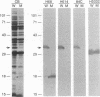
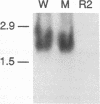
Abstract
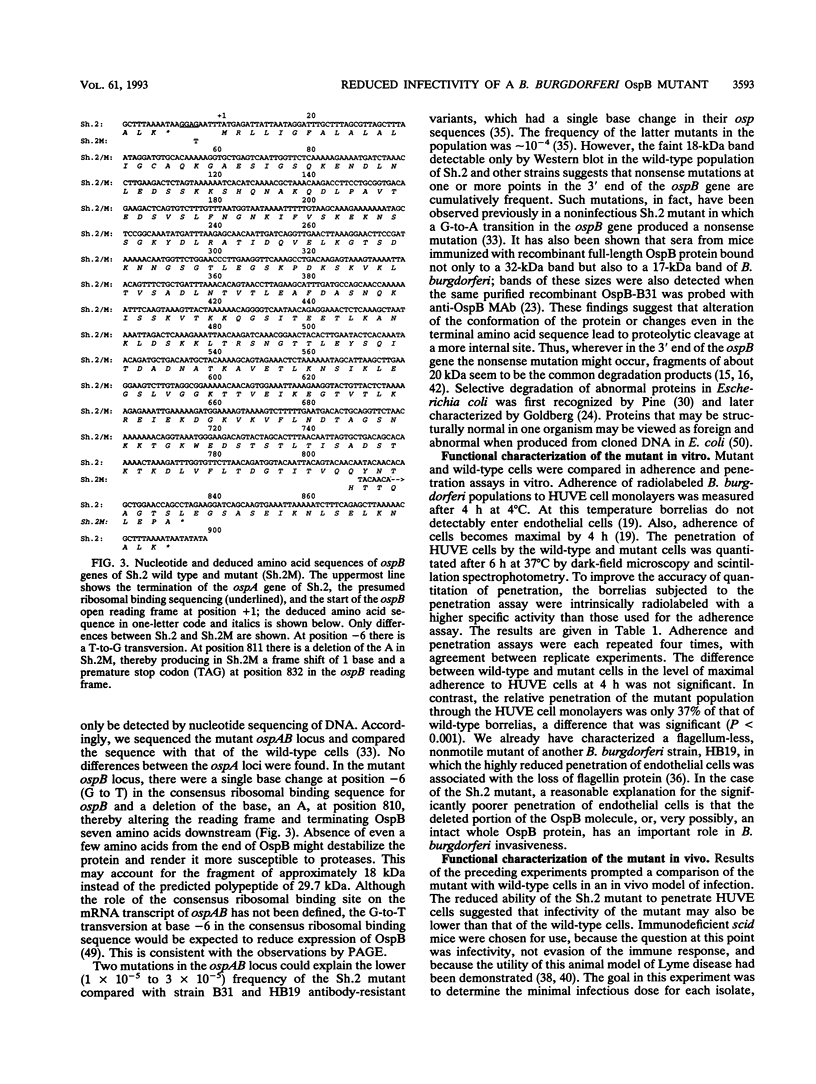
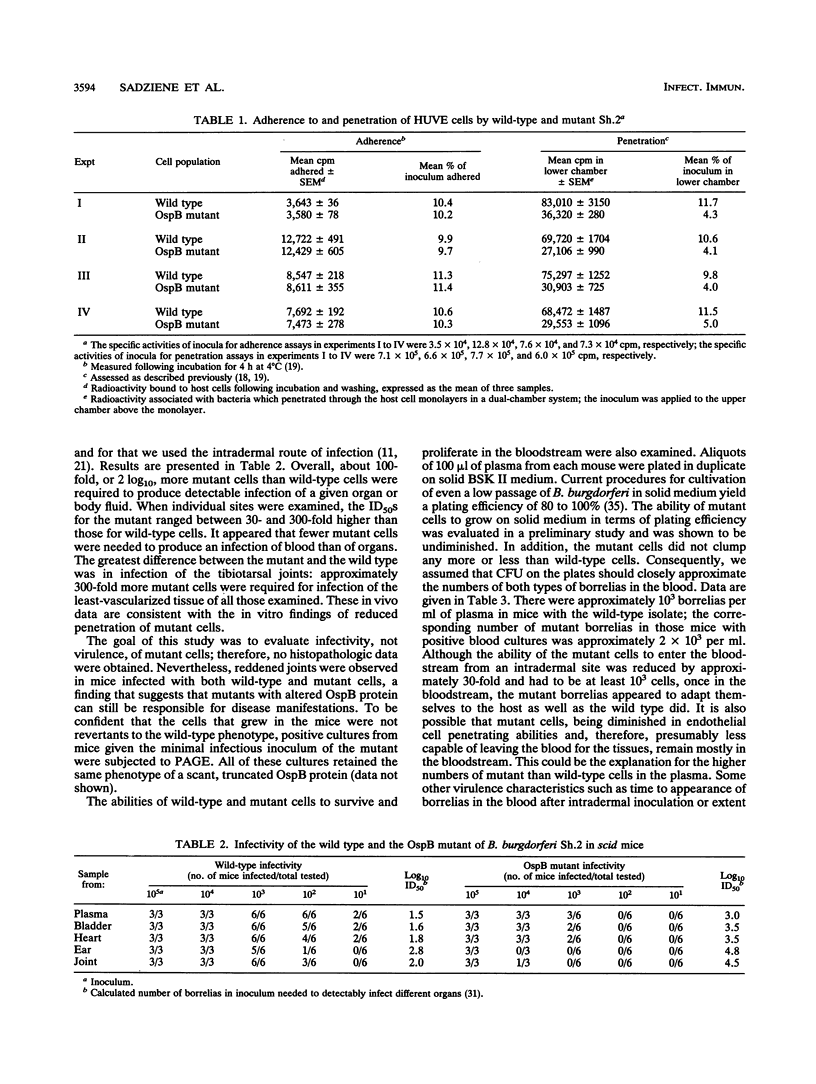
Most Borrelia burgdorferi strains have two major surface proteins, OspA and OspB. In the present study, we selected from a clonal population of infectious B. burgdorferi an OspB escape mutant, identified the genetic basis for this phenotype, and evaluated its functional activities. Selection with the anti-OspB antibody H614 was performed in vitro in medium and extended in vivo in scid mice. Mutants with a truncated OspB protein were selected at a frequency of 1 x 10(-5) to 3 x 10(-5). After no major rearrangements in DNA were detected, sequence analysis of the mutant's ospAB locus revealed a single base change in the consensus ribosomal binding sequence for ospB and a single nucleotide deletion in the ospB gene itself. The effect of these mutations was reduced expression of a truncated OspB protein. When functional abilities of the wild type and mutant were compared, the mutant had a threefold-lower capacity to penetrate a human endothelium umbilical vein cell monolayer. Infectivity of wild-type and mutant cells for scid mice was evaluated by culturing different organs, and the median infectious dose was calculated. The inoculum of mutant cells for infecting the mice was 30- to 300-fold higher than that of wild-type cells. This study shows that reduced size and expression of OspB are associated with lowered virulence of B. burgdorferi. Selection of mutants that to some degree remain infectious is one approach to defining the role of different surface proteins in the pathogenesis of Lyme disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Schrumpf M. E. Polymorphisms of major surface proteins of Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):83–91. doi: 10.1016/s0176-6724(86)80107-9. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991 Feb;163(2):419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Golightly M. G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988 Jan 1;140(1):265–272. [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Rogers R. C., Benach J. L. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992 Aug;60(8):3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock L. E., Fikrig E., Shoberg R. J., Flavell R. A., Thomas D. D. A monoclonal antibody to OspA inhibits association of Borrelia burgdorferi with human endothelial cells. Infect Immun. 1993 Feb;61(2):423–431. doi: 10.1128/iai.61.2.423-431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Characterization of Borrelia burgdorferi invasion of cultured endothelial cells. Microb Pathog. 1991 Feb;10(2):137–148. doi: 10.1016/0882-4010(91)90074-k. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G., Mays J. P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993 Jan;61(1):81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Marcantonio N., Deponte K., Kantor F. S., Flavell R. A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992 Feb;60(2):657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992 Aug;174(16):5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., Mayer L. W., Barbour A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985 Feb 8;227(4687):645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- Kurashige S., Bissett M., Oshiro L. Characterization of a tick isolate of Borrelia burgdorferi that possesses a major low-molecular-weight surface protein. J Clin Microbiol. 1990 Jun;28(6):1362–1366. doi: 10.1128/jcm.28.6.1362-1366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Hogan D., Schwan T. G. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J Clin Microbiol. 1991 Mar;29(3):524–532. doi: 10.1128/jcm.29.3.524-532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Thomas D. D., Bundoc V. G., Holt S. C., Barbour A. G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991 Jul;88(1):82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A., Thompson P. A., Barbour A. G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993 Jan;167(1):165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Gay S., Museteanu C., Kramer M. D., Zimmer G., Eichmann K., Museteanu U., Simon M. M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990 Oct;137(4):811–820. [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Karstens R. H., Schrumpf M. E., Simpson W. J. Changes in antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete, during persistent infection in mice. Can J Microbiol. 1991 Jun;37(6):450–454. doi: 10.1139/m91-074. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]