Abstract
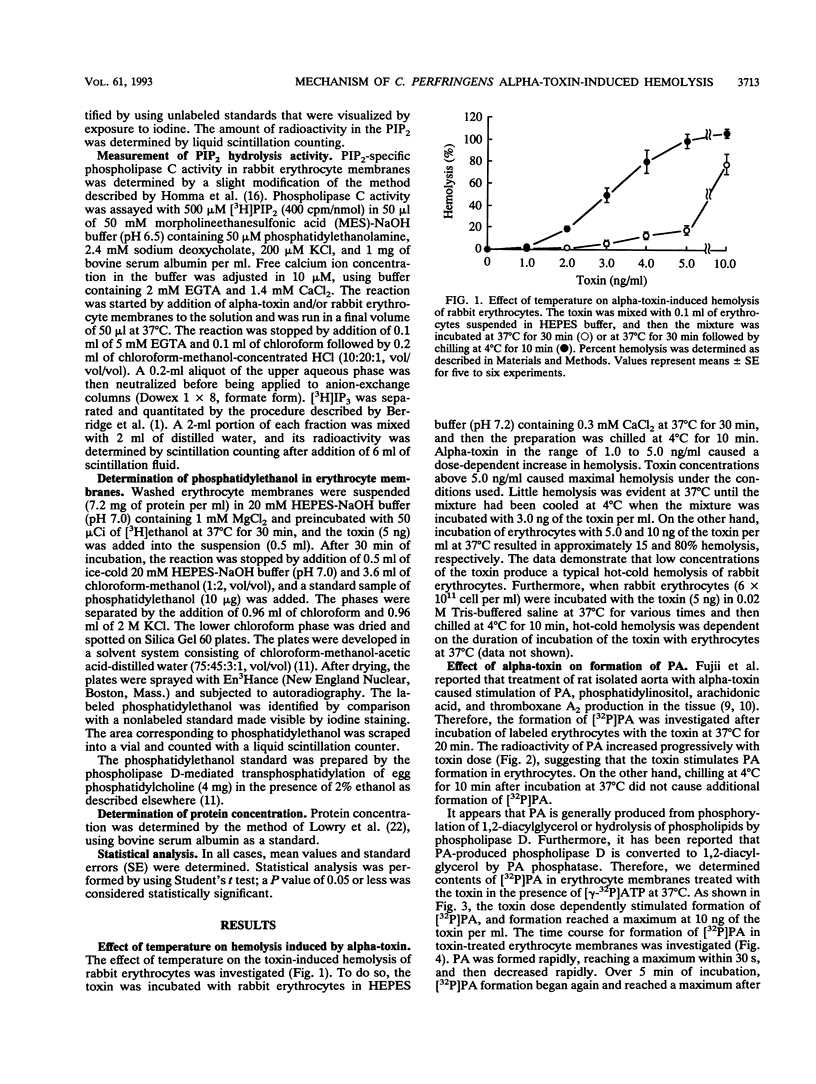
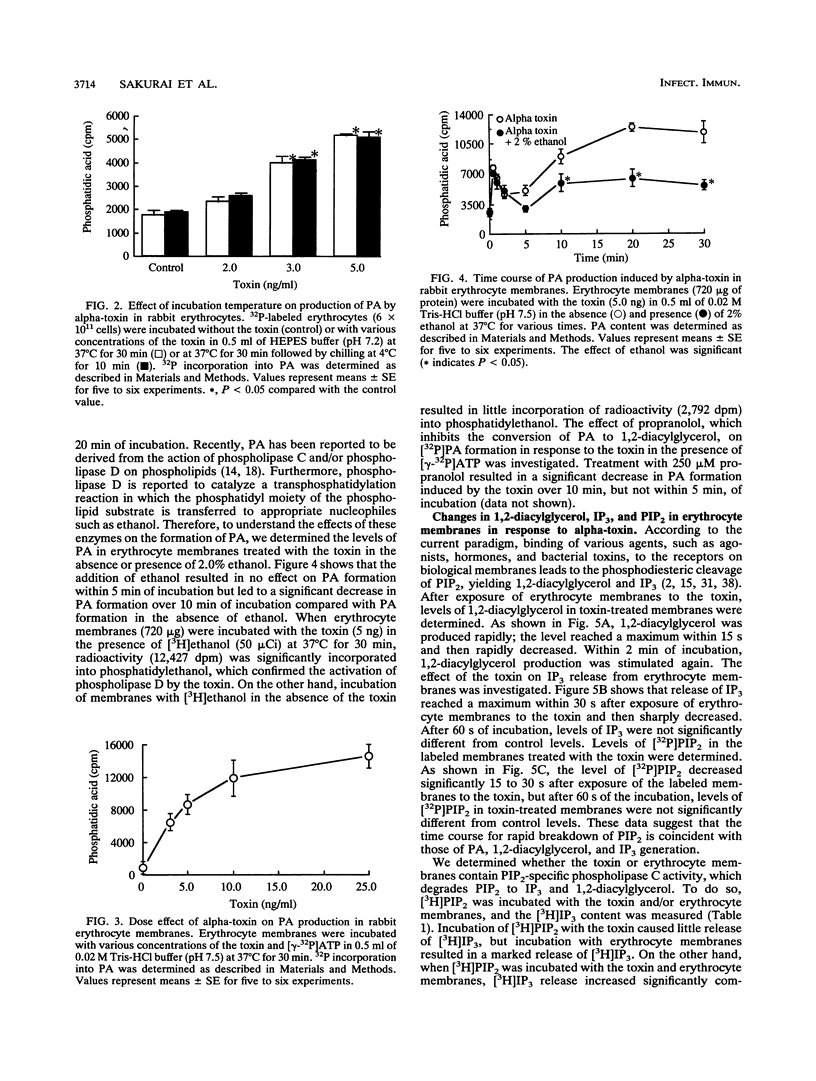
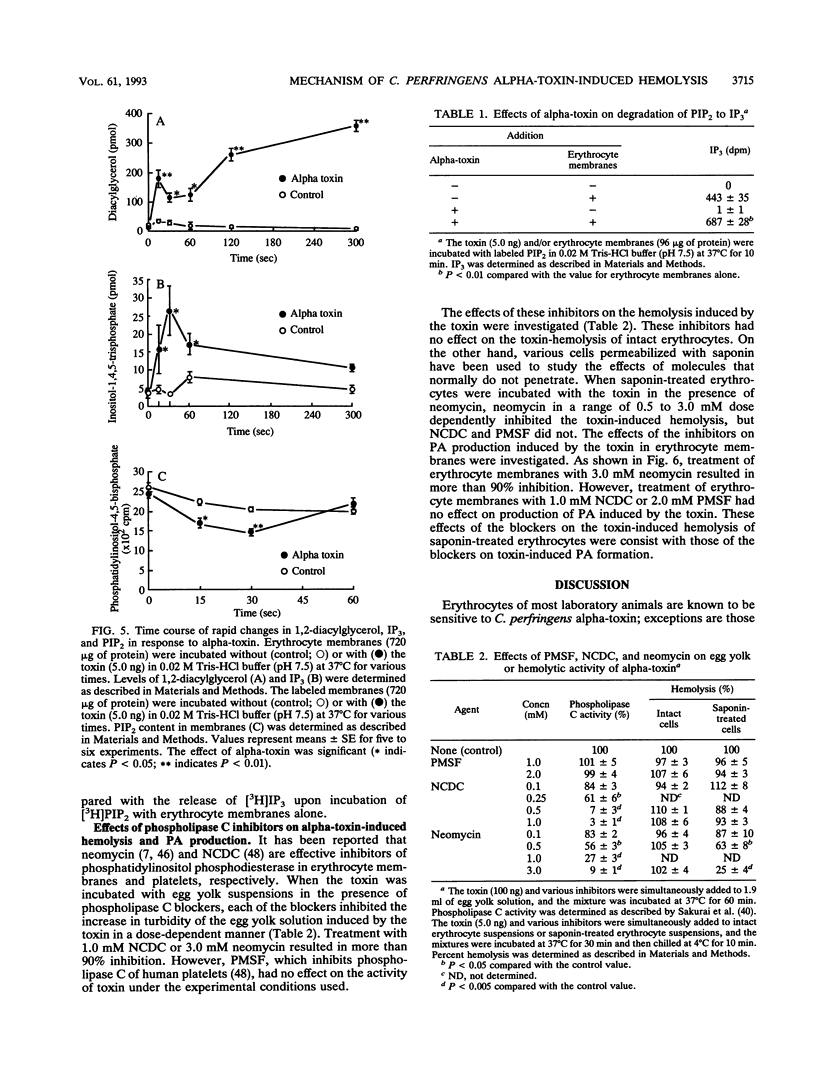
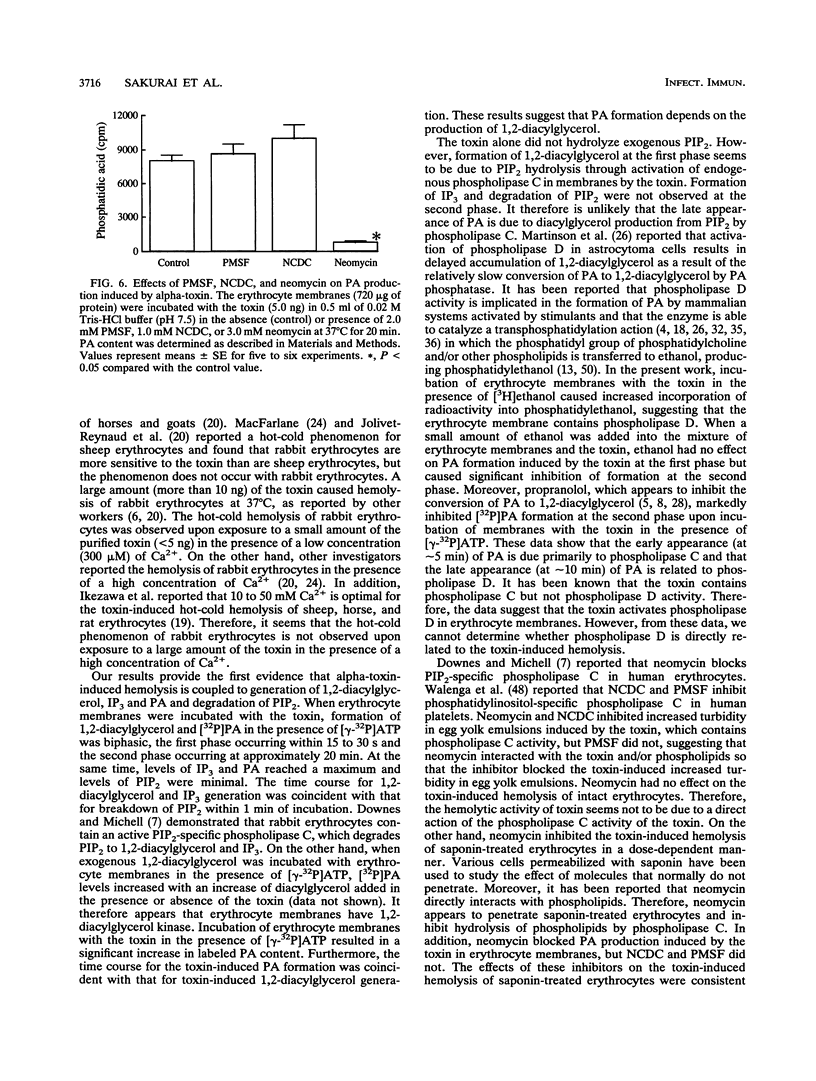
When rabbit erythrocytes were exposed to low concentrations of Clostridium perfringens alpha-toxin, hot-cold hemolysis was observed. The toxin induced production of phosphatidic acid (PA) in a dose-dependent manner when incubated with erythrocytes at 37 degrees C. When erythrocyte membranes were incubated with the toxin and [gamma-32P]ATP in the presence or absence of ethanol, [32P]PA formation was maximal within 30 s, then sharply decreased, and began again after 5 min of incubation. Ethanol had no effect on the early appearance (at approximately 5 min) of PA formation induced by the toxin but significantly inhibited formation of PA over 10 min of incubation. Treatment of erythrocyte membranes with alpha-toxin resulted in the biphasic formation of 1,2-diacylglycerol and PA as well as an increase of inositol-1,4,5-trisphosphate (IP3) and decrease of phosphatidylinositol-4,5-bisphosphate (PIP2) within 30 s. Neomycin inhibited the toxin-induced increase in turbidity of egg yolk suspensions but did not inhibit the toxin-induced hemolysis of intact erythrocytes. On the other hand, neomycin inhibited the toxin-induced hemolysis of saponin-treated erythrocytes. In addition, neomycin inhibited PA formation induced by the toxin in erythrocyte membranes. IP3 was released by incubation of PIP2 with erythrocyte membranes but not by incubation of PIP2 with the toxin. The toxin stimulated the membrane-induced release of IP3 from PIP2. These data suggest that the toxin-induced hemolysis is dependent on the action of phospholipase C in erythrocyte membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Chabot M. C., McPhail L. C., Wykle R. L., Kennerly D. A., McCall C. E. Comparison of diglyceride production from choline-containing phosphoglycerides in human neutrophils stimulated with N-formylmethionyl-leucylphenylalanine, ionophore A23187 or phorbol 12-myristate 13-acetate. Biochem J. 1992 Sep 15;286(Pt 3):693–699. doi: 10.1042/bj2860693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Bramley T. A. Hydrolysis of erythrocyte membrane phospholipids by a preparation of phospholipase C from Clostridium Welchii. Deactivation of (Ca-2+, Mg-2+)-ATPase and its reactivation by added lipids. Biochim Biophys Acta. 1975 Apr 8;382(4):565–575. doi: 10.1016/0005-2736(75)90223-0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Taylor G. S. Divergent effects of propranolol on neutrophil superoxide release: involvement of phosphatidic acid and diacylglycerol as second messengers. Biochem Biophys Res Commun. 1991 Mar 15;175(2):423–429. doi: 10.1016/0006-291x(91)91581-v. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Nomura S., Oshita Y., Sakurai J. Excitatory effect of Clostridium perfringens alpha toxin on the rat isolated aorta. Br J Pharmacol. 1986 Jul;88(3):531–539. doi: 10.1111/j.1476-5381.1986.tb10233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Sakurai J. Contraction of the rat isolated aorta caused by Clostridium perfringens alpha toxin (phospholipase C): evidence for the involvement of arachidonic acid metabolism. Br J Pharmacol. 1989 May;97(1):119–124. doi: 10.1111/j.1476-5381.1989.tb11931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geny B., Cockcroft S. Synergistic activation of phospholipase D by protein kinase C- and G-protein-mediated pathways in streptolysin O-permeabilized HL60 cells. Biochem J. 1992 Jun 1;284(Pt 2):531–538. doi: 10.1042/bj2840531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Halenda S. P., Rehm A. G. Evidence for the calcium-dependent activation of phospholipase D in thrombin-stimulated human erythroleukaemia cells. Biochem J. 1990 Apr 15;267(2):479–483. doi: 10.1042/bj2670479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T., Kato I. Mode of cytotoxic action of pseudomonal leukocidin on phosphatidylinositol metabolism and activation of lysosomal enzyme in rabbit leukocytes. Infect Immun. 1984 Jan;43(1):21–27. doi: 10.1128/iai.43.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma Y., Imaki J., Nakanishi O., Takenawa T. Isolation and characterization of two different forms of inositol phospholipid-specific phospholipase C from rat brain. J Biol Chem. 1988 May 15;263(14):6592–6598. [PubMed] [Google Scholar]
- Hostetler K. Y., Gardner M. F., Aldern K. A. Assay of phospholipases C and D in presence of other lipid hydrolases. Methods Enzymol. 1991;197:125–134. doi: 10.1016/0076-6879(91)97139-p. [DOI] [PubMed] [Google Scholar]
- Huang R., Kucera G. L., Rittenhouse S. E. Elevated cytosolic Ca2+ activates phospholipase D in human platelets. J Biol Chem. 1991 Jan 25;266(3):1652–1655. [PubMed] [Google Scholar]
- Jolivet-Reynaud C., Moreau H., Alouf J. E. Assay methods for alpha toxin from Clostridium perfringens: phospholipase C. Methods Enzymol. 1988;165:293–297. doi: 10.1016/s0076-6879(88)65044-0. [DOI] [PubMed] [Google Scholar]
- Krug E. L., Kent C. Phospholipase C from Clostridium perfringens: preparation and characterization of homogeneous enzyme. Arch Biochem Biophys. 1984 Jun;231(2):400–410. doi: 10.1016/0003-9861(84)90403-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACFARLANE M. G. The biochemistry of bacterial toxins; variation in haemolytic activity of immunologically distinct lecithinases towards erythrocytes from different species. Biochem J. 1950 Sep;47(3):270–279. doi: 10.1042/bj0470270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. G. The biochemistry of bacterial toxins: 2. The enzymic specificity of Clostridium welchii lecithinase. Biochem J. 1948;42(4):587–590. [PMC free article] [PubMed] [Google Scholar]
- Martinson E. A., Trilivas I., Brown J. H. Rapid protein kinase C-dependent activation of phospholipase D leads to delayed 1,2-diglyceride accumulation. J Biol Chem. 1990 Dec 25;265(36):22282–22287. [PubMed] [Google Scholar]
- Meduski J. W., Hochstein P. Hot-cold hemolysis: the role of positively charged membrane phospholipids. Experientia. 1972 May 15;28(5):565–566. doi: 10.1007/BF01931879. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Suganuma A., Matsui A., Hattori H., Sato M., Takenaka A., Nozawa Y. Primary role of calcium ions in arachidonic acid release from rat platelet membranes. Comparison with human platelet membranes. Biochem J. 1989 Apr 1;259(1):139–144. doi: 10.1042/bj2590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G. J. Lipid composition of erythrocytes in various mammalian species. Biochim Biophys Acta. 1967 Oct 2;144(2):221–232. doi: 10.1016/0005-2760(67)90152-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rubin R. Phosphatidylethanol formation in human platelets: evidence for thrombin-induced activation of phospholipase D. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1090–1096. doi: 10.1016/s0006-291x(88)80744-7. [DOI] [PubMed] [Google Scholar]
- Sabban E., Laster Y., Loyter A. Resolution of the hemolytic and the hydrolytic activities of phospholipase-C preparation from Clostridium perfringens. Eur J Biochem. 1972 Jul 24;28(3):373–380. doi: 10.1111/j.1432-1033.1972.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Saito M., Ando S., Mitsui K., Homma Y., Takenawa T. Activation of phosphatidic acid metabolism of human erythrocyte membranes by perfringolysin O. Biochem Biophys Res Commun. 1986 May 29;137(1):23–28. doi: 10.1016/0006-291x(86)91170-8. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Fujii Y., Shirotani M. Contraction induced by Clostridium perfringens alpha toxin in the isolated rat ileum. Toxicon. 1990;28(4):411–418. doi: 10.1016/0041-0101(90)90079-m. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Fujii Y., Torii K., Kobayashi K. Dissociation of various biological activities of Clostridium perfringens alpha toxin by chemical modification. Toxicon. 1989;27(3):317–323. doi: 10.1016/0041-0101(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nomura S., Fujii Y., Oshita Y. Effect of Clostridium perfringens alpha toxin on the isolated rat vas deferens. Toxicon. 1985;23(3):449–455. doi: 10.1016/0041-0101(85)90028-5. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Oshita Y., Fujii Y. Effect of Clostridium perfringens alpha toxin on the cardiovascular system of rats. Toxicon. 1985;23(6):903–912. [PubMed] [Google Scholar]
- Sato H., Chiba J., Sato Y. Monoclonal antibodies against alpha toxin of Clostridium perfringens. FEMS Microbiol Lett. 1989 May;50(1-2):173–176. doi: 10.1016/0378-1097(89)90480-1. [DOI] [PubMed] [Google Scholar]
- Schulze J. A., Nakamura M. Haemolytic activity of the alpha and theta toxins of Clostridium welchii. J Hyg (Lond) 1969 Mar;67(1):163–174. doi: 10.1017/s0022172400041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Freer J. H., Arbuthnott J. P. Interaction of Clostridium perfringens theta-haemolysin, a contaminant of commercial phospholipase C, with erythrocyte ghost membranes and lipid dispersions. A morphological study. Biochim Biophys Acta. 1975 Apr 8;382(4):479–493. doi: 10.1016/0005-2736(75)90216-3. [DOI] [PubMed] [Google Scholar]
- Tysnes O. B., Steen V. M., Holmsen H. Neomycin inhibits platelet functions and inositol phospholipid metabolism upon stimulation with thrombin, but not with ionomycin or 12-O-tetradecanoyl-phorbol 13-acetate. Eur J Biochem. 1988 Oct 15;177(1):219–223. doi: 10.1111/j.1432-1033.1988.tb14365.x. [DOI] [PubMed] [Google Scholar]
- Van Heyningen W. E. The biochemistry of the gas gangrene toxins: Partial purification of the toxins of Cl. welchii, type A. Separation of alpha and theta toxins. Biochem J. 1941 Nov;35(10-11):1257–1269. doi: 10.1042/bj0351257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenga R., Vanderhoek J. Y., Feinstein M. B. Serine esterase inhibitors block stimulus-induced mobilization of arachidonic acid and phosphatidylinositide-specific phospholipase C activity in platelets. J Biol Chem. 1980 Jul 10;255(13):6024–6027. [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


