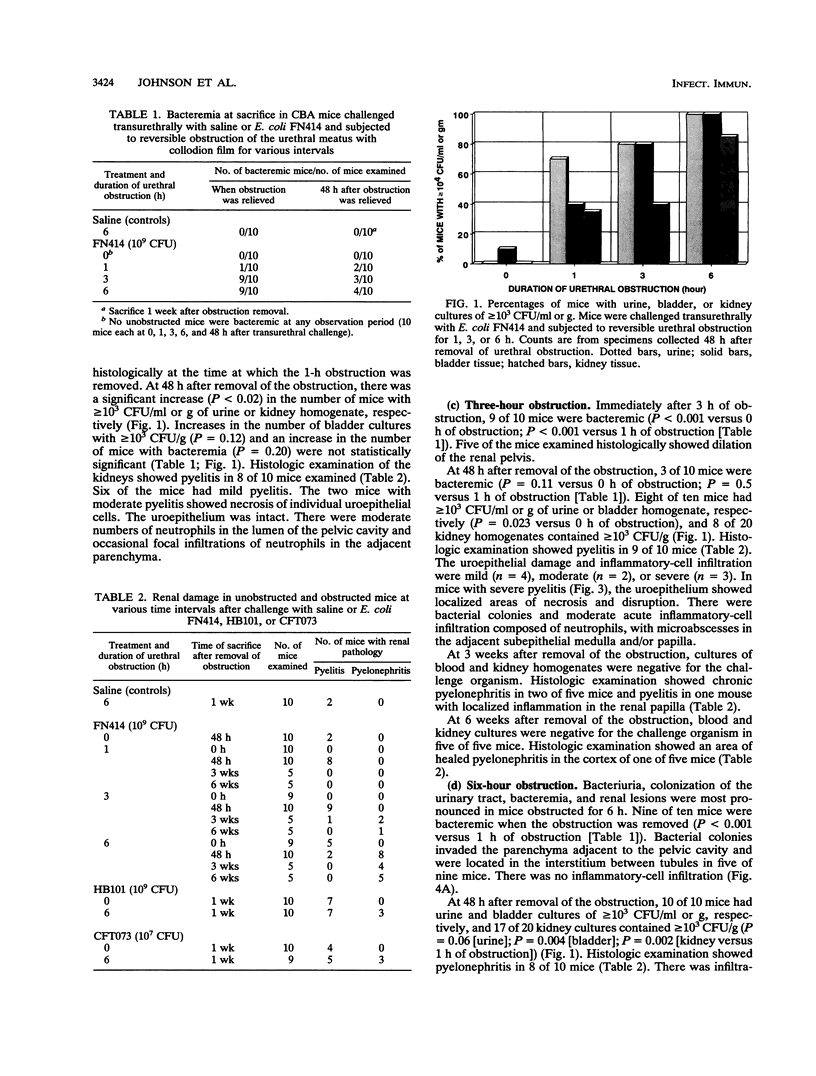
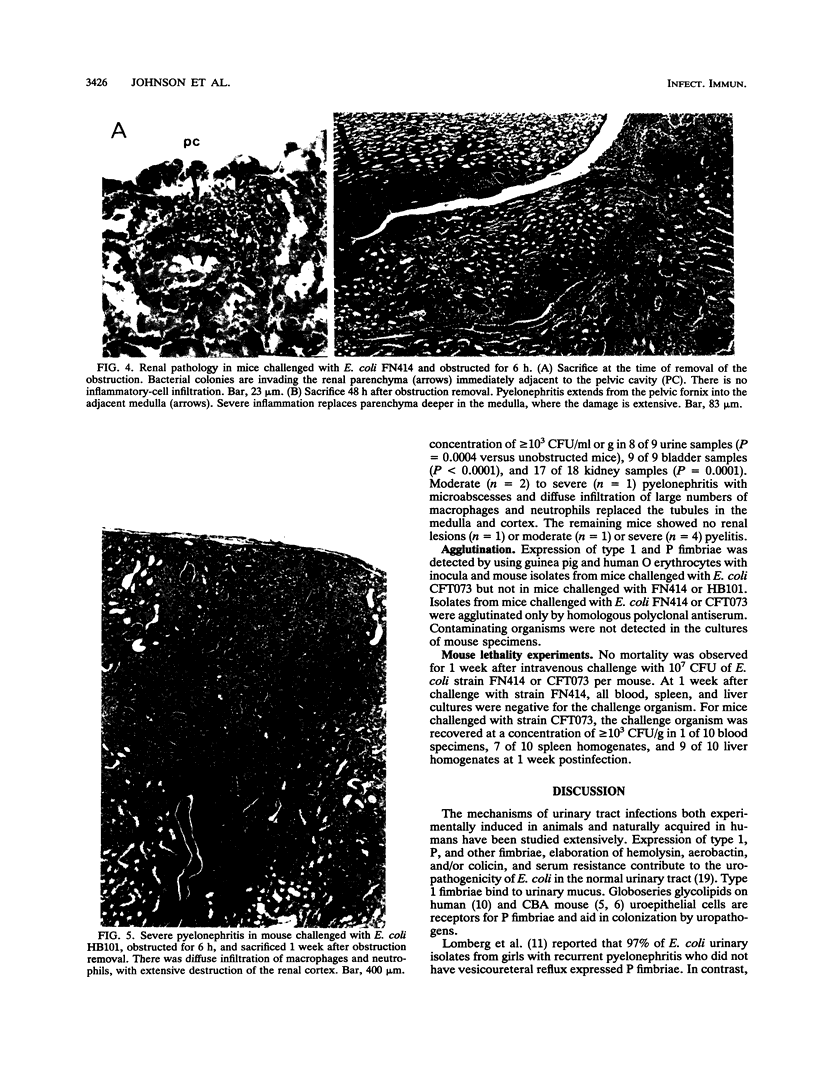
Urethral obstruction may be caused by prostatic hypertrophy, urethral stricture, or encrustation of a urethral-catheter lumen. Bacteriuria often complicates these obstructions. The sequelae include fever, acute pyelonephritis, chronic renal inflammation, and death. We hypothesized that even brief obstruction of the urinary tract containing a nonvirulent bacterium would result in these complications. Mice challenged transurethrally with Escherichia coli FN414, which is rapidly eliminated from normal mice without causing bacteriuria, bacteremia, or renal pathology, were subjected to reversible urethral obstruction by coating the urethral meatus with collodion for 1, 3, or 6 h. The majority of mice obstructed for 1 h demonstrated parenchymal renal inflammation 48 h later. At the end of 3 h of obstruction, 9 of 10 mice were bacteremic; some bacteremias were present at 48 h after removal of the obstruction. At that time, more severe renal inflammation was seen in these mice. As little as 6 h of obstruction resulted not only in the acute changes described above but also in chronic renal inflammation and fibrosis in the majority of animals sacrificed 3 and 6 weeks later. Additional studies demonstrated that urethral obstruction enhanced the uropathogenicity of another nonpathogenic E. coli strain (K-12 strain HB101) and caused more severe renal lesions in mice challenged with E. coli CFT073, isolated from a patient with symptoms of pyelonephritis. These findings demonstrate that brief urethral obstruction may (i) induce organisms which are cleared rapidly from the normal urinary tract to cause bacteriuria, bacteremia, and pyelonephritis and (ii) intensify the renal lesions caused by a uropathogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B. R., JACKSON G. G. Pyelitis, an important factor in the pathogenesis of retrograde pyelonephritis. J Exp Med. 1961 Sep 1;114:375–384. doi: 10.1084/jem.114.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille J., Glauser M. P. Protection against chronic pyelonephritis in rats by suppression of acute suppuration: effect of colchicine and neutropenia. J Infect Dis. 1982 Aug;146(2):220–226. doi: 10.1093/infdis/146.2.220. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Gleckman R., Blagg N., Hibert D., Hall A., Crowley M., Pritchard A., Warren W. Catheter-related urosepsis in the elderly: a prospective study of community-derived infections. J Am Geriatr Soc. 1982 Apr;30(4):255–257. doi: 10.1111/j.1532-5415.1982.tb07097.x. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lockatell C. V., Hall-Craigs M., Mobley H. L., Warren J. W. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J Urol. 1987 Sep;138(3):632–635. doi: 10.1016/s0022-5347(17)43287-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Roberts P. L., Stamm W. E. P fimbriae and other virulence factors in Escherichia coli urosepsis: association with patients' characteristics. J Infect Dis. 1987 Jul;156(1):225–229. doi: 10.1093/infdis/156.1.225. [DOI] [PubMed] [Google Scholar]
- Kunin C. M., Chin Q. F., Chambers S. Formation of encrustations on indwelling urinary catheters in the elderly: a comparison of different types of catheter materials in "blockers" and "nonblockers". J Urol. 1987 Oct;138(4):899–902. doi: 10.1016/s0022-5347(17)43412-4. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberg H., Hellström M., Jodal U., Leffler H., Lincoln K., Svanborg Edén C. Virulence-associated traits in Escherichia coli causing first and recurrent episodes of urinary tract infection in children with or without vesicoureteral reflux. J Infect Dis. 1984 Oct;150(4):561–569. doi: 10.1093/infdis/150.4.561. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. Tip proteins of pili associated with pyelonephritis: new candidates for vaccine development. Vaccine. 1988 Apr;6(2):110–112. doi: 10.1016/s0264-410x(88)80010-0. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Green D. M., Trifillis A. L., Johnson D. E., Chippendale G. R., Lockatell C. V., Jones B. D., Warren J. W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990 May;58(5):1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie H. L., Jr, Warren J. W. Reasons for replacement of long-term urethral catheters: implications for randomized trials. J Urol. 1990 Mar;143(3):507–509. doi: 10.1016/s0022-5347(17)40003-6. [DOI] [PubMed] [Google Scholar]
- Nishi T., Tsuchiya K. Experimental urinary tract infection with Pseudomonas aeruginosa in mice. Infect Immun. 1978 Nov;22(2):508–515. doi: 10.1128/iai.22.2.508-515.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Green G. M., Kass E. H. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968 Dec;47(12):2689–2700. doi: 10.1172/JCI105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecha B., Low D., O'Hanley P. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J Clin Invest. 1989 Jun;83(6):2102–2108. doi: 10.1172/JCI114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Sobel J. D. Bacterial adherence in the pathogenesis of urinary tract infection: a review. Rev Infect Dis. 1987 May-Jun;9(3):470–487. doi: 10.1093/clinids/9.3.470. [DOI] [PubMed] [Google Scholar]
- Tancrède C. H., Andremont A. O. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985 Jul;152(1):99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Damron D., Tenney J. H., Hoopes J. M., Deforge B., Muncie H. L., Jr Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987 Jun;155(6):1151–1158. doi: 10.1093/infdis/155.6.1151. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Muncie H. L., Jr, Hall-Craggs M. Acute pyelonephritis associated with bacteriuria during long-term catheterization: a prospective clinicopathological study. J Infect Dis. 1988 Dec;158(6):1341–1346. doi: 10.1093/infdis/158.6.1341. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Steinberg L., Hebel J. R., Tenney J. H. The prevalence of urethral catheterization in Maryland nursing homes. Arch Intern Med. 1989 Jul;149(7):1535–1537. [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]