Abstract
Estrogens influence the differentiation and maintenance of reproductive tissues and affect lipid metabolism and bone remodeling. Two estrogen receptors (ERs) have been identified to date, ERα and ERβ. We previously generated and studied knockout mice lacking estrogen receptor α and reported severe reproductive and behavioral phenotypes including complete infertility of both male and female mice and absence of breast tissue development. Here we describe the generation of mice lacking estrogen receptor β (ERβ −/−) by insertion of a neomycin resistance gene into exon 3 of the coding gene by using homologous recombination in embryonic stem cells. Mice lacking this receptor develop normally and are indistinguishable grossly and histologically as young adults from their littermates. RNA analysis and immunocytochemistry show that tissues from ERβ −/− mice lack normal ERβ RNA and protein. Breeding experiments with young, sexually mature females show that they are fertile and exhibit normal sexual behavior, but have fewer and smaller litters than wild-type mice. Superovulation experiments indicate that this reduction in fertility is the result of reduced ovarian efficiency. The mutant females have normal breast development and lactate normally. Young, sexually mature male mice show no overt abnormalities and reproduce normally. Older mutant males display signs of prostate and bladder hyperplasia. Our results indicate that ERβ is essential for normal ovulation efficiency but is not essential for female or male sexual differentiation, fertility, or lactation. Future experiments are required to determine the role of ERβ in bone and cardiovascular homeostasis.
Keywords: gene targeting, estrogen action, ovary, folliculogenesis, fertility
Estrogens are critical to the functioning and maintenance of a diverse array of tissues and physiological systems in mammals. The actions of estrogen on such classical targets as the reproductive tract, gonads, mammary tissue, and hypothalamic/pituitary axis have been well characterized. A role in nonreproductive tissues, such as maintenance of bone mineral density and cardiovascular health in women, also has been described (1, 2). The physiological responses to estrogen are known to be mediated within specific tissues by at least two estrogen receptors (ERs), ERα and ERβ (3–5). The ERs are a class I member of the nuclear hormone receptor family and act as ligand-activated nuclear transcription factors (6). Studies of the receptors’ tissue distribution and expression pattern indicate that ERα has a broad expression pattern, whereas ERβ has a more focused pattern with high levels in the ovary, prostate, epididymis, lung, and hypothalamus (7, 8). However, the exact physiological responses attributable to each receptor are unknown. We previously described the pleiotropic effects of disruption of the ERα gene in ERα knockout mice (αERKO), including absence of breast development in females and infertility caused by reproductive tract and gonadal and behavioral abnormalities in both sexes (9–13). Here, we describe the generation of mice homozygous for a disruption of the ERβ gene; initial characterizations indicate that the ERβ −/− mice exhibit phenotypes that are distinct from those of the αERKO mice.
MATERIALS AND METHODS
Disruption of the ERβ Gene.
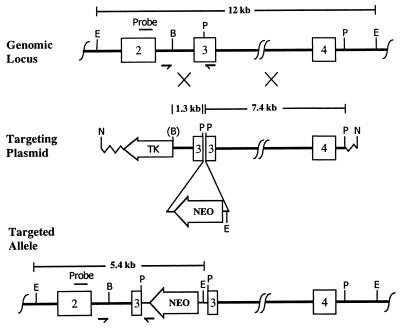
Genomic P1 clones of the mouse ERβ gene from a 129/SvJ library were isolated by Genome Systems (St. Louis) using primers and probes supplied by us. Sequence analysis confirmed the validity of the clones. A targeting construct (Fig. 1) was generated that included 1.3 kb and 7.4 kb of genomic sequence as the 5′ and 3′ homology regions. A copy of the neomycin-resistance gene (Neo) driven by a phosphoglucokinase promoter was inserted in the reverse orientation into the PstI site in exon 3. The targeting construct was linearized at the indicated NotI site and introduced into the BK4 subline of the E14TG2a strain 129 embryonic stem (ES) cell line as described (14). Screening for correctly targeted ES cells was accomplished by using PCR (15), which gave a 1,479-bp band with a primer from intron 2 (5′-GTGATGAGCTGAGGTGGTGCTT-3′) and a primer from the 3′ end of the Neo (5′-GCAGCCTCTGTTCCACATACAC-3′). Targeted ES cells were confirmed by using Southern blot analysis with EcoRI-digested ES cell genomic DNA and a probe specific to exon 2; the unmodified ERβ gene gives a 12-kb band, whereas the disrupted ERβ gene gives a 5.4-kb band. Genotyping of tail DNA was accomplished by using PCR with the intron 2 primer, the Neo primer, and a third primer from exon 3 (5′-CATCCTTCACAGGACCAGACAC-3′); a 1,435-bp band (intron 2 and exon 3 primers) is amplified for homozygous wild-type (+/+) mice, a 1,479-bp band (intron 2 and Neo primers) for homozygous mutant (−/−) mice, and both bands for heterozygous (+/−) mice.
Figure 1.
Targeted disruption of the ERβ gene. (Top) The unmodified genomic locus showing exons 2–4. The probe used for Southern blots is labeled above exon 2. The small arrows indicate the positions of primers. Restriction enzyme sites are EcoRI, BglII, and PstI, designated by E, B, and P. (Middle) The targeting plasmid with the left (1.3-kb) and right (7.4-kb) regions of homology. The Neo insertion site is indicated. NotI (N) was used to linearize the plasmid. Homologous recombination is indicated by the large Xs. (B) shows the BglII lost in the construct. (Bottom) The targeted allele with the Neo gene inserted into exon 3. Note the additional EcoRI site introduced during the targeting. Mouse genomic DNA is shown as a thick line, the inserted Neo sequences as a thinner line, and the plasmid vector as a zig-zag line.
ERβ mRNA Analysis.
Tissue was removed from animals immediately after sacrifice, snap-frozen, and stored at −70°C until further processing. RNA was extracted by using TRIzol (GIBCO/BRL) reagent according to the manufacturer’s protocol. Reverse-transcriptase generation of cDNA was performed on 1 μg of total RNA by using GeneAmp RNA PCR reagents (Perkin–Elmer) with random hexamers according to the manufacturer’s protocol in a final volume of 25 μl. Subsequent PCR analysis was carried out on 5 μl of the cDNA, and the products were analyzed by electrophoresis on a 1.5% agarose gel. Two primers (5′-GCCAATCATCGCTTCTCTAT-3′ and 5′-CCCTCTTTGCTCTTACTGTCCTCT-3′) (4) were used to generate a wild-type product of 1,291 bp. Primers specific for the mouse β-actin cDNA (CLONTECH) were used as a positive control and amplified a fragment of 540 bp. The reverse transcription–PCR (RT-PCR) products were cloned and sequenced by using primers specific to the vector and the ERβ cDNA.
Immunocytochemistry.
Adult wild-type and ERβ −/− females were sacrificed, and the ovaries were removed to 4% buffered formalin at 4°C. The tissue was processed for immunostaining by using the avidin–biotin peroxidase method as described (16). The primary antiserum was specific for the C-terminal amino acid residues 467–485 of the rat ERβ (Affinity Bioreagents, Golden, CO). Preabsorbed ERβ antiserum was prepared by incubating 1 ml of the antiserum with 20 mg of peptide for 24 hr at 4°C. The secondary antibody was biotin-labeled goat-antirabbit IgG. The final signal was generated with avidin–biotin peroxidase and diaminobenzidine (BioGenex Laboratories, San Ramon, CA). The sections were counterstained with hematoxylin.
Fertility Tests.
For evaluation of female fertility, known fertile males were placed with 4- to 6-week-old wild-type or ERβ −/− females for 8 weeks and then removed. Cages were monitored daily, and for an additional 23 days, and presence of seminal plugs, and number and size of litters were recorded. To evaluate male fertility, 6-week-old ERβ −/− males were placed with known fertile females for 6 weeks, and the same parameters were monitored.
Superovulation and Oocyte Quantitation.
Wild-type, heterozygous, and ERβ −/− female littermates 25–31 days old were treated s.c. with 5 units of pregnant mare serum gonadotropin followed by 5 units of human chorionic gonadotropin 48–52 hr later. Animals were sacrificed 12–16 hr after the second injection, the ovaries and oviduct were removed, the oocyte/cumulus mass was surgically extracted from the oviduct, and oocytes were counted after hyaluronidase disassociation from the surrounding cumulus.
RESULTS
Targeted Disruption of the ERβ Gene and Generation of Mice.
The targeting construct illustrated in Fig. 1 was used to disrupt the ERβ gene in ES cells. Of 83 cell lines doubly resistant to G418 and ganciclovir, 14 were positive for targeting as judged by using PCR and 7 were characterized by using Southern blot analysis. All gave the expected wild-type 12-kb band and a 5.4-kb band indicative of correct targeting. One of these cell lines was injected into blastocysts and yielded two male chimeras that when mated to C57BL/6J females transmitted the strain 129 genome to their wild-type and heterozygous-mutant F1 offspring. Intercrossing the F1 heterozygotes yielded F2 progeny including 122 ERβ +/+ (64 female, 58 male), 217 +/− (109 female, 108 male), and 97 −/− (50 female, 47 male), in agreement with Mendelian expectations (χ2, P = 0.25) and a normal sex ratio.
RT-PCR Analysis of the Disrupted ERβ Gene.
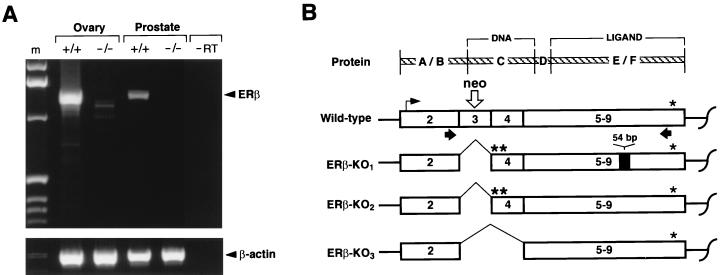
The targeting construct was designed so that the Neo insert would introduce stop codons into exon 3 (resulting in premature termination of translation of the ERβ mRNA) and prevent the formation of full-length functional ERβ polypeptide. However, it also was expected that some alternate RNA splicing might occur and result in the formation of shorter forms of the ERβ mRNA. We therefore carried out RT-PCR analysis with ovarian and prostate RNA from wild-type and mutant animals. The expected 1,291-bp product was obtained with RNA from wild-type ovary and prostate. No 1,291-bp product was detected in either of these same tissues from ERβ −/− mice, although small amounts of shorter PCR products were detected with RNA from their ovaries. Three of these shorter forms (Fig. 2) were cloned and sequenced: ERβ-KO1 (4 clones), ERβ-KO2 (4 clones), and ERβ-KO3 (1 clone). ERβ-KO1 and ERβ-KO2, 1,149 bp and 1,095 bp in length, respectively, lack the disrupted exon 3; both lead to a translational frameshift and the generation of multiple stop codons in exon 4; they differ by a 54-bp insertion equivalent to a natural ERβ mRNA variant that has been described in the rat (17). The ERβ-KO3 (979 bp in length) lacks exons 3 and 4 of the ERβ mRNA, and preserves the reading frame, but would yield a polypeptide lacking the residues known to function in DNA binding. We conclude that the ERβ −/− animals do not synthesize any mRNA coding for the normal ERβ polypeptide.
Figure 2.
RT-PCR for ERβ mRNA in +/+ and −/− tissues. (A) Gel electrophoresis of the products. The arrowheads indicate the bands corresponding to full-length ERβ mRNA and the β-actin control mRNA. The marker is labeled m. A lane loaded with PCR product in the absence of reverse transcriptase is labeled “−RT”. (B) Diagrammatic representation of the RT-PCR products (see text) from wild-type and mutant mRNA (ERβ-KO1, ERβ-KO2, and ERβ-KO3) derived by using the primers indicated by the black arrows. The top line (Protein) shows the domains of the protein corresponding to the exons 1–9. A/B, N-terminal domain; C, DNA-binding domain; D, hinge domain; and E/F, ligand-binding domain. The site of the Neo gene insertion is indicated by the open arrow.
Ovarian Histology and Function in the ERβ −/− Females.
Ovaries from young adult (7–9 weeks old) wild-type and ERβ −/− females were examined for indications of function. Fig. 3 A and B show representative sections of an ovary from an adult wild-type and from an ERβ −/− female. In both ovaries, follicles are seen at various stages of development ranging from primordial to fully developed with a clearly defined antrum. There are indications of more early atretic follicles and fewer corpora lutea in the ERβ −/− ovary compared with the wild-type ovary, suggesting partial arrest of follicular development and less frequent follicular maturation.
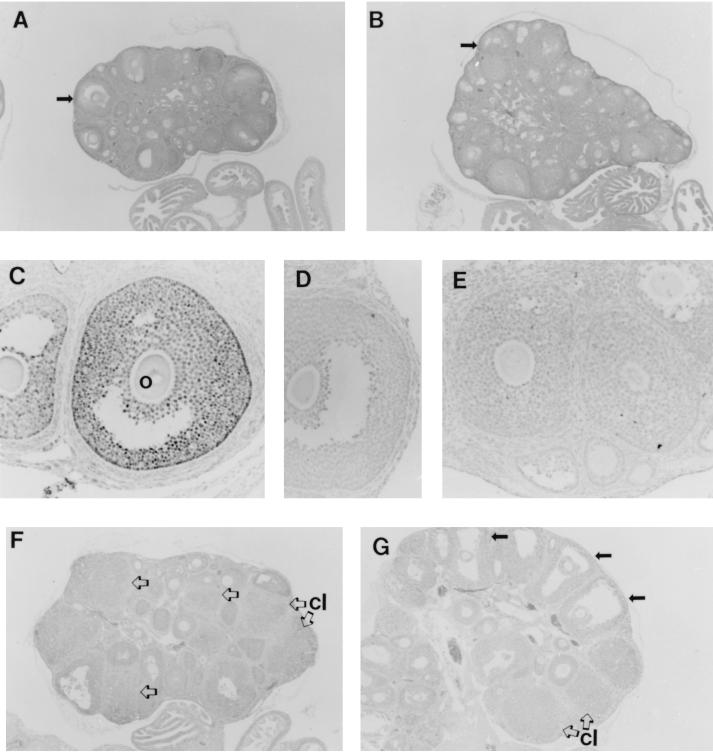
Figure 3.
Histology and ERβ immunocytochemistry in wild-type and ERβ −/− ovary. Hematoxylin-stained sections of adult wild-type (A) and ERβ −/− (B) ovary at ×40 magnification; the arrows indicate mature follicles. (C) Immunocytochemistry of wild-type ovary with an antiserum against ERβ; o, oocyte within the follicle. (D) Immunocytochemistry of wild-type ovary with the antiserum preabsorbed with immunogenic ERβ peptide. (E) Immunocytochemistry of adult ERβ −/− ovary with the ERβ antiserum. Histologic sections of a representative ovary from an immature wild-type female (F) and from an immature ERβ −/− female (G), both after superovulation; several unruptured preovulatory follicles in G are indicated by solid arrows. Corpora lutea (cl) are indicated in F and G by open arrows.
Immunocytochemistry for the ERβ protein (Fig. 3C) shows clearly detectable expression of ERβ in the granulosa cells of maturing wild-type follicles, with the highest levels appearing in granulosa cells lining the basement membrane of the follicle. Fig. 3D shows the absence of signal in wild-type follicles with antiserum preabsorbed with the antigenic peptide, thereby establishing the specificity of the assay. Fig. 3E shows immunostaining for ERβ with ovary from an adult ERβ −/− female; no specific staining is visible.
Reproductive Consequences of Lacking ERβ.
In a continuous mating study, F2 ERβ −/− females had significantly fewer litters (P < 0.05) than F2 wild-type females (Table 1), and the number of pups per litter was likewise significantly lower (P < 0.001). Thus, the ERβ −/− females exhibit reduced fertility. Two of the tested ERβ −/− females had no litters despite the detection of vaginal plugs on multiple occasions, suggesting that the infertility was not caused by any impairment of sexual behavior. Young homozygous mutant females (7–9 weeks) lacking ERβ have normal mammary histology and appear to lactate effectively, as judged by normal nursing behavior and growth of their pups.
Table 1.
Testing of female fertility
| Female genotype | n | Litters | Pups | Litters per female | Pups per litter |
|---|---|---|---|---|---|
| +/+ | 6 | 17 | 150 | 2.8 ± 0.4 | 8.8 ± 2.5 |
| −/− | 11 | 19 | 59 | 1.7 ± 1.0* | 3.1 ± 1.8** |
Results are presented as mean ± SD. ∗, P < 0.05 vs. +/+, Student’s two-tailed t-test. ∗∗, P < 0.001 vs. +/+, Student’s two-tailed t-test.
Males lacking ERβ are normally fertile, as judged by test matings with known fertile F1 females during which they produced litters every 21 days, with an average litter size of 10.2 pups per litter.
In light of the breeding results indicating a reduced fertility in the ERβ −/− females, additional experiments to assess ovarian function were carried out. Immature (25–31 days) ERβ −/− females and their wild-type littermates were hormonally stimulated (superovulated), and the oocyte yield per animal was determined. The results of three independent trials with animals of all three ERβ genotypes are shown in Table 2. The wild-type and heterozygous females exhibited an average yield of 33.7 ± 4.8 and 52.5 ± 5.7 oocytes per female, with the oocyte count from the heterozygotes being significantly greater than wild type (P < 0.05). The ERβ −/− females ovulated less efficiently, yielding 6.0 ± 1.5 oocytes per female (P < 0.001), with 2 of 11 animals tested yielding no detectable ova. In addition, the cumulus mass surrounding the ovulated follicles from the ERβ −/− females was composed of fewer cells when compared with those from wild-type and ERβ +/− mice. The histology of the wild-type and ERβ −/− ovaries from these superovulation experiments are illustrated in Fig. 3 F and G. In comparison with wild-type mice, the ovary from the ERβ −/− mice shows a large number of mature oocytes (solid arrows), indicating an intact response to pregnant mare serum gonadotropin. However, there are fewer corpora lutea (open arrows) in the ovaries of the stimulated ERβ −/− ovary than in the wild-type ovary, suggesting that some follicles failed to fully respond and discharge their oocytes in response to the ovulatory surge of hCG.
Table 2.
Superovulation of ERβ +/+, +/−, −/− genotypes
| Genotype | n | Oocyte count
|
|
|---|---|---|---|
| Average | Range | ||
| +/+ | 10 | 33.7 ± 4.8 | 9–57 |
| +/− | 11 | 52.5 ± 5.7* | 20–77 |
| −/− | 11 | 6.0 ± 1.5** | 0–13 |
Results are presented as means ± SD. ∗, P < 0.05 vs. +/+, Student’s two-tailed t-test. ∗∗, P < 0.001 vs. +/+, Student’s two-tailed t-test.
Male Urogenital Tract.
High levels of ERβ have been reported in the male urogenital tract [especially in the prostate and epididymis (7, 8, 18)]. Histological analysis of these tissues from 2- to 3-month-old ERβ −/− males showed no marked abnormalities when compared with age-matched wild-type littermates. However, older ERβ −/− males exhibited indications of epithelial hyperplasia in the prostatic collecting ducts as well as the bladder wall (data not shown).
DISCUSSION
Several recent discoveries and developments, including the generation of αERKO (9) and aromatase knockout (ArKO) mice (19), have changed our understanding of estrogen action and have offered new insights into the mechanisms by which endogenous estrogens and exogenous estrogenic and antiestrogenic compounds exert their effects. The mouse models have been complemented by the discovery of a human male lacking functional ERα (20) and of human males and females with mutations of the aromatase gene (21, 22). However, the discovery of a second estrogen receptor, ERβ, has introduced a new level of uncertainty in our understanding of the mechanisms and role of estrogen and raises the possibility that the two receptors have different actions. In the αERKO mice, some estrogenic effects are preserved in tissues such as the brain (23), cardiovascular system (24), and uterus (25), suggesting that in these tissues ERβ may mediate the effects of estrogens. Here we have described the generation and initial characterization of a mouse homozygous for a targeted disruption of the ERβ gene, which in combination with the models described above, should prove useful in further dissecting the roles of the individual estrogen receptors.
Breeding of mice heterozygous for the ERβ gene disruption yielded ERβ −/− progeny in expected Mendelian proportions and with normal sex ratios. The ERβ −/− mice survive to adulthood and do not exhibit any obvious abnormalities. Assays for ERβ mRNA demonstrate the lack of wild-type ERβ transcripts but indicate the presence of very small amounts of three distinct splicing variants. Sequencing clones corresponding to these variants showed that two of them not only are missing sequences that encode the essential first zinc finger of the DNA-binding domain but also cause a frameshift that would result in a shortened form of the ERβ protein. The third and least common variant retains the normal translational reading frame but would be missing the entire functional portion of the DNA-binding domain, thought to be critical to the function of nuclear steroid receptors (26). Thus the ERβ −/− mutant animals lack any means of synthesizing normal ERβ. Immunocytochemistry employing an antibody specific for the C-terminal amino acids of rat ERβ confirms this conclusion by demonstrating the absence of detectable ERβ protein in the ovaries of the ERβ −/− mice compared with the strong staining observed in the granulosa cells of the wild-type ovary.
The role of estrogens in male reproduction is complex. Previous studies have shown that male mice lacking ERα are completely infertile (10, 12), whereas male mice lacking aromatase are fully fertile (16). In the present work, we found that male mice lacking ERβ are fully fertile. We conclude that ERα, but not aromatase or ERβ, are essential for normal male fertility. Nevertheless, we have observed indications of age-related abnormalities in the male urogenital tract—specifically in the prostate and bladder—but a more complete analysis of the nature and incidence of this phenotype is needed.
Fertility in females is much more clearly dependent on a multitude of estrogen actions mediated by both aromatase and ERα, as demonstrated by the infertility of ArKO (16) and αERKO (9, 11, 27) mice. Although the ERβ −/− females are not infertile, their fertility is compromised, as demonstrated by the reduced number and size of litters compared with normally fertile females in a continuous mating study. The occurrence of successful pregnancies and seminal plugs in ERβ −/− females that never produced a litter suggests, however, that the sexual behaviors necessary for reproduction are intact in the ERβ −/− females. This is in marked contrast to the severe deficit in sexual behavior described in the αERKO females (11, 28). Furthermore, the mammary glands from both virgin and parous ERβ −/− females did not reveal any notable differences from the wild-type mice.
The most likely cause of the subfertility in the ERβ −/− females is a direct loss of ERβ-mediated estrogen actions in the ovary. Recent studies using in situ hybridization (3, 29), RNase protection assay (8), and immunohistochemical data (15) and the data we have presented here demonstrate that ERβ is the predominant form of ER in the ovary and that it is localized to the granulosa cells of maturing follicles. Disruption of the ERβ gene, therefore, would be expected to have effects on ovarian function and subsequent fertility in the female. The general histology of ovaries from our adult ERβ −/− mice revealed a reduced number of corpora lutea, with indications that follicular development may be partially arrested and that completed follicular maturation occurs at a reduced frequency. A more dramatic ovarian phenotype became evident when the animals were stimulated to ovulate with exogenous hormones. After superovulation, the number of oocytes recovered from the ERβ −/− mice was less than 1/5 that seen from wild-type or heterozygous mice. The number of oocytes recovered from the heterozygous ERβ +/− superovulated females was greater than from wild-type females but was still within the range of normal animals. The ovaries from the superovulated ERβ −/− females exhibited several mature but unruptured follicles, resulting in trapped oocytes. The number of corpora lutea detected in the ovary of ERβ −/− animals after superovulation was considerably lower than in the wild-type animals. These results suggest an attenuated response to the ovulatory hormone surge after human chorionic gonadotropin administration.
The conclusion we draw from the ovarian histology, the continuous mating studies, and the superovulation experiments is that the primary reproductive defect in the ERβ −/− females is impaired ovarian function. Over two decades ago, Richards (30) identified specific estradiol-binding sites in the nuclei of granulosa cells that now appear to be most likely caused by ERβ rather than ERα. The ability of estradiol—through an appropriate receptor—to enhance follicular responsiveness to gonadotropins also has long been known. Thus, several studies have described estradiol effects in the ovary, including an increase in granulosa cell growth and number (31–33), increased synthesis of granulosa cell insulin-like growth factor 1 (34), maintenance of follicle-stimulating hormone receptor (35), induction of luteinizing hormone receptor (36, 37), augmentation of aromatase activity and subsequent estradiol production (38), and attenuation of granulosa cell apoptosis (39). The abnormalities we have observed in the ERβ −/− ovary (decreased rates of spontaneous ovulation, increased atresia, decreased cellular mass of the oocyte cumulus, and an inadequate response to exogenous hormones) may well be the result of a loss of one or more of the previously described estrogen actions.
Similar ovarian phenotypes have been reported in mice with a disruption of the progesterone receptor gene (40), the cell cycle-regulating cyclin-D2 gene (41), or the cyclooxygenase-2 gene (42). All have ovulatory defects. The ovarian phenotype of the ERβ −/− female is somewhat similar to that of the ArKO female, which shows a complete termination of folliculogenesis at the preovulatory stage (16). However, the ovarian phenotype of the ERβ −/− female is markedly less severe than that of the completely infertile αERKO female, in which follicular arrest is complete and occurs at stages earlier than in the ERβ −/− ovary (10, 43). Furthermore, the enlarged and hemorrhagic cystic follicles that are a hallmark of the αERKO ovary are not present in the ERβ −/− ovaries, indicating separate mechanisms behind the phenotypes in the two mutants. Studies of αERKO (44) and ArKO mice (16) show that estrogen action via ERα is critical for the proper regulation of gonadotropin synthesis and secretion in the hypothalamic/pituitary system. However, because ERβ is normally present in the hypothalamus (8, 45), some disturbance in the normal action of estrogen in the hypothalamic/pituitary system of the ERβ −/− mice cannot be ruled out at this time.
Breast development and function is not compromised by absence of ERβ as judged by normal mammary histology and lactation. In contrast, female mice lacking aromatase (16) or ERα (10) show absence of breast tissue development beyond that of prepubertal females. We conclude that estrogen acting through ERα, but not through ERβ, is essential for normal breast development and function.
In summary, ERβ −/− mice of both sexes appear to develop normally and survive to adulthood. The urogenital tract structures of both sexes undergo normal prenatal development, although initial studies of the prostate and bladder of older ERβ −/− males indicate some abnormal hyperplasia. Female mice lacking ERβ have normal breast histology and function but reduced fertility, with the predominating factors apparently originating in the ovary. The similarities and differences that we have noted between the previously described αERKO and ArKO mice and ERβ −/− mice emphasize the complexity of the estrogen signaling system and its diverse physiological roles. Future work is needed to determine the effects of a lack of ERβ in aging animals, particularly in relation to the cardiovascular system and bone homeostasis.
Acknowledgments
We thank Wayne Bocchinfuso, Geoffrey Greene, John Hagaman, Kimberly Kluckman, Joel Mahler, Sari Makela, Li Peng, David Schomberg, and Todd Washburn for their help. This work was supported by Grant GM20069 from the National Institutes of Health (O.S.), and by The Swedish Cancer Society (J.-A.G.). We are grateful to the W. M. Keck Foundation for a grant to the University of North Carolina at Chapel Hill to support work with animals.
ABBREVIATIONS
- ER
estrogen receptor
- ES cell
embryonic stem cell
- Neo
neomycin resistance gene
- αERKO
α estrogen receptor knockout
- ArKO
aromatase knockout
- RT-PCR
reverse transcription–PCR
References
- 1.The Writing Group for the PEPI Trial. J Am Med Assoc. 1996;276:1389–1396. [PubMed] [Google Scholar]
- 2.The Writing Group for the PEPI Trial. J Am Med Assoc. 1995;273:199–208. [PubMed] [Google Scholar]
- 3.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay G B, Tremblay A, Copeland N G, Gilbert D J, Jenkins N A, Labrie F, Giguere V. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 5.Mosselman S, Polman J, Dijkema R. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuiper G G J M, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-Å. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 8.Couse J F, Lindzey J, Grandien K, Gustafsson J- Å, Korach K S. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 9.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korach K S, Couse J F, Curtis S W, Washburn T F, Lindzey J, Kimbro K S, Eddy E M, Migliaccio S, Snedeker S M, Lubahn D B, et al. Recent Prog Horm Res. 1996;51:159–188. [PubMed] [Google Scholar]
- 11.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Taylor J A, Lubahn D B, Korach K S, Pfaff D W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smithies O, Kim H-S. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H-S, Smithies O. Nucleic Acids Res. 1988;16:8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sar M. Tech Immunocytochem. 1985;3:43–54. [Google Scholar]
- 17.Peterson D N, Tkalcevic G T, Koza-Taylor P H, Turi T G, Brown T A. Endocrinology. 1998;139:1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- 18.Saunders P T, Maguire S M, Gaughan J, Millar M R. J Endocrinol. 1997;154:R13–R16. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- 19.Fisher C R, Graves K H, Parlow A F, Simpson E. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith E P, Boyd J, Frank G R, Takahashi H, Cohen R M, Specker B, Williams T C, Lubahn D B, Korach K S. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 21.Conte F A, Grumbach M M, Ito Y, Fisher C R, Simpson E R. J Clin Endocrinol Metab. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- 22.Bulun S E. J Clin Endocrinol Metab. 1996;81:867–871. doi: 10.1210/jcem.81.3.8772541. [DOI] [PubMed] [Google Scholar]
- 23.Shughrue P J, Lubahn D B, Negro-Vilar A, Korach K S, Merchenthaler I. Proc Natl Acad Sci USA. 1997;94:11008–11012. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iafrati M D, Karas R H, Aronovitz M, Kim S, Sullivan T R, Jr, Lubahn D B, O’Donnell T F, Jr, Korach K S, Mendelsohn M E. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 25.Das S K, Taylor J A, Korach K S, Paria B C, Dey S K, Lubahn D B. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzenellenbogen J A, Katzenellenbogen B S. Chem Biol. 1996;3:529–536. doi: 10.1016/s1074-5521(96)90143-x. [DOI] [PubMed] [Google Scholar]
- 27.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 28.Rissman E F, Early A H, Taylor J A, Korach K S, Lubahn D B. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 29.Byers M, Kuiper G G J M, Gustafsson J-Å, Park-Sarge O-K. Mol Endocrinol. 1997;11:172–182. doi: 10.1210/mend.11.2.9887. [DOI] [PubMed] [Google Scholar]
- 30.Richards J S. Endocrinology. 1975;97:1174–1184. doi: 10.1210/endo-97-5-1174. [DOI] [PubMed] [Google Scholar]
- 31.Richards J S, Ireland J J, Rao M C, Bernath G A, Midgley A R, Jr, Reichert L E., Jr Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg R L, Vaitukaitis J L, Ross G T. Endocrinology. 1972;90:1492–1498. doi: 10.1210/endo-90-6-1492. [DOI] [PubMed] [Google Scholar]
- 33.Richards J S. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez E R, Roberts C T, Jr, LeRoith D, Adashi E Y. Endocrinology. 1989;125:572–574. doi: 10.1210/endo-125-1-572. [DOI] [PubMed] [Google Scholar]
- 35.Tonetta S A, diZerga G S. Endocr Rev. 1989;10:205–229. doi: 10.1210/edrv-10-2-205. [DOI] [PubMed] [Google Scholar]
- 36.Wang X-N, Greenwald G S. Biol Reprod. 1993;48:595–605. doi: 10.1095/biolreprod48.3.595. [DOI] [PubMed] [Google Scholar]
- 37.Farhookhi R, Desjardins J. Mol Cell Endocrinol. 1986;47:13–24. doi: 10.1016/0303-7207(86)90011-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang L-Z, Adashi E Y, Hsueh A J W. Endocrinology. 1982;110:2219. doi: 10.1210/endo-110-6-2219. [DOI] [PubMed] [Google Scholar]
- 39.Kaipia A, Hsueh A J W. Annu Rev Physiol. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- 40.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Jr, Shyamala G, Conneely O M, O’Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 41.Sicinski P, Donaher J L, Geng Y, Parker S B, Gardner H, Park M Y, Robker R L, Richards J S, McGinnis L K, Biggers J D, et al. Nature (London) 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 42.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 43.Couse J F, Curtis S W, Washburn T F, Eddy E M, Schomberg D W, Korach K S. Biochem Soc Trans. 1995;23:929–935. doi: 10.1042/bst0230929. [DOI] [PubMed] [Google Scholar]
- 44.Scully K M, Gleiberman A S, Lindzey J, Lubahn D B, Korach K S, Rosenfeld M G. Mol Endocrinol. 1997;11:674–681. doi: 10.1210/mend.11.6.0019. [DOI] [PubMed] [Google Scholar]
- 45.Shughrue P J, Komm B, Merchenthaler I. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]