Abstract
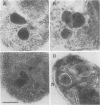
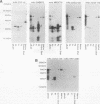
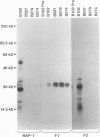
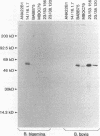
Babesia bovis merozoite apical membrane polypeptide Bv60 was found to be rhoptry associated by immuno-electron microscopy and was redesignated rhoptry-associated protein 1 (RAP-1). The N-terminal 300 amino acids of RAP-1 have a high level of sequence similarity to the same N-terminal region of p58, its homolog from Babesia bigemina. However, the interspecies conserved sequences did not include RAP-1 surface-exposed B-cell epitopes as defined by monoclonal antibodies. Furthermore, neither heterologous B. bigemina immune nor monospecific anti-p58 bovine serum binds to whole RAP-1, indicating that the major B-cell epitopes recognized by these sera are also not encoded by the conserved sequences. Truncated RAP-1, expressed by a subclone encoding the N-terminal 235 amino acids, is weakly bound by antibodies in heterologous sera. A peptide representing the longest conserved amino acid sequence (amino acids 121 to 134) in this region is also weakly bound by antibodies in immune bovine sera, and rabbit antibodies induced by and strongly reactive with the peptide alone fail to bind native or denatured RAP-1. Thus, although the conserved region may contain one or more poorly immunogenic B-cell epitopes, these epitopes are inaccessible to antibody in whole RAP-1. The results indicate that the major immunogenic B-cell epitopes of RAP-1, including surface-accessible epitopes bound by monoclonal antibodies, are distinct from the conserved sequences representing putative functional domains.
Full text
PDF
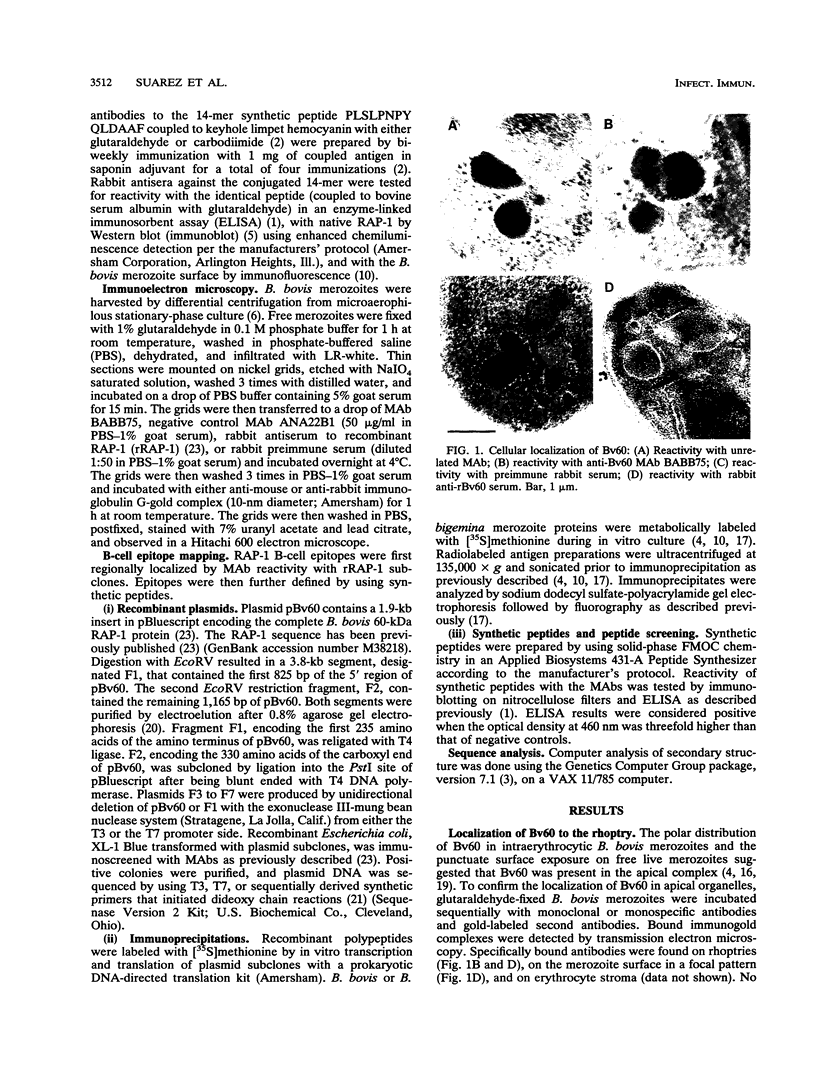

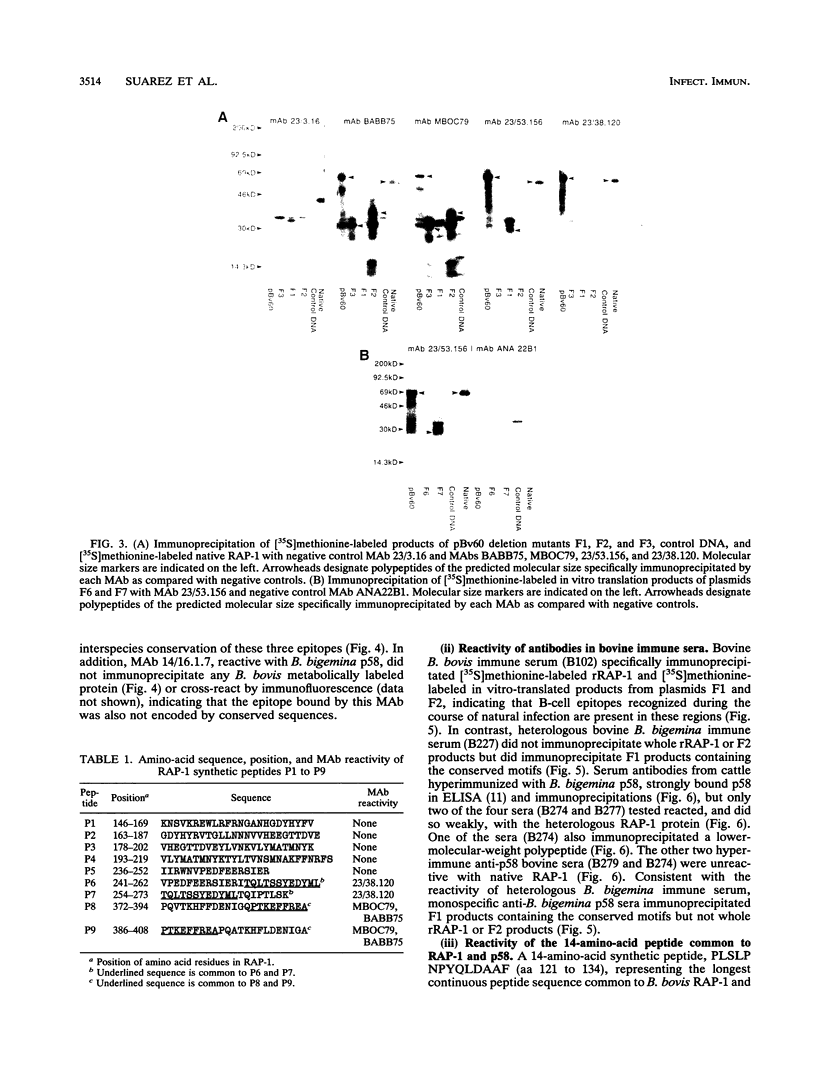
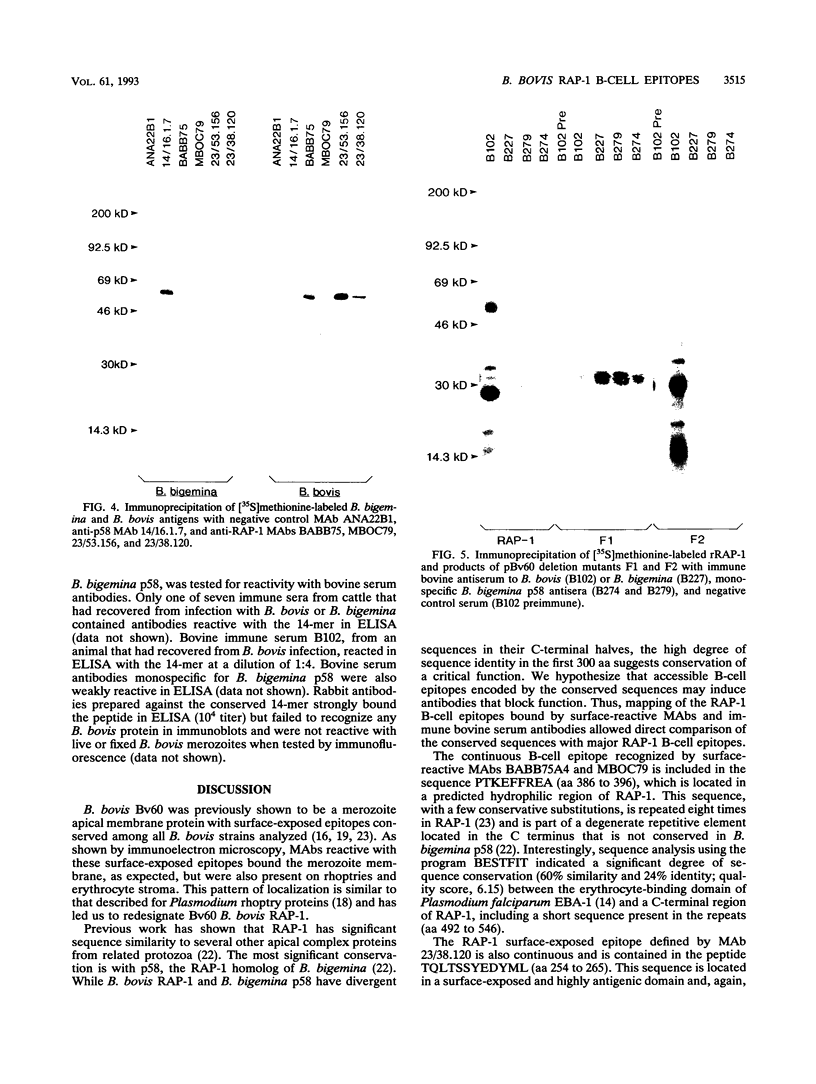

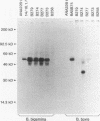
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., McGuire T. C., Palmer G. H., Leib S. R., Harkins T. M., McElwain T. F., Barbet A. F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Levy M. G., Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980 Mar 14;207(4436):1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Kerr J. D., Goodger B. V., Wright I. G. The immune response of cattle to Babesia bovis (syn. B. argentina). Studies on the nature and specificity of protection. Int J Parasitol. 1979 Aug;9(4):297–306. doi: 10.1016/0020-7519(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Goodger B. V. Immunity in cattle to Babesia bovis after single infections with parasites of various origin. Aust Vet J. 1979 Jan;55(1):10–12. doi: 10.1111/j.1751-0813.1979.tb09535.x. [DOI] [PubMed] [Google Scholar]
- McElwain T. F., Palmer G. H., Goff W. L., McGuire T. C. Identification of Babesia bigemina and Babesia bovis merozoite proteins with isolate- and species-common epitopes recognized by antibodies in bovine immune sera. Infect Immun. 1988 Jun;56(6):1658–1660. doi: 10.1128/iai.56.6.1658-1660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain T. F., Perryman L. E., Davis W. C., McGuire T. C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987 Apr 1;138(7):2298–2304. [PubMed] [Google Scholar]
- McElwain T. F., Perryman L. E., Musoke A. J., McGuire T. C. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. 1991 Aug;47(2):213–222. doi: 10.1016/0166-6851(91)90181-5. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V. S., Stephens E. B., Dame J. B., Perryman L. E., McGuire T. C., McElwain T. F. Immunogenicity and sequence analysis of recombinant p58: a neutralization-sensitive, antigenically conserved Babesia bigemina merozoite surface protein. Mol Biochem Parasitol. 1991 Aug;47(2):207–212. doi: 10.1016/0166-6851(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Sim B. K., Chulay J. D., Haynes J. D. Characterization of the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990 May;40(2):285–294. doi: 10.1016/0166-6851(90)90050-v. [DOI] [PubMed] [Google Scholar]
- Palmer D. A., Buening G. M., Carson C. A. Cryopreservation of Babesia bovis for in vitro cultivation. Parasitology. 1982 Jun;84(Pt 3):567–572. doi: 10.1017/s0031182000052835. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McElwain T. F., Perryman L. E., Davis W. C., Reduker D. R., Jasmer D. P., Shkap V., Pipano E., Goff W. L., McGuire T. C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect Immun. 1991 Sep;59(9):3340–3342. doi: 10.1128/iai.59.9.3340-3342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Perkins M. E. Rhoptry organelles of apicomplexan parasites. Parasitol Today. 1992 Jan;8(1):28–32. doi: 10.1016/0169-4758(92)90308-o. [DOI] [PubMed] [Google Scholar]
- Reduker D. W., Jasmer D. P., Goff W. L., Perryman L. E., Davis W. C., McGuire T. C. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol Biochem Parasitol. 1989 Jul;35(3):239–247. doi: 10.1016/0166-6851(89)90210-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C. E., McElwain T. F., Stephens E. B., Mishra V. S., Palmer G. H. Sequence conservation among merozoite apical complex proteins of Babesia bovis, Babesia bigemina and other apicomplexa. Mol Biochem Parasitol. 1991 Dec;49(2):329–332. doi: 10.1016/0166-6851(91)90077-j. [DOI] [PubMed] [Google Scholar]
- Suarez C. E., Palmer G. H., Jasmer D. P., Hines S. A., Perryman L. E., McElwain T. F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol Biochem Parasitol. 1991 May;46(1):45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]