Abstract
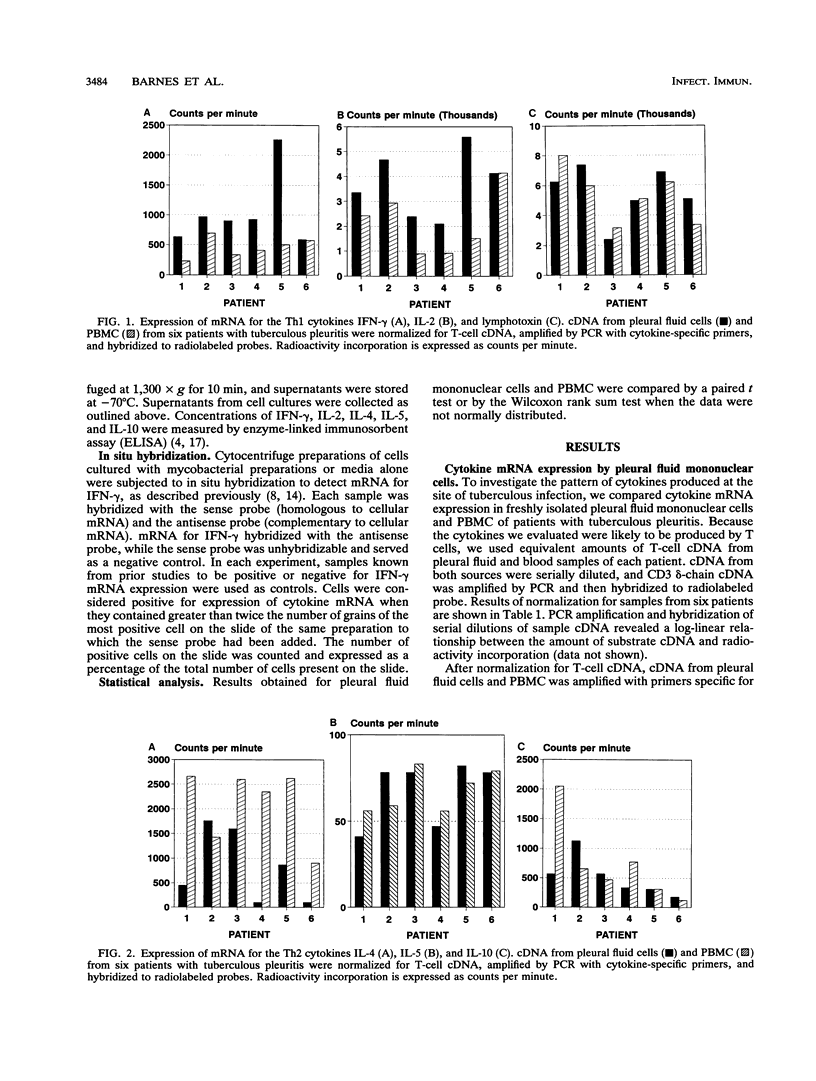
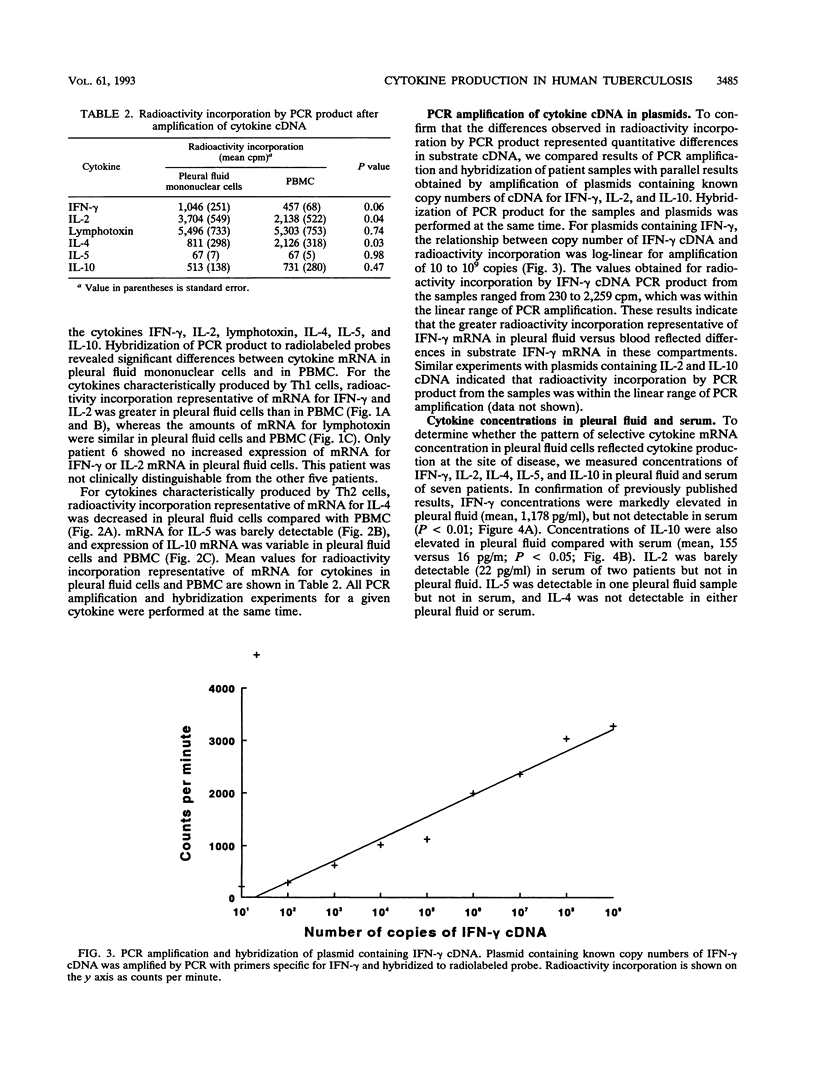
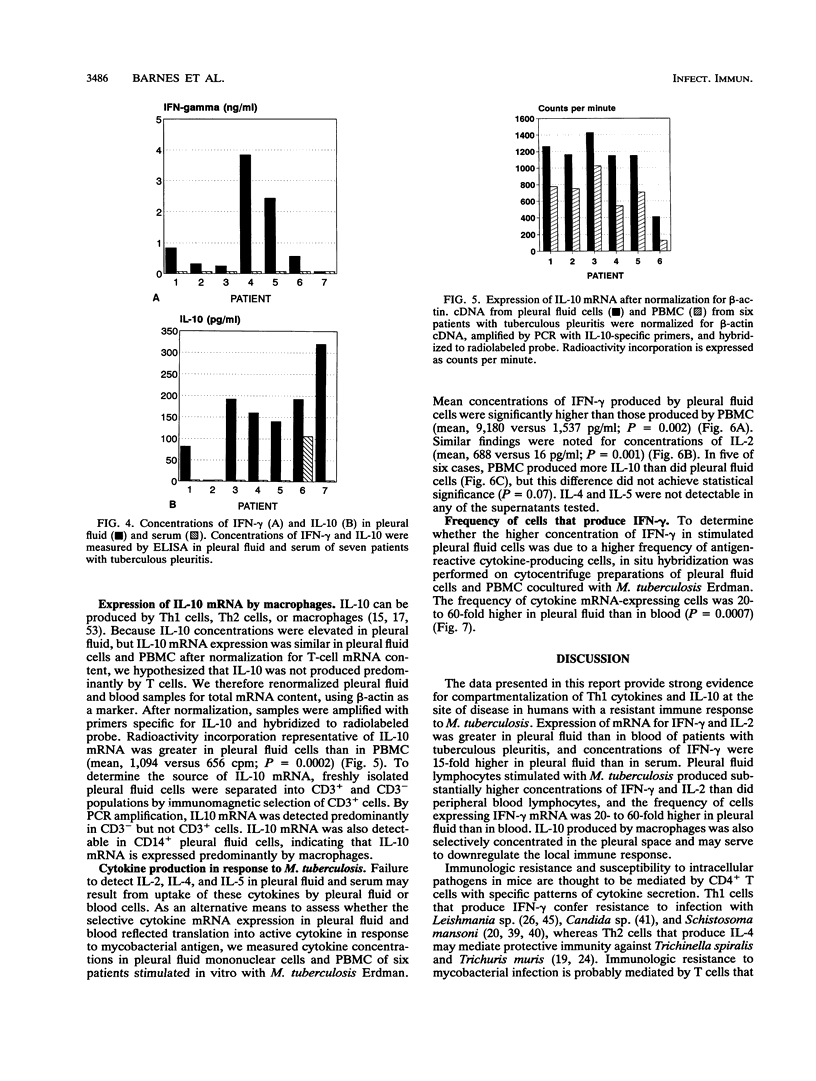
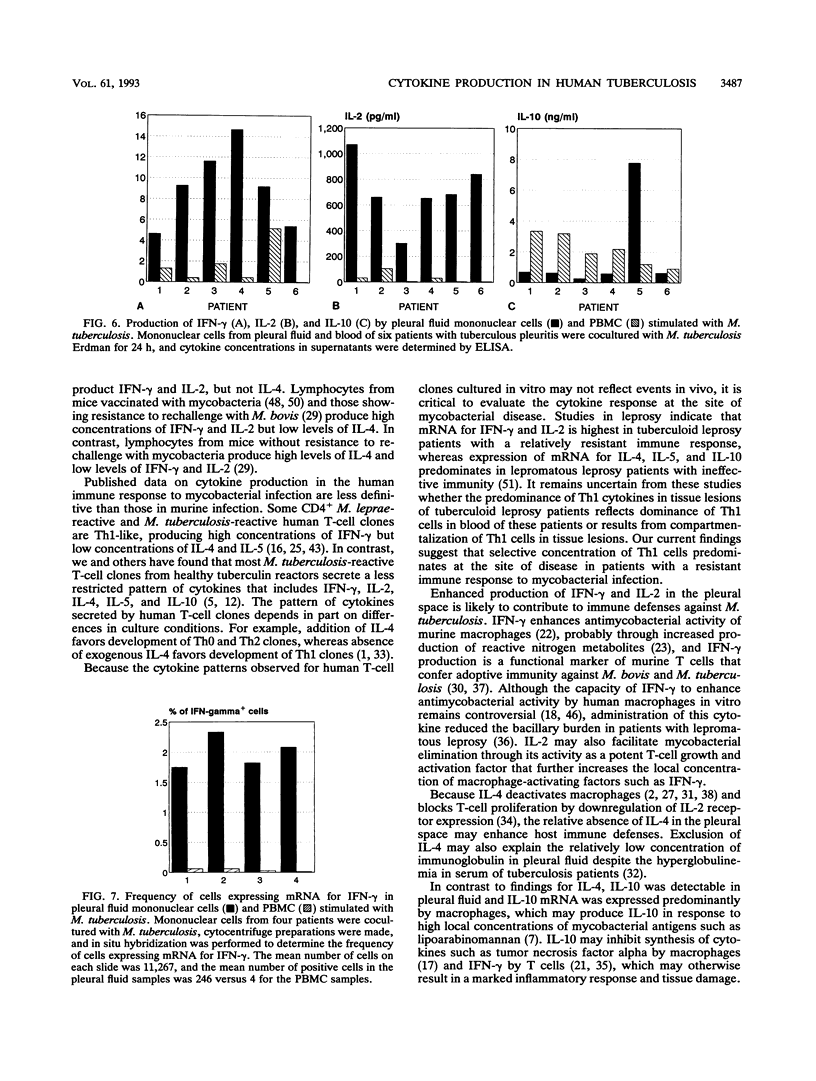
Clinical and immunologic evidence suggests that tuberculous pleuritis provides a model to understand protective immune mechanisms against Mycobacterium tuberculosis. We therefore evaluated the pattern of cytokine mRNA expression and cytokine production in pleural fluid and blood of patients with tuberculous pleuritis. RNA was extracted from mononuclear cells, reverse transcribed to cDNA, and amplified by polymerase chain reaction (PCR). After normalization for T-cell cDNA, cDNA from pleural fluid cells and peripheral blood mononuclear cells (PBMC) was amplified with cytokine-specific primers. PCR product was quantified by Southern blot. For the Th1 cytokines gamma interferon (IFN-gamma) and interleukin-2 (IL-2), PCR product was greater in pleural fluid than in blood, whereas PCR product for the Th2 cytokine IL-4 was decreased in pleural fluid compared with blood. Concentrations of IFN-gamma were elevated in pleural fluid compared with serum, but IL-2, IL-4, and IL-5 were not detectable. Mean concentrations of IFN-gamma and IL-2 in supernatants of M. tuberculosis-stimulated pleural fluid cells were significantly greater than corresponding concentrations in supernatants of stimulated PBMC. In situ hybridization showed that increased IFN-gamma production by pleural fluid cells was associated with a 20- to 60-fold increase in the frequency of antigen-reactive IFN-gamma-mRNA-expressing cells. Because IL-10 can be produced by T cells and macrophages, pleural fluid cells and PBMC were normalized for beta-actin cDNA content and then amplified by PCR with IL-10-specific primers. IL-10 mRNA was greater in pleural fluid cells than in PBMC and was expressed predominantly by macrophages. IL-10 concentrations were elevated in pleural fluid versus serum. These data provide strong evidence for compartmentalization of Th1 cytokines and IL-10 at the site of disease in humans with a resistant immune response to mycobacterial infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abehsira-Amar O., Gibert M., Joliy M., Thèze J., Jankovic D. L. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992 Jun 15;148(12):3820–3829. [PubMed] [Google Scholar]
- Abramson S. L., Gallin J. I. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol. 1990 Jan 15;144(2):625–630. [PubMed] [Google Scholar]
- Bacchetta R., de Waal Malefijt R., Yssel H., Abrams J., de Vries J. E., Spits H., Roncarolo M. G. Host-reactive CD4+ and CD8+ T cell clones isolated from a human chimera produce IL-5, IL-2, IFN-gamma and granulocyte/macrophage-colony-stimulating factor but not IL-4. J Immunol. 1990 Feb 1;144(3):902–908. [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Rivoire B., Fong S. J., Brennan P. J., Voegtline M. S., Minden P., Houghten R. A., Bloom B. R., Modlin R. L. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol. 1992 Mar 15;148(6):1835–1840. [PubMed] [Google Scholar]
- Barnes P. F., Mistry S. D., Cooper C. L., Pirmez C., Rea T. H., Modlin R. L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989 Feb 15;142(4):1114–1119. [PubMed] [Google Scholar]
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else K. J., Grencis R. K. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology. 1991 Apr;72(4):508–513. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Pearce E. J., Urban J. F., Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991 Mar;12(3):A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Grencis R. K., Hültner L., Else K. J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991 Oct;74(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. L., He S. H., Rios M. J., Wick E. A. Interleukin-4 inhibits human macrophage activation by tumor necrosis factor, granulocyte-monocyte colony-stimulating factor, and interleukin-3 for antileishmanial activity and oxidative burst capacity. J Infect Dis. 1992 Feb;165(2):344–351. doi: 10.1093/infdis/165.2.344. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Billing R. J., Parker J. W., Taylor C. R. Cytoplasmic as opposed to surface Ia antigens expressed on human peripheral blood lymphocytes and monocytes. Clin Exp Immunol. 1982 Aug;49(2):355–363. [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura I., Tsukada H., Yoshikawa H., Fujita M., Nomoto K., Mitsuyama M. IFN-gamma-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992 May 1;148(9):2887–2893. [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Levy H., Wayne L. G., Anderson B. E., Barnes P. F., Light R. W. Antimycobacterial antibody levels in pleural fluid as reflection of passive diffusion from serum. Chest. 1990 May;97(5):1144–1147. doi: 10.1378/chest.97.5.1144. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Martinez O. M., Gibbons R. S., Garovoy M. R., Aronson F. R. IL-4 inhibits IL-2 receptor expression and IL-2-dependent proliferation of human T cells. J Immunol. 1990 Mar 15;144(6):2211–2215. [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Kaplan G., Levis W. R., Nusrat A., Witmer M. D., Sherwin S. A., Job C. K., Horowitz C. R., Steinman R. M., Cohn Z. A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986 Jul 3;315(1):6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPER W. H., WARING J. J. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955 May;71(5):616–634. doi: 10.1164/artpd.1955.71.5.616. [DOI] [PubMed] [Google Scholar]
- Reynolds S. R., Kunkel S. L., Thomas D. W., Higashi G. I. T cell clones for antigen selection and lymphokine production in murine Schistosomiasis mansoni. J Immunol. 1990 Apr 1;144(7):2757–2762. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Grohmann U., Mocci S., Mosci P., Puccetti P., Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992 Jul 1;176(1):19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Moreira A. L., Sarno E. N., Malta A. M., Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992 Jun 1;175(6):1729–1737. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Toba H., Ellner J. J. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-gamma. J Infect Dis. 1990 Oct;162(4):932–938. doi: 10.1093/infdis/162.4.932. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Tsuyuguchi I. Analysis of T cell subsets by monoclonal antibodies in patients with tuberculosis after in vitro stimulation with purified protein derivative of tuberculin. Clin Exp Immunol. 1984 Aug;57(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Singh I. G., Mukherjee R., Talwar G. P., Kaufmann S. H. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun. 1992 Jan;60(1):257–263. doi: 10.1128/iai.60.1.257-263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Walker K. B., Butler R., Colston M. J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1992 Mar 15;148(6):1885–1889. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992 Aug 15;149(4):1470–1475. [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]


