Abstract
Selectivity rules in organic chemistry have largely been inferred from non-aqueous environments. In contrast, enzymes operate in water, and the chemical impact of the medium change remains only partially understood. Structural characterization of the “ladder” polyether marine natural products raised a puzzle that persisted for 20 years: Although the stereochemistry of adjacent tetrahydropyran (THP) cycles would seem to arise from a biosynthetic cascade of epoxide-opening reactions, experience in organic solvents argued consistently that such a pathway would be kinetically disfavored. We report that neutral water acts as an optimal promoter for the requisite ring-opening selectivity, once a single templating THP is appended to a chain of epoxides. This strategy offers a high-yielding route to the naturally occurring ladder core and highlights the likely significance of aqueous medium effects in underpinning certain apparently remarkable enzymatic selectivities.
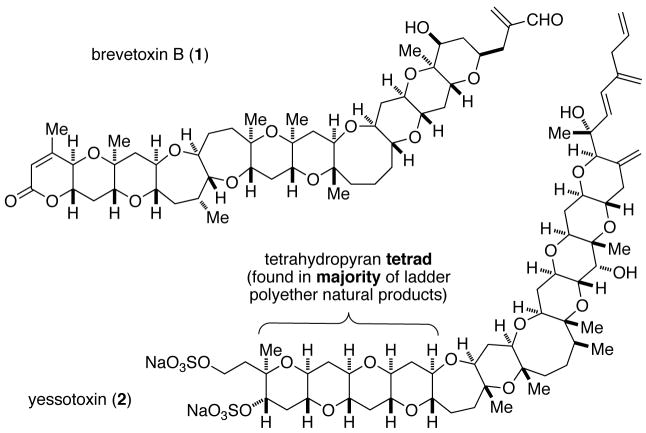
Brevetoxin B (Figure 1, 1), yessotoxin (2), the ciguatoxins, and related ladder polyether natural products are the active constituents of many harmful algal blooms, marine phenomena also known collectively as the Red Tide. Since the isolation and structural elucidation of 1 by Nakanishi and Clardy in 1981 (1) the distinctive molecular architecture and extreme lethality to marine life of these toxins continue to stimulate the development of methods for their chemical synthesis (2, 3, 4) and research into their mode of action. (5)
Fig. 1.
Representative ladder polyether natural products. Me, methyl.
How these molecules are assembled by the dinoflagellates that produce them has also been an active area of investigation (6, 7, 8) and, to be sure, speculation. Although they are among the most complex secondary metabolites ever characterized, all ladder polyethers possess a structural pattern and stereochemical regularity that confer upon them a certain degree of simplicity. A backbone of repeating oxygen–carbon–carbon (O–C–C) units extends from one end of the polyether network to the other (e.g., gymnocin B (3), Figure 2A, red bonds and O atoms), regardless of the size of the intervening rings and of any functional groups present on the rings. The “ladder” topography is the consequence of consistently trans stereochemistry across the carbon-carbon bonds of the ring junctions, coupled with the relative syn configuration of adjacent junctions.
Fig. 2.
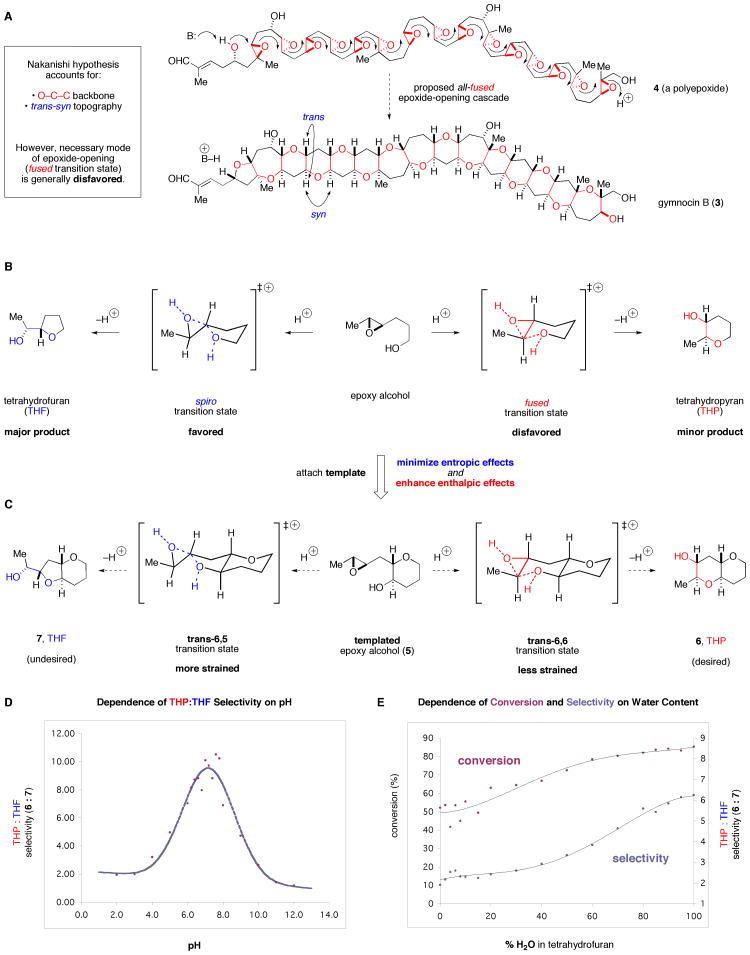
(A) Structural and stereochemical patterns explained by the Nakanishi hypothesis of ladder polyether biosynthesis. (B) The two transition states (fused and spiro) and products (THP (6) and THF (7), respectively) for cyclization of an exemplary epoxy alcohol via epoxide opening. (C) Templated epoxide-opening cyclizations. (D) Dependence of THP:THF (6:7) selectivity on pH in cyclization of 5. (E) Enhancement of conversion of 5 and of THP:THF selectivity by H2O. Me, methyl; THF, tetrahydrofuran; THP, tetrahydropyran.
The Nakanishi cascade hypothesis
Over twenty years ago, Nakanishi put forth a hypothesis that accounts for these structural and stereochemical similarities – the transformation of a polyepoxide (e.g., 4) into a ladder polyether via a series or “cascade” of epoxide-opening events (Figure 2A). (9) The oxygen and two carbon atoms of each epoxide comprise the O–C–C backbone, and with the proviso that all of the ring openings proceed with inversion of configuration, the trans-syn topography is explained by this mechanism. That there is little evidence to support this two-decade-old hypothesis has not deterred significant efforts to emulate such cascades.
Despite its intellectual appeal, however, the hypothesis relies upon a ring-opening process generally regarded to be disfavored (Figure 2B). With few exceptions, epoxide-opening reactions of this type favor the smaller heterocycle (e.g., a tetrahydrofuran (THF, 7) likely arising from a spiro transition state), not the larger one (a tetrahydropyran (THP, 6), from fused transition state). (10, 11, 12) The majority of the approaches to promote the desired outcome use “directing groups” that must be covalently attached to the epoxides. (13) In contrast, catalytic antibodies (14) and transition metal complexes (15, 16) can be particularly effective in certain cases involving a single epoxide-opening event. To date, however, all existing THP-selective cascades that open more than one epoxide have required a directing group at every epoxide. (17, 18, 19) These directing groups either are not found in the natural products or for other reasons clearly cannot be the natural solution. Therefore, though amenable to the synthesis of certain polyether ring systems, these methods do not provide evidence in support of or against the Nakanishi hypothesis.
Thus, if epoxide-opening reactions are used in ladder polyether biosynthesis, then how is this preference for the smaller ring overcome? Enzymatic control is a logical supposition, but there is as yet no evidence for such an intervention. With the joint aims of addressing this question and accelerating the synthesis of ladder polyethers, we have focused our recent efforts in this area on “directing-group-free”, THP-selective cascades. (20) Our approach stems from an analysis of the potential factors governing the regioselectivity of epoxide opening in these reactions and uses a template to modulate them in the desired fashion.
We reasoned that in 5, where one THP is already in place, entropic issues that might normally favor the undesired spiro transition state would be minimized and instead enthalpic contributions to the energies of the competing transition states would play a more important role (Figure 2C). Trans-bicyclo[4.4.0]decane derivatives are typically less strained than their trans-bicyclo[4.3.0]nonane counterparts, and were this difference in developing ring strain reflected in the transition states, then the desired THP product (6) might be favored in this templated system under the appropriate reaction conditions.
Water as a reaction promoter
To test these hypotheses we prepared 5 and exposed it to a wide range of combinations of acids, bases, solvents, and other additives. (21) Bases tended to favor the THF product 7, whereas acids exhibited a slight preference for the desired THP product (6). Combinations of acids and bases (Lewis or Brønsted) also favored 7. In order to better understand the requirements for activation of the nucleophile and the electrophile, we examined the pH dependence of THP-to-THF selectivity (various buffers (phosphate, tris, His, e.g.) and ionic strengths). These experiments revealed a clear and provocative trend (Figure 2D): In all cases the selectivity for the desired THP product increases significantly as the pH of the reaction environment approaches neutrality, even exceeding 10:1 THP:THF selectivity near pH 7.
Several lines of evidence clearly implicate water in both the acceleration and increase in selectivity in these reactions. In less polar solvents (CH2Cl2, toluene, among others), low conversion of 5 is observed, and in polar aprotic solvents (e.g. CH3CN, DMSO, DMF), although higher conversion to 6 and 7 occurs, the selectivity is greatly reduced (≤3:1 6:7). Furthermore, both increased THP:THF selectivity and increased conversion of 5 correlate with increased water content in the reaction milieu (Figure 2E). Finally, deionized H2O, in which both the ionic strength and percentage of impurities are near zero, provides the highest selectivity (>11:1 THP:THF). The only other promoters that we have found that approach the selectivity and rate exhibited by water are ethylene glycol (9:1) and methanol (8:1), which along with water are capable of both providing and accepting hydrogen bonds.
Water-promoted cascades
Having developed a THP-selective epoxide-opening method, we turned our attention to the possibility of using this approach in epoxide-opening cascades. However, we were well aware from our own work and from case studies reported by others that many highly selective epoxide ring-opening methods summarily fail when extended to cascades. (20)
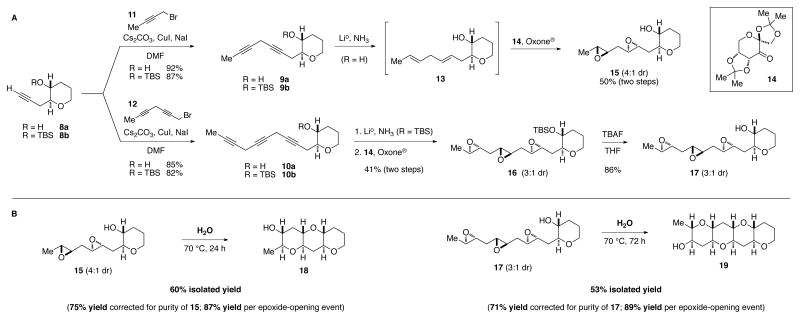
The synthesis of the epoxides is shown in Figure 3A and emulates another aspect of Nakanishi’s hypothesis, polyepoxidation of a polyene. Alkyne 8a was extended to diyne 9a and triyne 10a in high yield by alkylation with the appropriate propargyl bromide (11 and 12, respectively). Alkyne 8b, in which the hydroxyl group is protected as a silyl ether, was converted to alkynes 9b and 10b in the same manner and similar yield. Dissolving metal reduction (Li/NH3) of 9a provided a skipped diene (13) that was unstable enough to prohibit prolonged storage. The corresponding triene (not shown) from 10b was even less robust, requiring hydroxyl protection prior to reduction.
Fig. 3.
(A) Synthesis of polyepoxides. (B) Epoxide-opening cascades promoted by H2O. DMF, dimethylformamide; Me, methyl; TBS, tert-butyldimethylsilyl; THF, tetrahydrofuran.
An epoxidation method developed by Shi that hinges upon fructose-derived ketone 14 and Oxone® converted the diene and triene to the corresponding polyepoxides (15 and 16). (22) The moderate stereoselectivity appears to be due to the alkene proximal to the hydroxyl group; more remote alkenes do not suffer from this mismatched double diastereoselection. Diepoxide 15 and triepoxide 17 (after removal of the TBS protective group) were obtained in 50% and 35% overall yield from the diyne (9a) and triyne (10b).
The suboptimal stereochemical purity of 15 and 17 turned out to be of little concern (Figure 3B). Heating 15 in deionized water for 24 h at 70 °C afforded a THP triad (18) in 60% isolated yield (75% yield when corrected for the purity of 15). Similarly, a THP tetrad (19) representative of that found in more than half of the known ladder polyethers (see Figure 1) was obtained in 53% isolated yield (71% when corrected for the purity of 17).
These THP-selective, epoxide-opening cascades are far higher-yielding than those that rely upon covalently attached directing groups. The per-epoxide yields of 87% (for 18) and 89% (for 19), are in line with the ~10:1 selectivity seen with monoepoxide 5 and do not change as a function of the number of epoxides or the number of THP rings preceding a given epoxide. In addition to validating the “template-by-a-THP” concept, this invariance would seem to support a mechanism for the cascade involving attack of an activated epoxide by the hydroxyl group attached to the preceding THP ring (right-to-left, as drawn in Figure 3). The alternative mechanism (activated epoxide attacked by the next epoxide) has the opposite directionality (left to right) and is often invoked, despite the fact that it involves a highly strained epoxonium intermediate. (17, 20, 23)
The effect of temperature upon the reaction rate is substantial, but its impact on selectivity is remarkably minimal, consistent with the template concept of minimization of entropic contributions to the competing transition states. For example, approximately one month (28 d) was required for complete consumption of 17 at room temperature in pH 7 phosphate buffer (1.0 M), but polyether triad was nonetheless afforded in identical isolated yield (60%).
Mechanism and implications
Just as the development of all-THF epoxide-opening cascades (24, 25) is taken as support of the Cane-Celmer-Westley hypothesis of monensin biosynthesis, (26) we believe that the all-THP cascades herein represent long-sought solid pieces of evidence in favor of Nakanishi’s hypothesis of ladder polyether biosynthesis (or at the very least the feasibility thereof) for several reasons: They are high yielding and highly THP-selective (yield per epoxide > 85% in all cases), require no directing groups on any of the epoxides, and are most effectively promoted by H2O.
The template may be functioning as a surrogate for conformational constraints imposed by an enzyme active site, and since water is the superior promoter of the all-THP cascades, it is reasonable to propose that such cascades would be promoted by hydrogen bond activation of the epoxide in the natural systems. (27) Monensin and related polyethers are produced via epoxide ring-opening reactions promoted by epoxide hydrolases (EHs), and based on sequence homology to other EHs, the epoxide appears to be activated by hydrogen bonds donated by two conserved tyrosine residues. (28) Currently far less is known about brevetoxin and other ladder polyether toxins in this regard, but it is possible that dinoflagellates possess a similar set of polyketide synthase enzymes, (8) though with epoxide hydrolases that are selective for the larger ring.
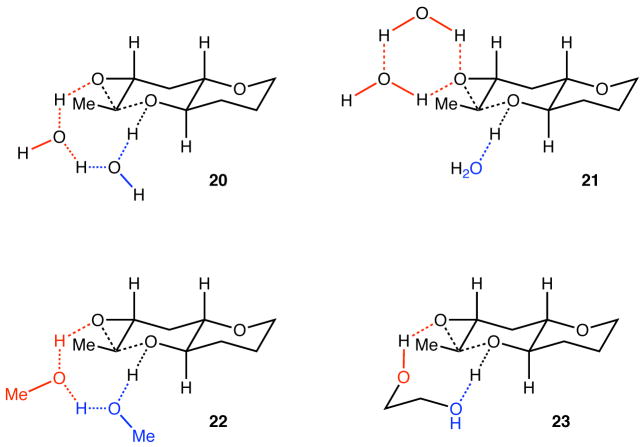
Water is one of the most heavily studied molecules on Earth, and its colligative structure and catalytic properties are still a subject of intense investigation. (29, 30, 31) Thus the means by which it affects selectivity in these cascades and organic reactions in general (32, 33, 34, 35) is not without uncertainty at this point. Moreover, although the reactions appear to be homogeneous, we cannot rule out surface effects or the formation of micelles that impact the conformation and reactivity of the epoxy alcohols (5, 15, and 17). Nevertheless, as shown in Figure 4 (20) activation of the nucleophile (OH group) and electrophile (epoxide) may be achieved by two water molecules (red and blue H2O, respectively) in a cooperative network of hydrogen bonds that would account for not only the enhanced regioselectivity in water (relative to other solvents), but also the marginal effects of temperature on selectivity. Another possibility (21) is analogous to the dual-H-bond mode of activation (red H2O) in epoxide hydrolases, but because activation of the electrophile and nucleophile are disconnected, this model less easily explains the selectivity.
Fig. 4.
Models of epoxide-opening reactions promoted by water (20 and 21), methanol (22), and ethylene glycol (23). Me, methyl.
More complex hybrids that unite the attributes of these two models can also be posited, but at this stage we favor 20 for several reasons. Its relative simplicity (i.e., lower molecularity) constitutes a more easily testable structural hypothesis, and the results observed with methanol and ethylene glycol are also adequately explained, in the forms of 22 and 23, respectively. Furthermore, as illustrated in 23, ethylene glycol represents an attractive starting point for the development of small molecules that activate the nucleophile and electrophile in such a way as to effect cyclizations and cascades of even higher selectivity and efficiency. In the meantime, templated, water-promoted, THP-selective epoxide-opening cascades provide a straightforward means for efficient and rapid assembly of ladder polyethers, enabling investigations directed toward understanding the mode of action of these extraordinary natural products. (36)
Supplementary Material
Footnotes
References
- 1.Lin YY, et al. J Am Chem Soc. 1981;103:6773–6775. [Google Scholar]
- 2.Nicolaou KC, et al. J Am Chem Soc. 1995;117:1173–1174. [Google Scholar]
- 3.Nicolaou KC, et al. Nature. 1998;392:264–269. doi: 10.1038/32623. [DOI] [PubMed] [Google Scholar]
- 4.Hirama M, et al. Science. 2001;294:1904–1907. doi: 10.1126/science.1065757. [DOI] [PubMed] [Google Scholar]
- 5.Trainer VL, Baden DG, Catterall WA. J Biol Chem. 1994;269:19904–19909. [PubMed] [Google Scholar]
- 6.Lee MS, Repeta DJ, Nakanishi K, Zagorski MG. J Am Chem Soc. 1986;108:7855–7856. doi: 10.1021/ja00284a072. [DOI] [PubMed] [Google Scholar]
- 7.Chou HN, Shimizu Y. J Am Chem Soc. 1987;109:2184–2185. [Google Scholar]
- 8.Snyder RV, et al. Phytochemistry. 2005;66:1767–1780. doi: 10.1016/j.phytochem.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakanishi K. Toxicon. 1985;23:473–479. doi: 10.1016/0041-0101(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 10.Coxon JM, Hartshorn MP, Swallow WH. Aust J Chem. 1973;26:2521–2526. [Google Scholar]
- 11.The usual terminology (“exo/endo”) applied to described THF/THP selectivity is a misleading and generally incorrect invocation of Baldwin’s rules for ring closure. (Both epoxide ring-opening products are the result of “exo-tet” processes according to the Baldwin construct.) See: Baldwin JE. J Chem Soc, Chem Commun. 1976;18:734.
- 12.The fused/spiro nomenclature is commonly used in cyclopropane-opening reactions: Danishefsky SJ, Dynak J, Hatch E, Yamamoto M. J Am Chem Soc. 1974;96:1256–1259.
- 13.Many effective directing groups have been developed. For the pioneering work in this area see: Nicolaou KC, et al. J Am Chem Soc. 1989;111:5330–5334.
- 14.Janda KD, Shevlin CG, Lerner RA. Science. 1993;259:490–493. doi: 10.1126/science.8424171. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN. Science. 1997;277:936–938. doi: 10.1126/science.277.5328.936. [DOI] [PubMed] [Google Scholar]
- 16.Wu MH, Hansen KB, Jacobsen EN. Angew Chem Int Ed. 1999;38:2012–2014. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<2012::AID-ANIE2012>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Tokiwano T, Fujiwara K, Murai A. Synlett. 2000:335–338. [Google Scholar]
- 18.Bravo F, McDonald FE, Neiwert WA, Do B, Hardcastle KI. Org Lett. 2003;5:2123–2126. doi: 10.1021/ol034539o. [DOI] [PubMed] [Google Scholar]
- 19.Simpson GL, Heffron TP, Merino E, Jamison TF. J Am Chem Soc. 2006;128:1056–1057. doi: 10.1021/ja057973p. [DOI] [PubMed] [Google Scholar]
- 20.Heffron TP, Jamison TF. Synlett. 2006:2329–2333. [Google Scholar]
- 21.Materials and methods are available as supporting online material in Science Online.
- 22.Tu Y, Wang ZX, Shi Y. J Am Chem Soc. 1996;118:9806–9807. [Google Scholar]
- 23.Wan S, Gunaydin H, Houk KN, Floreancig PE. J Am Chem Soc. 2007;129 doi: 10.1021/ja0709674. in press, (available at http://pubs3.acs.org/acs/journals/doilookup?in_doi=10.1021/ja0709674) [DOI] [PMC free article] [PubMed]
- 24.Still WC, Romero AG. J Am Chem Soc. 1986;108:2105–2106. [Google Scholar]
- 25.Schreiber SL, et al. J Am Chem Soc. 1986;108:2106–2108. [Google Scholar]
- 26.Cane DE, Celmer WB, Westley JW. J Am Chem Soc. 1983;105:3594–3600. [Google Scholar]
- 27.Kleiner CM, Schreiner PR. Chem Commun. 2006:4315–4317. doi: 10.1039/b605850g. [DOI] [PubMed] [Google Scholar]
- 28.Gallimore AR, et al. Chem Biol. 2006;13:453–460. doi: 10.1016/j.chembiol.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Wernet Ph, et al. Science. 2004;304:995–999. doi: 10.1126/science.1096205. [DOI] [PubMed] [Google Scholar]
- 30.Smith JD, et al. Science. 2004;306:851–853. doi: 10.1126/science.1102560. [DOI] [PubMed] [Google Scholar]
- 31.Vöhringer-Martinez E, et al. Science. 2007;315:497–501. doi: 10.1126/science.1134494. [DOI] [PubMed] [Google Scholar]
- 32.Rideout DC, Breslow R. J Am Chem Soc. 1980;102:7817–7818. [Google Scholar]
- 33.Lindstrom UM. Chem Rev. 2002;102:2751–2772. doi: 10.1021/cr010122p. [DOI] [PubMed] [Google Scholar]
- 34.Li CJ, Chen L. Chem Soc Rev. 2006;35:68–82. doi: 10.1039/b507207g. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Xu Y. Angew Chem. 2007;46 doi: 10.1002/anie.200701966. in press, (available at http://dx.doi.org/10.1002/anie.200701966) [DOI] [PubMed]
- 36.Financial support was provided by the NIH National Institute of General Medical Sciences (R01 GM-72566) and by gifts from Merck Research Laboratories and Boehringer Ingelheim. I.V. is grateful to MIT for a Nicholas A. Milas Fellowship.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.