Abstract
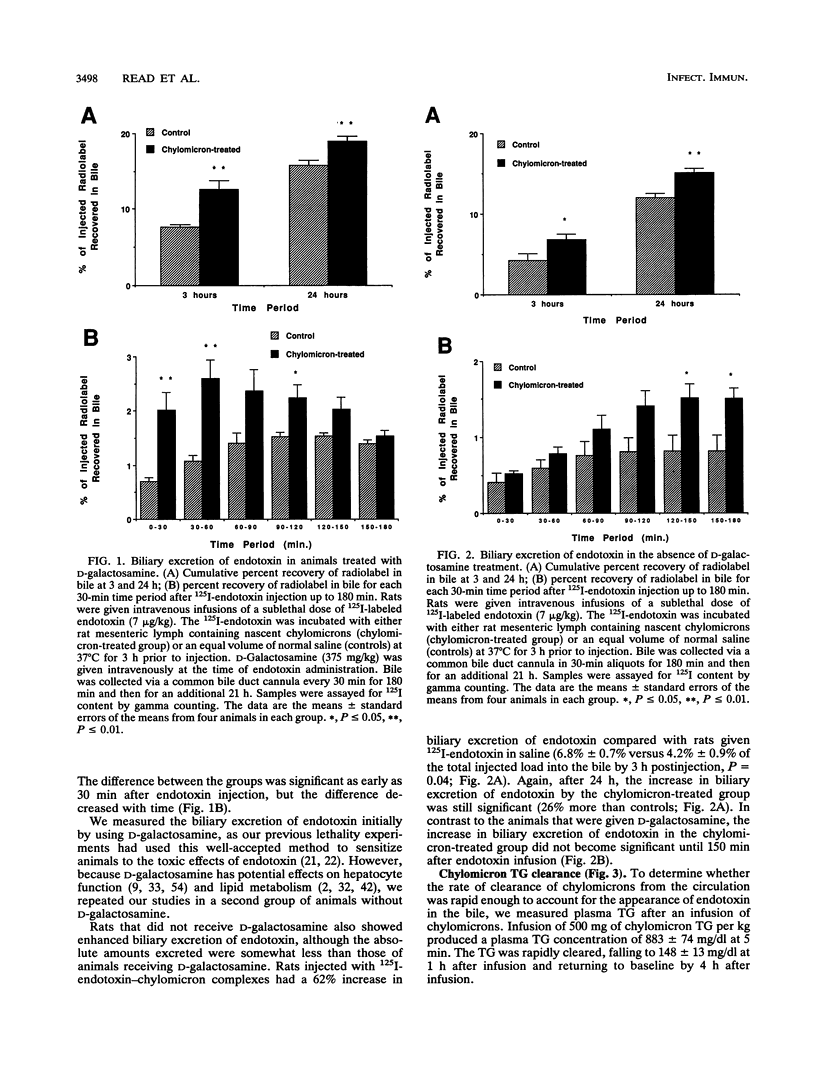
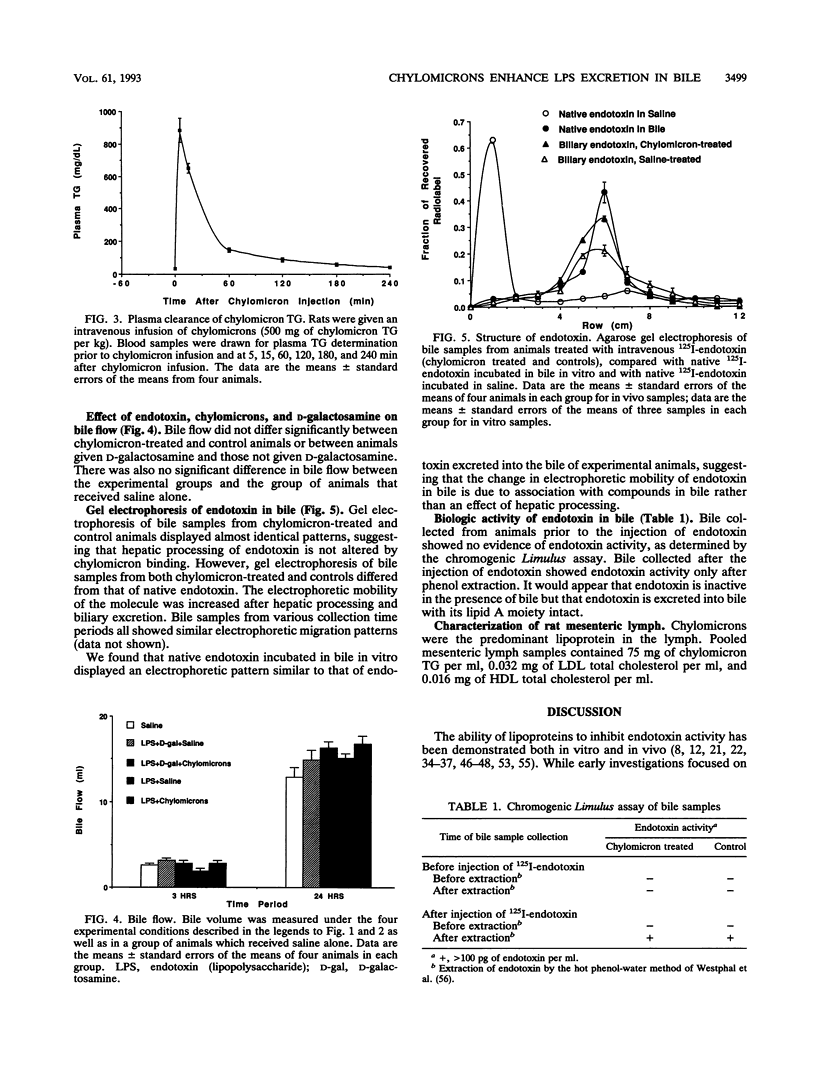
Chylomicrons prevent endotoxin toxicity and increase endotoxin uptake by hepatocytes. As a consequence, less endotoxin is available to activate macrophages, thereby reducing tumor necrosis factor secretion. To determine whether the chylomicron-mediated increase in hepatocellular uptake of endotoxin results in increased endotoxin excretion into bile, we examined bile after endotoxin administration. A sublethal dose (7 micrograms/kg) of 125I-endotoxin was incubated with either rat mesenteric lymph containing nascent chylomicrons (500 mg of chylomicron triglyceride per kg of body weight) or an equal volume of normal saline (controls) for 3 h and then infused into male Sprague-Dawley rats. Bile samples were collected via a common bile duct catheter for 24 h. Infusion of endotoxin incubated with chylomicrons increased biliary excretion of endotoxin by 67% at 3 h (P < or = 0.006) and by 20% at 24 h (P < or = 0.01) compared with infusion of endotoxin incubated in saline. Endotoxin activity, as measured by the Limulus assay, was not detected in the bile of test animals. However, endotoxin activity was detected after hot phenol-water extraction of bile, demonstrating that endotoxin is inactive in the presence of bile but retains bioactivity after hepatic processing. Since the majority of an intravenous endotoxin load has been shown to be cleared by the liver, acceleration of hepatocyte clearance and biliary excretion of endotoxin may represent a component of the mechanism by which chylomicrons protect against endotoxin-induced lethality.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUDE A. I., CAREY F. J., ZALESKY M. Studies with radioactive endotoxin. II. Correlation of physiologic effects with distribution of radioactivity in rabbits injected with radioactive sodium chromate. J Clin Invest. 1955 Jun;34(6):858–866. doi: 10.1172/JCI103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Black D. D., Tso P., Weidman S., Sabesin S. M. Intestinal lipoproteins in the rat with D-(+)-galactosamine hepatitis. J Lipid Res. 1983 Aug;24(8):977–992. [PubMed] [Google Scholar]
- Blackburn G. L. Lipid metabolism in infection. Am J Clin Nutr. 1977 Aug;30(8):1321–1332. doi: 10.1093/ajcn/30.8.1321. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Bryn K., Kierulf P., Ovstebø R., Namork E., Aase B., Jantzen E. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Invest. 1992 Mar;89(3):816–823. doi: 10.1172/JCI115660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana V. G., Siegel J. N., Sabesin S. M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989 Jan;30(1):39–49. [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Haeffner-Cavaillon N., Kirsch S. J., Warren H. S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990 Jul;58(7):2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine induced liver injury. Prog Liver Dis. 1972;4:183–199. [PubMed] [Google Scholar]
- Decker K., Keppler D., Rudigier J., Domschke W. Cell damage by trapping of biosynthetic intermediates. The role of uracil nucleotides in experimental hepatitis. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):412–418. doi: 10.1515/bchm2.1971.352.1.412. [DOI] [PubMed] [Google Scholar]
- Eichbaum E. B., Harris H. W., Kane J. P., Rapp J. H. Chylomicrons can inhibit endotoxin activity in vitro. J Surg Res. 1991 Nov;51(5):413–416. doi: 10.1016/0022-4804(91)90143-a. [DOI] [PubMed] [Google Scholar]
- Emancipator K., Csako G., Elin R. J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992 Feb;60(2):596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Staprans I., Memon R. A., Moser A. H., Shigenaga J. K., Doerrler W., Dinarello C. A., Grunfeld C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res. 1992 Dec;33(12):1765–1776. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Alterations in rats in vivo of the chemical structure of lipopolysaccharide from Salmonella abortus equi. Eur J Biochem. 1985 Oct 15;152(2):353–359. doi: 10.1111/j.1432-1033.1985.tb09205.x. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Kleine B., Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984 Jul-Aug;6(4):483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- Freudenberg M., Galanos C. Metabolic fate of endotoxin in rat. Adv Exp Med Biol. 1990;256:499–509. doi: 10.1007/978-1-4757-5140-6_44. [DOI] [PubMed] [Google Scholar]
- Freudenberg N., Freudenberg M. A., Bandara K., Galanos C. Distribution and localization of endotoxin in the reticulo-endothelial system (RES) and in the main vessels of the rat during shock. Pathol Res Pract. 1985 Mar;179(4-5):517–527. doi: 10.1016/S0344-0338(85)80193-X. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Eichbaum E. B., Kane J. P., Rapp J. H. Detection of endotoxin in triglyceride-rich lipoproteins in vitro. J Lab Clin Med. 1991 Aug;118(2):186–193. [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Rapp J. H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990 Sep;86(3):696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Read T. E., Kane J. P., Jones A. L., Eichbaum E. B., Bland G. F., Rapp J. H. Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J Clin Invest. 1993 Mar;91(3):1028–1034. doi: 10.1172/JCI116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. I., Stone P. C., Stuart J. An improved chromogenic substrate endotoxin assay for clinical use. J Clin Pathol. 1983 Oct;36(10):1145–1149. doi: 10.1136/jcp.36.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf U., Ramadori G., Möller B., Galanos C. Hepatocellular clearance function of bacterial lipopolysaccharides and free lipid A in mice with endotoxic shock. Am J Emerg Med. 1984 Jan;2(1):13–19. doi: 10.1016/0735-6757(84)90105-0. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Hornick C., Renaud G., Windler E. E., Havel R. J. Uptake and processing of remnants of chylomicrons and very low density lipoproteins by rat liver. J Lipid Res. 1984 Nov;25(11):1151–1158. [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine B., Freudenberg M. A., Galanos C. Excretion of radioactivity in faeces and urine of rats injected with 3H,14C-lipopolysaccharide. Br J Exp Pathol. 1985 Jun;66(3):303–308. [PMC free article] [PubMed] [Google Scholar]
- Maitra S. K., Rachmilewitz D., Eberle D., Kaplowitz N. The hepatocellular uptake and biliary excretion of endotoxin in the rat. Hepatology. 1981 Sep-Oct;1(5):401–407. doi: 10.1002/hep.1840010506. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Fat metabolism in normal and abnormal states. Am J Clin Nutr. 1977 Aug;30(8):1311–1320. doi: 10.1093/ajcn/30.8.1311. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Matsuura J. E., Swaney J. B. High density lipoprotein subpopulations from galactosamine-treated rats and their transformation by lecithin:cholesterol acyltransferase. J Lipid Res. 1991 Apr;32(4):581–594. [PubMed] [Google Scholar]
- Mourelle M., Meza M. A. Colchicine prevents D-galactosamine-induced hepatitis. J Hepatol. 1989 Mar;8(2):165–172. doi: 10.1016/0168-8278(89)90004-4. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Dietschy J. M. Effects of specific antibodies, hormones, and lipoproteins on bacterial lipopolysaccharides injected into the rat. J Infect Dis. 1985 Jul;152(1):177–184. doi: 10.1093/infdis/152.1.177. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Van Lenten B. J., Berliner J. A., Fogelman A. M. Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. J Clin Invest. 1988 Feb;81(2):601–605. doi: 10.1172/JCI113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praaning-van Dalen D. P., Brouwer A., Knook D. L. Clearance capacity of rat liver Kupffer, Endothelial, and parenchymal cells. Gastroenterology. 1981 Dec;81(6):1036–1044. [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., van der Meulen J., Brouwer A., Hummel M. J., Mauw B. J., van der Ploeg J. C., Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest. 1981 Jul;45(1):38–45. [PubMed] [Google Scholar]
- Rustgi V. K., Jones D. B., Dinarello C. A., Hoofnagle J. H. Lymphokines and bile secretion in the rat. Liver. 1987 Jun;7(3):149–154. doi: 10.1111/j.1600-0676.1987.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Sabesin S. M., Kuiken L. B., Ragland J. B. Lipoprotein and lecithin: cholesterol acyltransferase changes in galactosamine-induced rat liver injury. Science. 1975 Dec 26;190(4221):1302–1304. doi: 10.1126/science.812181. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Goodman D. S., Fidge N. H. The metabolism of chylomicron cholesteryl ester in rat liver. A combined radioautographic-electron microscopic and biochemical study. J Cell Biol. 1969 Dec;43(3):410–431. doi: 10.1083/jcb.43.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., Morrison D. C. The physical-chemical characterization and biologic activity of serum released lipopolysaccharides. J Immunol. 1988 Nov 15;141(10):3523–3531. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Utili R., Abernathy C. O., Zimmerman H. J. Cholestatic effects of Escherichia coli endotoxin endotoxin on the isolated perfused rat liver. Gastroenterology. 1976 Feb;70(2):248–253. [PubMed] [Google Scholar]
- Utili R., Abernathy C. O., Zimmerman H. J. Studies on the effects of C. coli endotoxin on canalicular bile formation in the isolated perfused rat liver. J Lab Clin Med. 1977 Mar;89(3):471–482. [PubMed] [Google Scholar]
- Van Bossuyt H., De Zanger R. B., Wisse E. Cellular and subcellular distribution of injected lipopolysaccharide in rat liver and its inactivation by bile salts. J Hepatol. 1988 Dec;7(3):325–337. doi: 10.1016/s0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Van Bossuyt H., Wisse E. Cultured Kupffer cells, isolated from human and rat liver biopsies, ingest endotoxin. J Hepatol. 1988 Aug;7(1):45–56. doi: 10.1016/s0168-8278(88)80505-1. [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Haberland M. E., Edwards P. A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Wendel A. Studies on the hepatotoxicity of galactosamine/endotoxin or galactosamine/TNF in the perfused mouse liver. Biochem Pharmacol. 1990 Jan 15;39(2):267–270. doi: 10.1016/0006-2952(90)90025-g. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Knights C. V., Siber G. R. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986 Nov;154(5):784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985 Jun;248(6 Pt 1):E732–E740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]


