Abstract
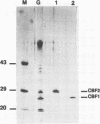
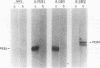
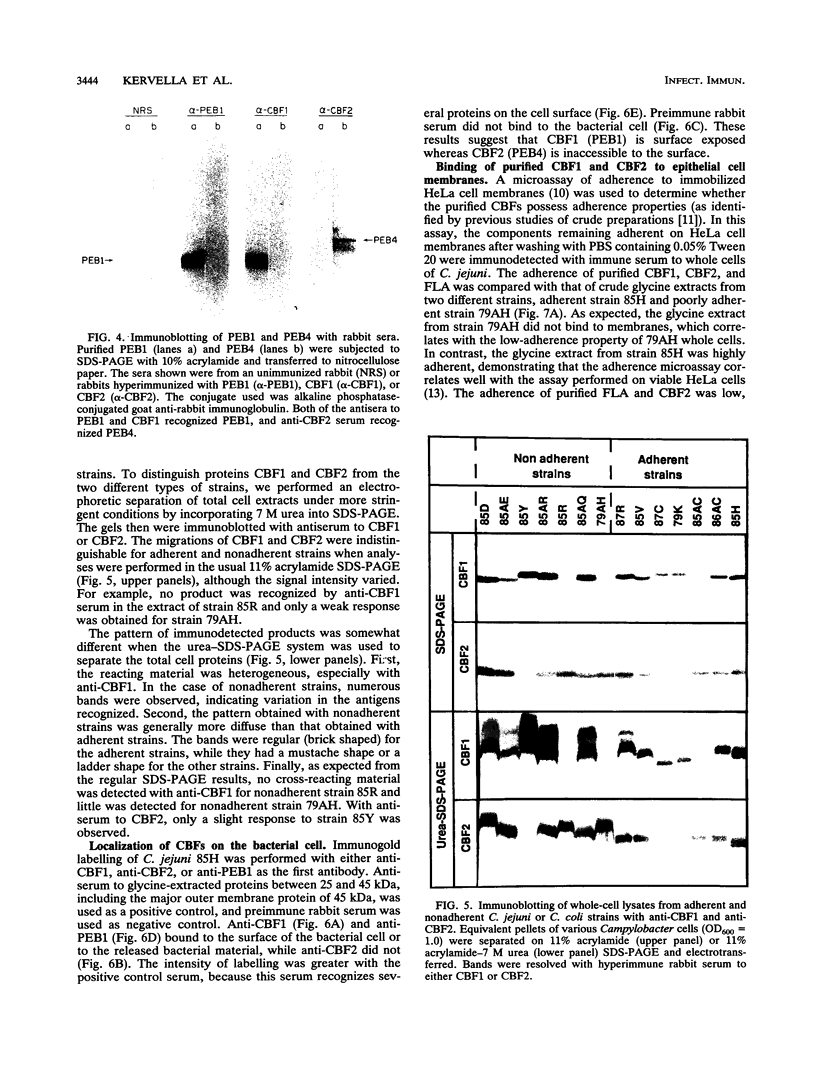
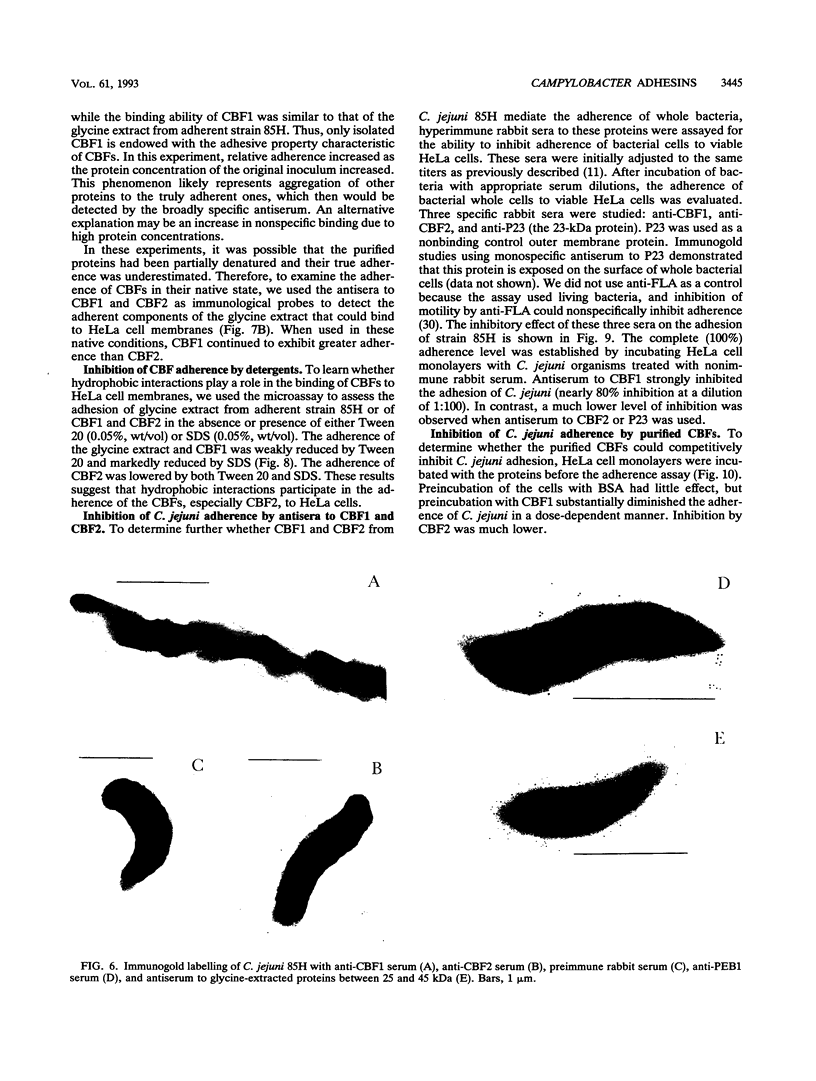
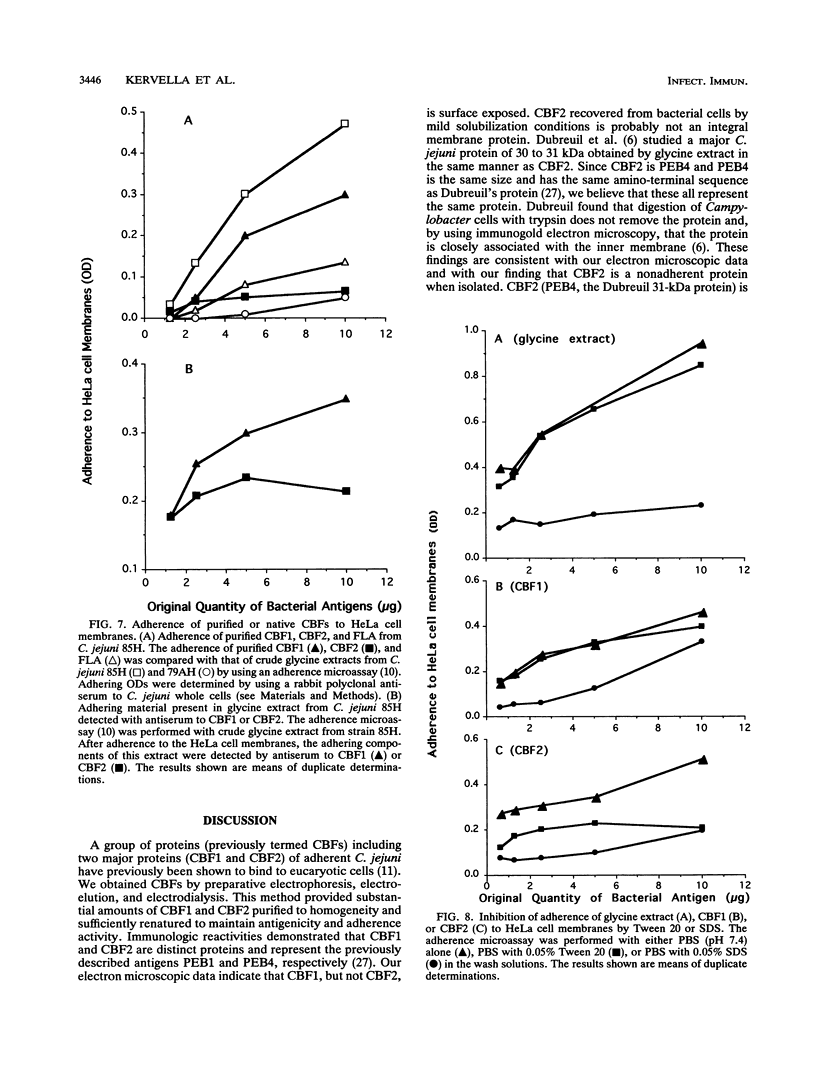
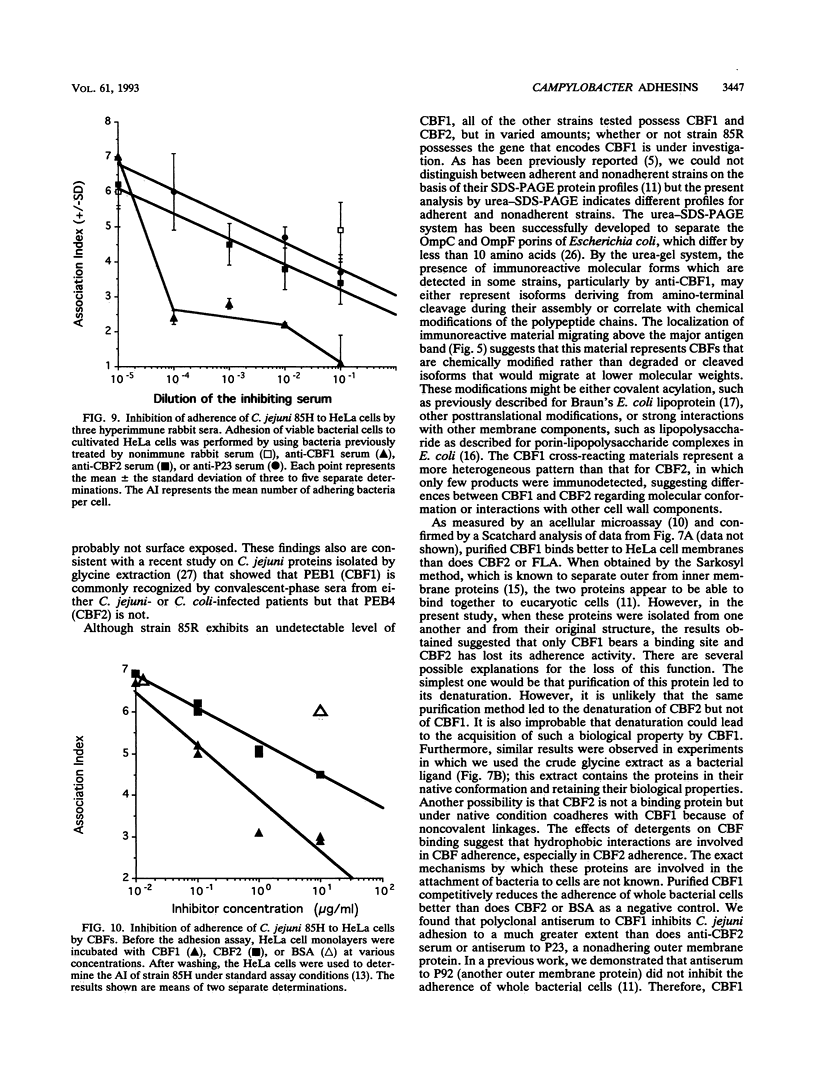
Two immunogenic proteins of 27 (CBF1) and 29 (CBF2) kDa from enteropathogenic Campylobacter species appear to bind to mammalian cells. We purified these two proteins from a pathogenic and adherent Campylobacter jejuni strain to homogeneity by using acid extraction, preparative gel electrophoresis, and electroelution. Polyclonal rabbit antisera to these proteins were prepared. Immunologic studies indicate that CBF1 corresponds to the PEB1 and CBF2 corresponds to the PEB4 described by Pei et al. (Z. Pei, R. T. Ellison, and M. Blaser, J. Biol. Chem. 226:16363-16369, 1991). Immunogold labeling of a C. jejuni adherent strain with anti-CBF1, anti-CBF2, and anti-PEB1 suggested that CBF1 (PEB1) is surface exposed while CBF2 (PEB4) is not. Analysis of whole-cell extracts from 14 strains by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with 7 M urea and immunoblotting with antisera to CBF1 and CBF2 suggests that CBF proteins from adherent and nonadherent strains are different. Use of purified proteins in a microassay of adherence to cellular membranes indicated that CBF1 was much more adherent than CBF2. Adherence of C. jejuni to viable HeLa cells was markedly reduced with the antiserum to CBF1, whereas the CBF2 antiserum was a poor inhibitor. Purified CBF1 competitively inhibited adherence of whole bacteria to HeLa cells, whereas purified CBF2 was no better a competitor than bovine serum albumin. Adherence of CBF2 was markedly reduced in the presence of Tween 20 or SDS, whereas adherence of CBF1 was reduced only by SDS. We conclude that (i) CBF1 (PEB1) is surface exposed and may be the key protein for C. jejuni adhesion and (ii) CBF2 (PEB4) may be complexed with CBF1 and may passively coadhere with CBF1 under certain experimental conditions. Adherent and nonadherent strains contain different isotypes of these two proteins which could be useful markers of C. jejuni adhesion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Vasil M. L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- De Melo M. A., Gabbiani G., Pechère J. C. Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect Immun. 1989 Jul;57(7):2214–2222. doi: 10.1128/iai.57.7.2214-2222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil J. D., Kostrzynska M., Logan S. M., Harris L. A., Austin J. W., Trust T. J. Purification, characterization, and localization of a protein antigen shared by thermophilic campylobacters. J Clin Microbiol. 1990 Jun;28(6):1321–1328. doi: 10.1128/jcm.28.6.1321-1328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Blaser M. J., Snyder E. L. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter outer membrane proteins. Infect Immun. 1987 Jul;55(7):1564–1572. doi: 10.21236/ada265461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Barratt D. L., Wise G. E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchere J. L., Rosenau A., Veron M., Moyen E. N., Richard S., Pfister A. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect Immun. 1986 Nov;54(2):283–287. doi: 10.1128/iai.54.2.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchère J. L., Blaser M. J. Adherence of Helicobacter pylori cells and their surface components to HeLa cell membranes. Microb Pathog. 1990 Dec;9(6):427–439. doi: 10.1016/0882-4010(90)90061-t. [DOI] [PubMed] [Google Scholar]
- Fauchère J. L., Kervella M., Rosenau A., Mohanna K., Véron M. Adhesion to HeLa cells of Campylobacter jejuni and C. coli outer membrane components. Res Microbiol. 1989 Jul-Aug;140(6):379–392. doi: 10.1016/0923-2508(89)90014-4. [DOI] [PubMed] [Google Scholar]
- Fauchère J. L., Véron M., Lellouch-Tubiana A., Pfister A. Experimental infection of gnotobiotic mice with Campylobacter jejuni: colonisation of intestine and spread to lymphoid and reticulo-endothelial organs. J Med Microbiol. 1985 Oct;20(2):215–224. doi: 10.1099/00222615-20-2-215. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Holzenburg A., Engel A., Kessler R., Manz H. J., Lustig A., Aebi U. Rapid isolation of OmpF porin-LPS complexes suitable for structure-function studies. Biochemistry. 1989 May 16;28(10):4187–4193. doi: 10.1021/bi00436a010. [DOI] [PubMed] [Google Scholar]
- Konkel M. E., Babakhani F., Joens L. A. Invasion-related antigens of Campylobacter jejuni. J Infect Dis. 1990 Oct;162(4):888–895. doi: 10.1093/infdis/162.4.888. [DOI] [PubMed] [Google Scholar]
- Konkel M. E., Joens L. A. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect Immun. 1989 Oct;57(10):2984–2990. doi: 10.1128/iai.57.10.2984-2990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982 Dec;38(3):898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Dolby J. M. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond) 1985 Oct;95(2):217–227. doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès C., Princé P., Pagès J. M. Immunological comparison of major outer membrane proteins from different strains of Escherichia coli. Ann Inst Pasteur Microbiol. 1987 Jul-Aug;138(4):393–406. doi: 10.1016/0769-2609(87)90057-3. [DOI] [PubMed] [Google Scholar]
- Pei Z. H., Ellison R. T., 3rd, Blaser M. J. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J Biol Chem. 1991 Sep 5;266(25):16363–16369. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tessmer U., Dernick R. Preparative separation of poliovirus structural polypeptides by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, copper staining and electroelution, and induction of monospecific antisera. Electrophoresis. 1989 Apr;10(4):277–279. doi: 10.1002/elps.1150100414. [DOI] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Schmauder-Chock E. A., Parker J. L., Burr D. Selective association and transport of Campylobacter jejuni through M cells of rabbit Peyer's patches. Can J Microbiol. 1988 Oct;34(10):1142–1147. doi: 10.1139/m88-201. [DOI] [PubMed] [Google Scholar]
- de Melo M. A., Pechère J. C. Identification of Campylobacter jejuni surface proteins that bind to Eucaryotic cells in vitro. Infect Immun. 1990 Jun;58(6):1749–1756. doi: 10.1128/iai.58.6.1749-1756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]