Abstract
The surface properties of Legionella pneumophila were examined by analyzing outer membrane (OM) proteins, lipopolysaccharides (LPS), and cellular fatty acids after growth within Acanthamoeba polyphaga and in vitro under various nutrient-depleted conditions. Intra-amoeba-grown legionellae were found to differ in several respects from cells grown in vitro; most notably, they contained a 15-kDa OM protein and a monounsaturated straight-chain fatty acid (18:1(9)). These compounds were also found in abundant quantities in the host amoeba. Immunoblot analysis of intra-amoeba-grown legionellae with antiacanthamoebic serum revealed that both the bacterial whole cells and Sarkosyl-extracted OMs contained amoebic antigens. The findings suggest that the 15-kDa OM protein is likely to be of amoebic origin and associates with the OM of the bacterium. It is proposed that disruption of amoebic membranes, as a result of intra-amoebic infection, may liberate macromolecules, including a 15-kDa polypeptide, a major constituent of the amoebic membrane, which adhere to the surface of the legionellae. Growth under specific nutrient depletions also had a significant effect on the surface composition of L. pneumophila. Cells grown under phosphate depletion were markedly sensitive to protease K digestion and contained lower levels of LPS, as observed by silver staining of the digests on polyacrylamide gels. Intra-amoeba-grown cells contained more bands than the in vitro-grown organisms, reflecting further differences in the nature of the LPS. The whole-cell fatty acids of the phosphate-depleted cells were appreciably different from those of cells grown under other nutritional conditions. We found no evidence for expression of iron-regulated OM proteins under iron depletion.
Full text
PDF


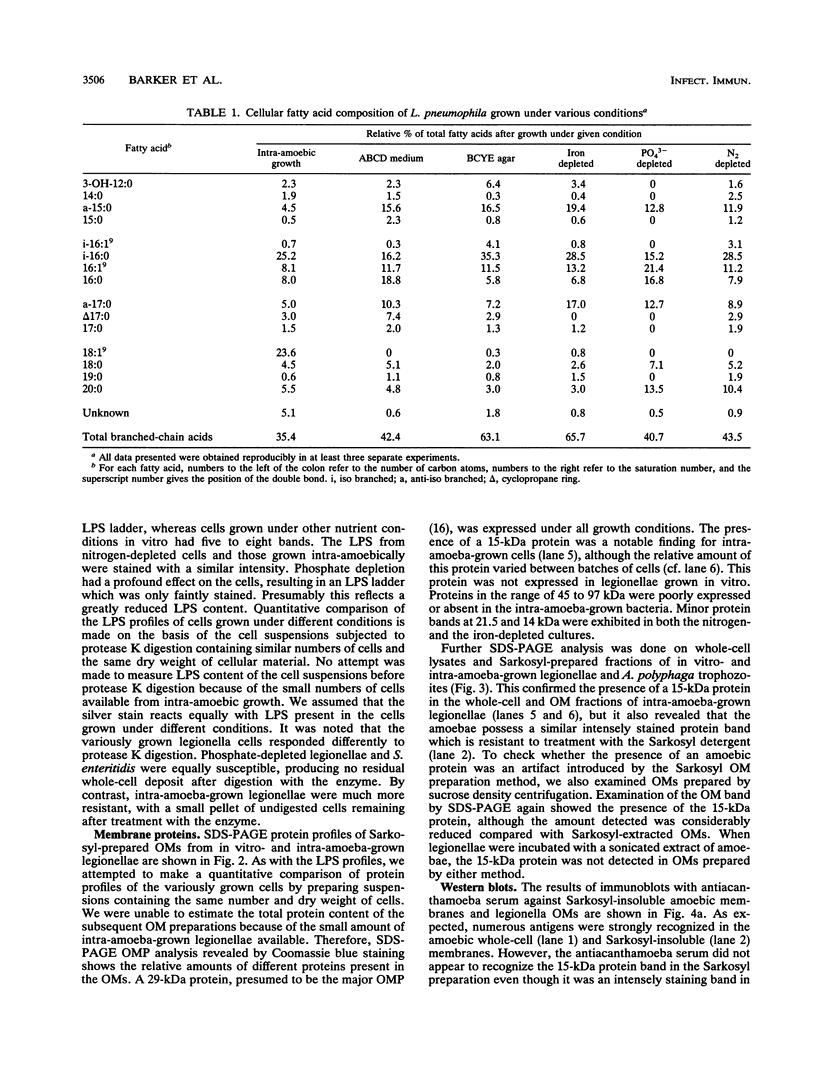

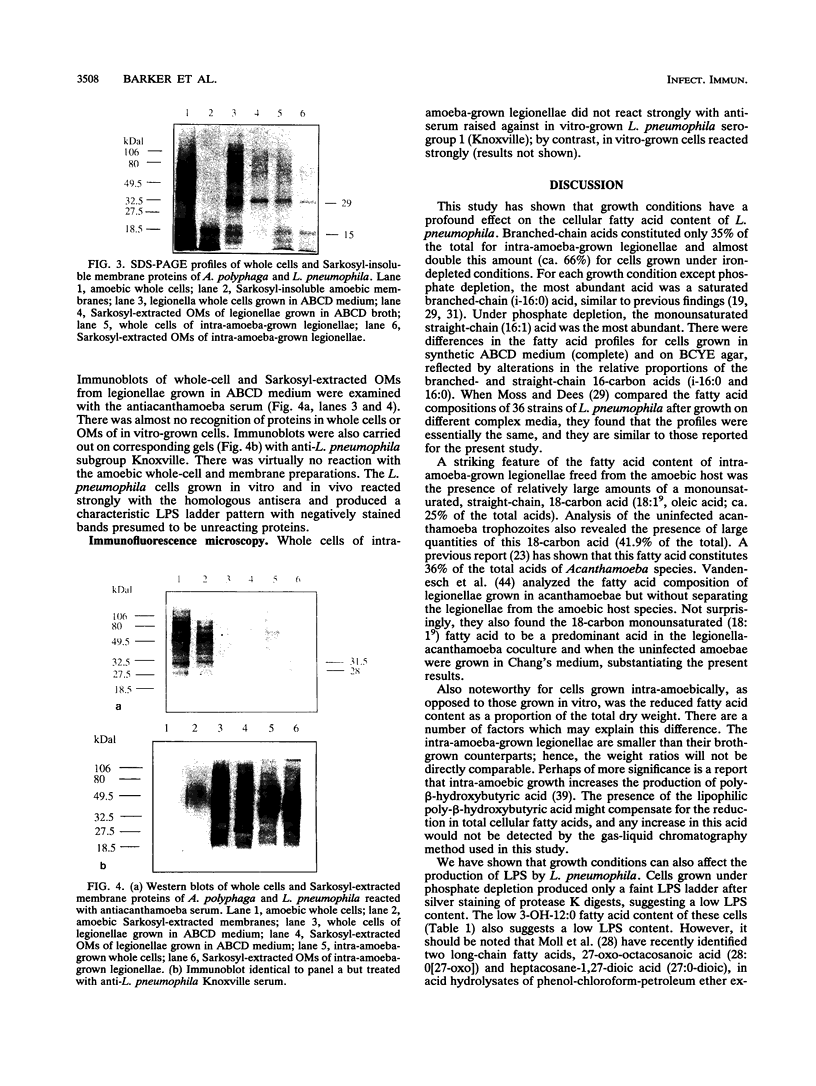


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand C. M., Skinner A. R., Malic A., Kurtz J. B. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J Hyg (Lond) 1983 Oct;91(2):167–178. doi: 10.1017/s0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J., Farrell I. D., Hutchison J. G. Factors affecting growth of Legionella pneumophila in liquid media. J Med Microbiol. 1986 Sep;22(2):97–100. doi: 10.1099/00222615-22-2-97. [DOI] [PubMed] [Google Scholar]
- Barker J., Till D. H. Survival of Legionella pneumophila. Med Lab Sci. 1986 Oct;43(4):388–389. [PubMed] [Google Scholar]
- Barthe C., Joly J. R., Ramsay D., Boissinot M., Benhamou N. Common epitope on the lipopolysaccharide of Legionella pneumophila recognized by a monoclonal antibody. J Clin Microbiol. 1988 May;26(5):1016–1023. doi: 10.1128/jcm.26.5.1016-1023.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat U. R., Carlson R. W., Busch M., Mayer H. Distribution and phylogenetic significance of 27-hydroxy-octacosanoic acid in lipopolysaccharides from bacteria belonging to the alpha-2 subgroup of Proteobacteria. Int J Syst Bacteriol. 1991 Apr;41(2):213–217. doi: 10.1099/00207713-41-2-213. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Collier P. J., Gilbert P. Influence of growth rate on susceptibility to antimicrobial agents: modification of the cell envelope and batch and continuous culture studies. Antimicrob Agents Chemother. 1990 Sep;34(9):1623–1628. doi: 10.1128/aac.34.9.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Role of divalent cations in the action of polymyxin B and EDTA on Pseudomonas aeruginosa. J Gen Microbiol. 1969 Dec;59(2):263–274. doi: 10.1099/00221287-59-2-263. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest. 1991 Oct;88(4):1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. J., Hohman T. C., Bowers B. Purification of plasma membrane from Acanthamoeba castellanii. J Protozool. 1988 Aug;35(3):408–413. doi: 10.1111/j.1550-7408.1988.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., Ashworth L. A. The relationship between the serogroup antigen and lipopolysaccharide of Legionella pneumophila. J Hyg (Lond) 1986 Feb;96(1):39–48. doi: 10.1017/s0022172400062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981 Sep;14(3):298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret W., Ruckdeschel G. Membrane proteins of legionellaceae. I. Membrane proteins of different strains and serogroups of Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Jul;259(4):433–445. doi: 10.1016/s0176-6724(85)80075-4. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R., Makula R. A., Feeley J. C. Cellular lipids of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):631–634. doi: 10.7326/0003-4819-90-4-631. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D. FATTY ACIDS OF ACANTHAMOEBA SP. J Biol Chem. 1963 Nov;238:3584–3587. [PubMed] [Google Scholar]
- Korn E. D., Wright P. L. Macromolecular composition of an amoeba plasma membrane. J Biol Chem. 1973 Jan 25;248(2):439–447. [PubMed] [Google Scholar]
- Lambert P. A. Enterobacteriaceae: composition, structure and function of the cell envelope. Soc Appl Bacteriol Symp Ser. 1988;17:21S–34S. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Moll H., Sonesson A., Jantzen E., Marre R., Zähringer U. Identification of 27-oxo-octacosanoic acid and heptacosane-1,27-dioic acid in Legionella pneumophila. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):1–6. doi: 10.1016/0378-1097(92)90354-q. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Further studies of the cellular fatty acid composition of Legionnaires disease bacteria. J Clin Microbiol. 1979 May;9(5):648–649. doi: 10.1128/jcm.9.5.648-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Karr D. E., Dees S. B. Cellular fatty acid composition of Legionella longbeachae sp. nov. J Clin Microbiol. 1981 Dec;14(6):692–694. doi: 10.1128/jcm.14.6.692-694.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome A. L., Baker R. L., Miller R. D., Arnold R. R. Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun. 1985 Nov;50(2):449–452. doi: 10.1128/iai.50.2.449-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte F. S., Conlin C. A., Motley M. A. Electrophoretic and serological characterization of the lipopolysaccharides of Legionella pneumophila. Infect Immun. 1986 Jun;52(3):676–681. doi: 10.1128/iai.52.3.676-681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Hoffman P. S., Malcolm G. B., Benson R. F., Franzus M. J. Role of keto acids and reduced-oxygen-scavenging enzymes in the growth of Legionella species. J Clin Microbiol. 1986 Jan;23(1):33–42. doi: 10.1128/jcm.23.1.33-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986 Sep;22(9):678–689. [PubMed] [Google Scholar]
- Rowbotham T. J. Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J Clin Pathol. 1983 Sep;36(9):978–986. doi: 10.1136/jcp.36.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Maler T., Chambers J. A., Wilkins J. A. Evidence for a cytosol counterpart of the major plasma membrane protein in Acanthamoeba castellanii. J Gen Microbiol. 1977 Apr;99(2):257–261. doi: 10.1099/00221287-99-2-257. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vandenesch F., Surgot M., Bornstein N., Paucod J. C., Marmet D., Isoard P., Fleurette J. Relationship between free amoeba and Legionella: studies in vitro and in vivo. Zentralbl Bakteriol. 1990 Mar;272(3):265–275. doi: 10.1016/s0934-8840(11)80027-7. [DOI] [PubMed] [Google Scholar]
- Wadowsky R. M., Wilson T. M., Kapp N. J., West A. J., Kuchta J. M., States S. J., Dowling J. N., Yee R. B. Multiplication of Legionella spp. in tap water containing Hartmannella vermiformis. Appl Environ Microbiol. 1991 Jul;57(7):1950–1955. doi: 10.1128/aem.57.7.1950-1955.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]