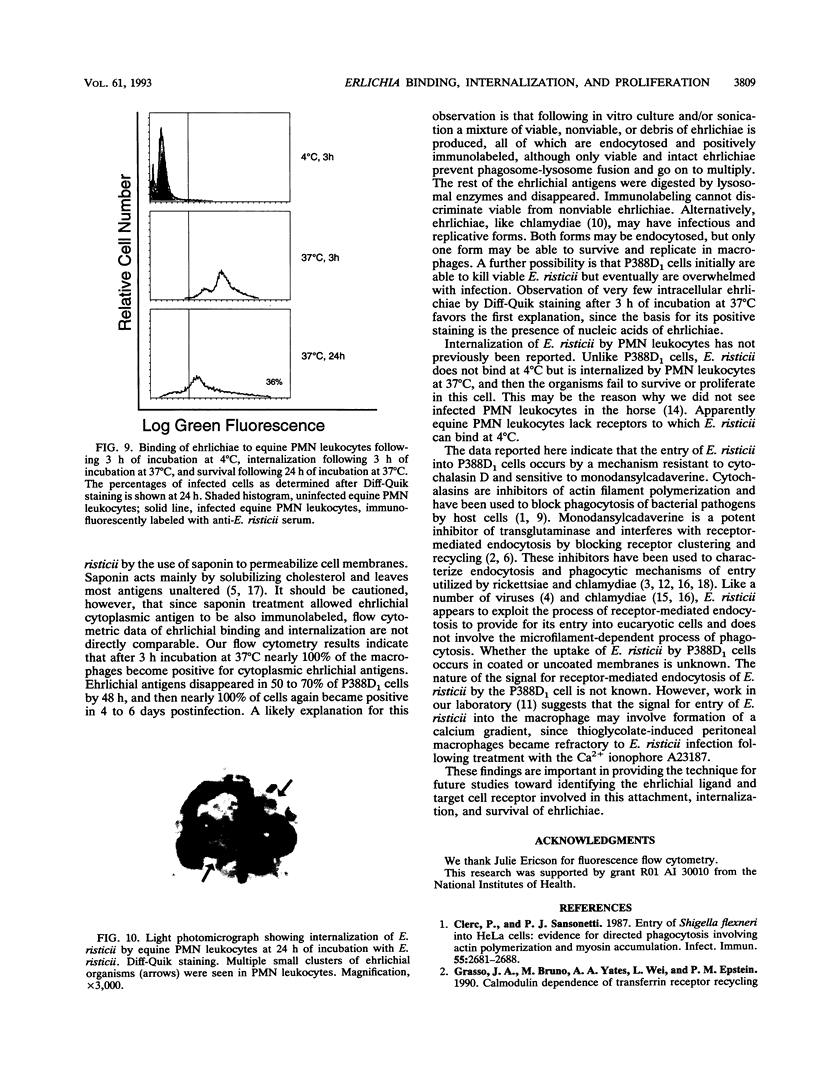
Abstract
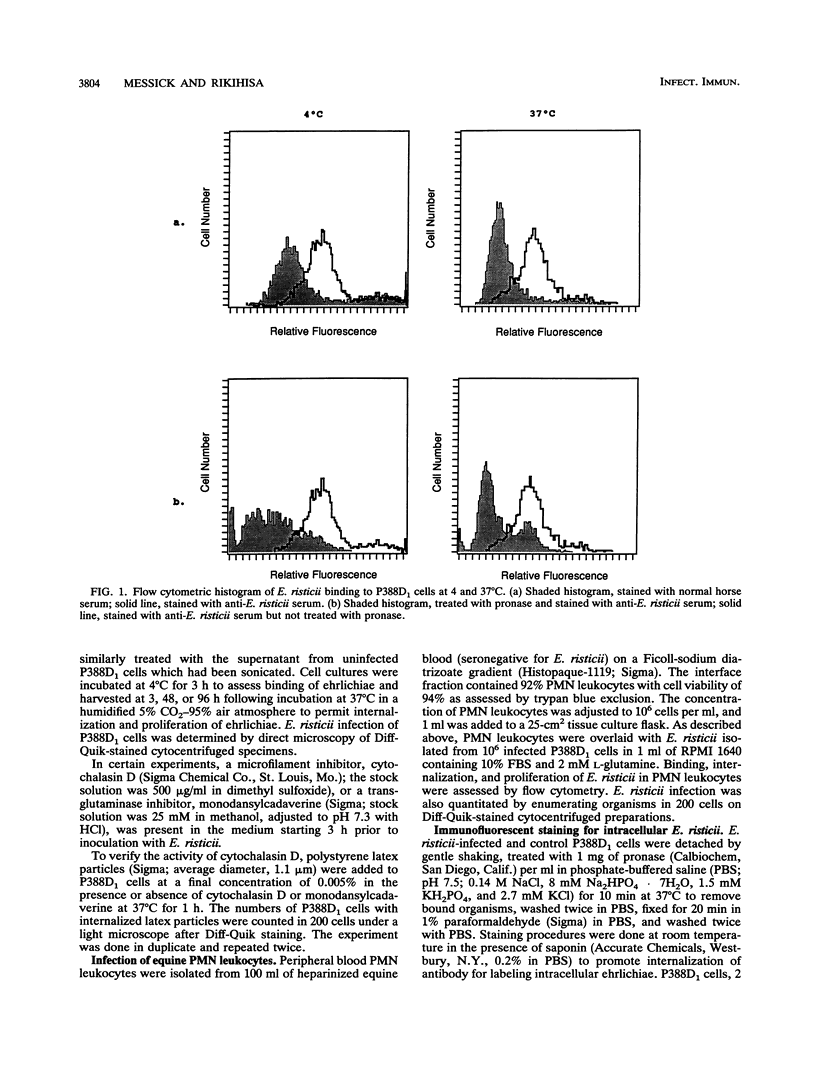
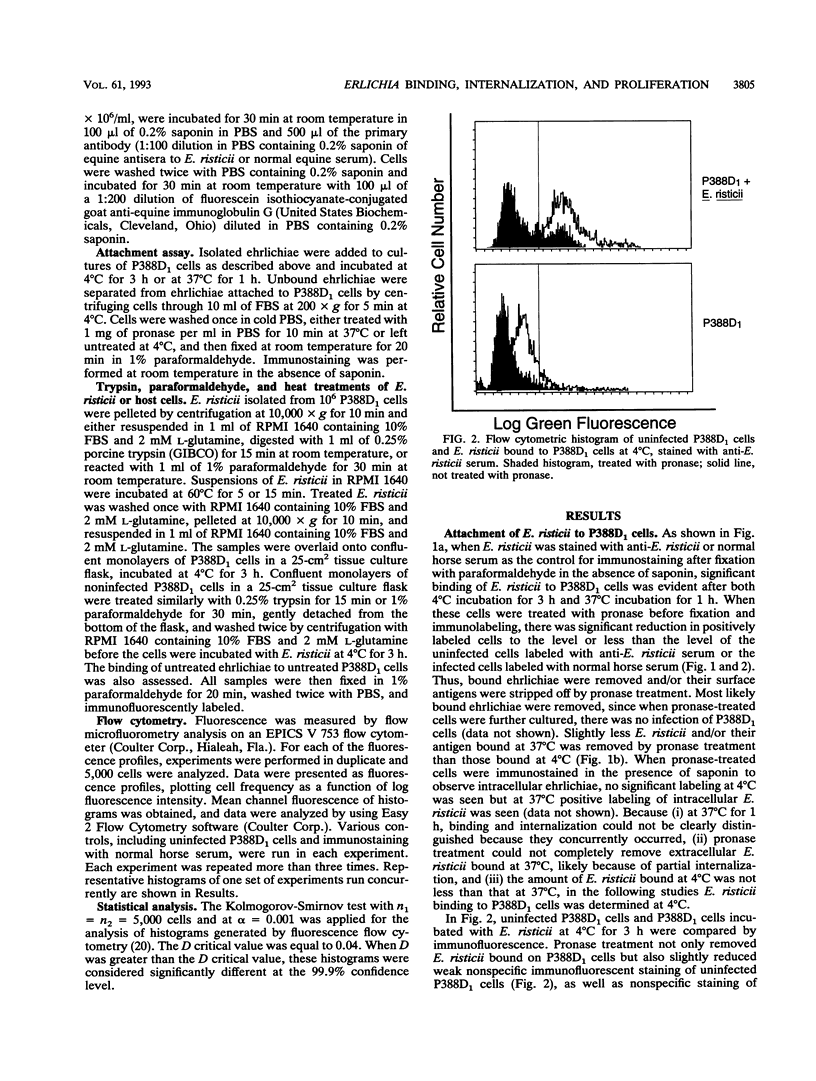
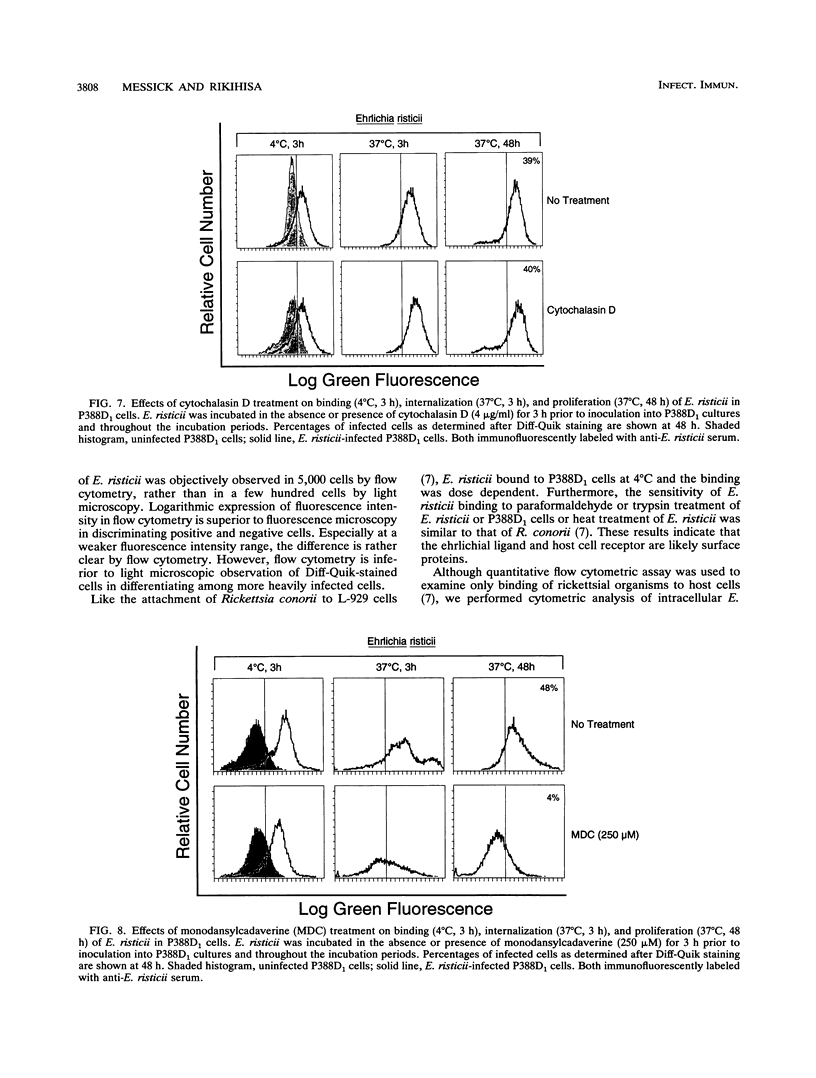
The binding, internalization, and proliferation of Ehrlichia risticii in P388D1 cells and equine polymorphonuclear (PMN) leukocytes were studied by immunofluorescent staining and flow cytometric analysis. The binding of ehrlichiae to P388D1 cells at 4 degrees C was dose dependent, and the antigens of bound organisms were susceptible to pronase treatment. Additionally, the binding of ehrlichiae to P388D1 cells was diminished when either P388D1 cells or ehrlichiae were treated with 1% paraformaldehyde for 30 min or 0.25% trypsin for 15 min. These results indicate that the ehrlichial ligand and host cell receptor are likely surface proteins. Following incubation at 37 degrees C, bound E. risticii and/or its antigens were removed with pronase and indirect immunofluorescent staining in the presence of saponin was used to examine intracellular ehrlichiae. Our results indicate that E. risticii was internalized into P388D1 cells within 3 h and proliferated by 48 h of incubation. The microfilament-disrupting agent cytochalasin D and the transglutaminase inhibitor monodansylcadaverine were used to differentiate between phagocytosis (sensitive to cytochalasin) and receptor-mediated endocytosis (sensitive to monodansylcadaverine) of E. risticii by P388D1 cells. In concentrations that produced distinctive morphological changes and inhibited phagocytosis of polystyrene latex beads, cytochalasin D did not suppress the infectivity of E. risticii. Binding, internalization, or proliferation of E. risticii was not affected by cytochalasin D. However, monodansylcadaverine inhibited infection of E. risticii in a dose-dependent manner. The agent did not affect the attachment of ehrlichiae to host cells, but it did suppress internalization and proliferation. These results suggest that E. risticii is internalized by receptor-mediated endocytosis and that productive infection by E. risticii does not depend on phagocytosis by the P388D1 cells. Although E. risticii did not bind to the surface of equine PMN leukocytes at 4 degrees C, organisms were taken up by this cell at 37 degrees C. E. risticii, however, failed to survive in equine PMN leukocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso J. A., Bruno M., Yates A. A., Wei L. T., Epstein P. M. Calmodulin dependence of transferrin receptor recycling in rat reticulocytes. Biochem J. 1990 Feb 15;266(1):261–272. doi: 10.1042/bj2660261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Kok J. W. Entry mechanisms of enveloped viruses. Implications for fusion of intracellular membranes. Biosci Rep. 1989 Jun;9(3):273–305. doi: 10.1007/BF01114682. [DOI] [PubMed] [Google Scholar]
- Jacob M. C., Favre M., Bensa J. C. Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry. 1991;12(6):550–558. doi: 10.1002/cyto.990120612. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Willingham M., Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1980 May;77(5):2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Walker D. H. Characterization of rickettsial attachment to host cells by flow cytometry. Infect Immun. 1992 May;60(5):2030–2035. doi: 10.1128/iai.60.5.2030-2035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messick J. B., Rikihisa Y. Presence of parasite antigen on the surface of P388D1 cells infected with Ehrlichia risticii. Infect Immun. 1992 Aug;60(8):3079–3086. doi: 10.1128/iai.60.8.3079-3086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Rikihisa Y. Inhibition of Ehrlichia risticii infection in murine peritoneal macrophages by gamma interferon, a calcium ionophore, and concanavalin A. Infect Immun. 1991 Oct;59(10):3418–3423. doi: 10.1128/iai.59.10.3418-3423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. J., Pearce J. H. Characterization of the cytochalasin D-resistant (pinocytic) mechanisms of endocytosis utilized by chlamydiae. Infect Immun. 1990 Oct;58(10):3208–3216. doi: 10.1128/iai.58.10.3208-3216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991 Jul;4(3):286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman F. M., Hadley W. K., Fulwyler M. J., Schachter J. Flow cytometric analysis of Chlamydia trachomatis interaction with L cells. Cytometry. 1987 Jan;8(1):55–59. doi: 10.1002/cyto.990080109. [DOI] [PubMed] [Google Scholar]
- Walker T. S. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect Immun. 1984 May;44(2):205–210. doi: 10.1128/iai.44.2.205-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988 Dec;56(12):3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. T. Proof without prejudice: use of the Kolmogorov-Smirnov test for the analysis of histograms from flow systems and other sources. J Histochem Cytochem. 1977 Jul;25(7):935–941. doi: 10.1177/25.7.894009. [DOI] [PubMed] [Google Scholar]



