Abstract
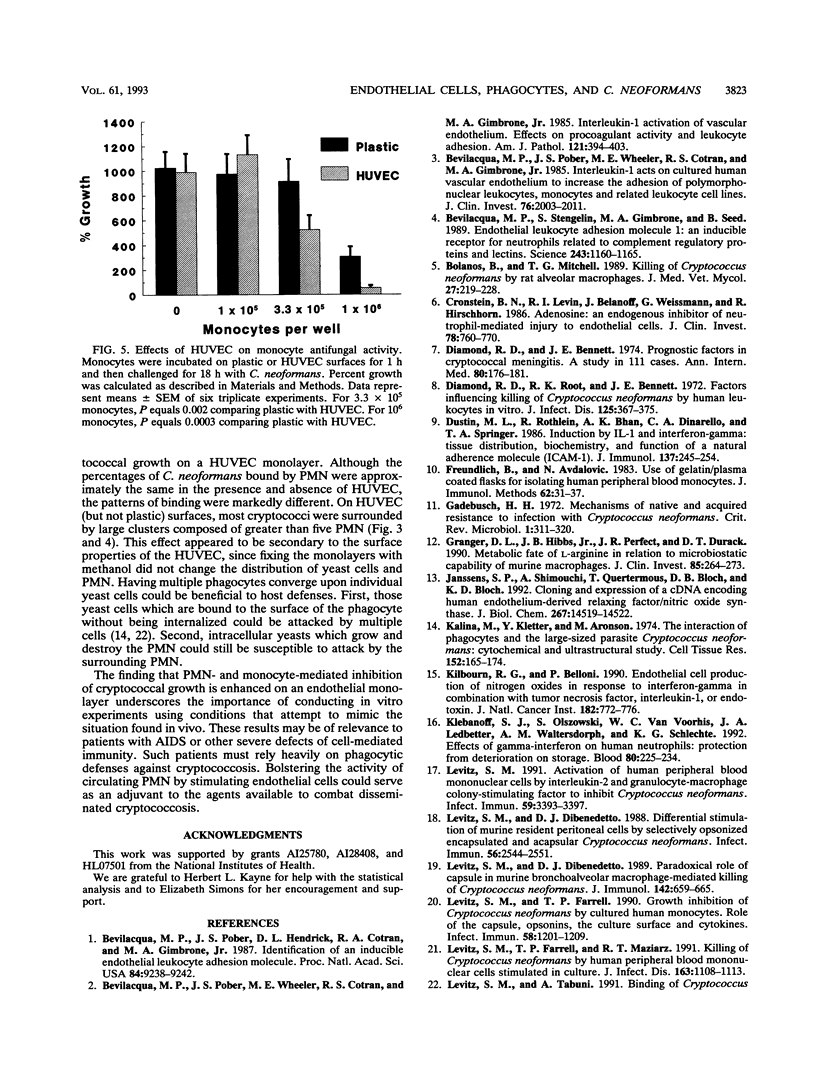
The anticryptococcal activity of peripheral blood polymorphonuclear leukocytes (PMN) and monocytes was compared on plastic versus human umbilical vein endothelial cell surfaces. Various amounts of PMN and monocytes were incubated on plastic or endothelial surfaces and then challenged for 18 h with Cryptococcus neoformans. Both phagocyte populations exhibited significantly more anticryptococcal activity on an endothelial cell monolayer than on plastic. Prestimulating the endothelial cell monolayer with interleukin-1 augmented the antifungal activity of PMN but not that of monocytes. In the absence of phagocytes, endothelial cells lacked activity. Blocking antibodies directed against endothelial adhesion molecules ICAM-1 and ELAM-1 did not affect PMN-mediated inhibition of fungal growth. Recombinant interleukin-1 and interleukin-8 (two cytokines secreted by endothelial cells) activated neutrophils for modestly enhanced antifungal activity. However, supernatants derived from endothelial cells, as well as neutralizing antibodies directed against the endothelial cell-derived cytokines interleukin-8 and granulocyte-macrophage colony-stimulating factor failed to augment PMN antifungal activity. PMN viability after 18 h was diminished on plastic compared with endothelial surfaces. While the percentages of C. neoformans bound to neutrophils were similar on both surfaces, the patterns of binding were markedly different: on endothelial (but not plastic) surfaces, most cryptococci were surrounded by greater than five PMN. Thus, phagocyte-mediated inhibition of cryptococcal growth is enhanced on endothelial monolayers compared with plastic surfaces, possibly as a result of differences in phagocyte viability and patterns of binding. Bolstering the activity of circulating phagocytes by stimulating endothelial cells may be of relevance in the treatment of patients with or at risk for cryptococcemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bolaños B., Mitchell T. G. Killing of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27(4):219–228. [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Kalina M., Kletter Y., Aronson M. The interaction of phagocytes and the large-sized parasite Cryptococcus neoformans: cytochemical and ultrastructural study. Cell Tissue Res. 1974;152(2):165–174. doi: 10.1007/BF00224692. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Olszowski S., Van Voorhis W. C., Ledbetter J. A., Waltersdorph A. M., Schlechte K. G. Effects of gamma-interferon on human neutrophils: protection from deterioration on storage. Blood. 1992 Jul 1;80(1):225–234. [PubMed] [Google Scholar]
- Levitz S. M. Activation of human peripheral blood mononuclear cells by interleukin-2 and granulocyte-macrophage colony-stimulating factor to inhibit Cryptococcus neoformans. Infect Immun. 1991 Oct;59(10):3393–3397. doi: 10.1128/iai.59.10.3393-3397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J Immunol. 1989 Jan 15;142(2):659–665. [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990 May;58(5):1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P., Maziarz R. T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991 May;163(5):1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Brock A. F., Arnaout M. A., Gimbrone M. A., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J Immunol. 1989 Apr 1;142(7):2257–2263. [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991 Jan;59(1):24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T., Gallis H. A. Cryptococcemia. Medicine (Baltimore) 1983 Mar;62(2):98–109. doi: 10.1097/00005792-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C., Wiessner J. H. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J Infect Dis. 1991 Jul;164(1):137–142. doi: 10.1093/infdis/164.1.137. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Hoffman P. A., Tomita J. K., Dickinson E. S., Jasman R. L., St John T., Gallatin W. M. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature. 1992 Dec 3;360(6403):485–488. doi: 10.1038/360485a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. P., Levitz S. M., Tabuni A., Kornfeld H. HIV-1 envelope protein (gp120) inhibits the activity of human bronchoalveolar macrophages against Cryptococcus neoformans. Am Rev Respir Dis. 1992 Dec;146(6):1434–1438. doi: 10.1164/ajrccm/146.6.1434. [DOI] [PubMed] [Google Scholar]
- Weinberg P. B., Becker S., Granger D. L., Koren H. S. Growth inhibition of Cryptococcus neoformans by human alveolar macrophages. Am Rev Respir Dis. 1987 Nov;136(5):1242–1247. doi: 10.1164/ajrccm/136.5.1242. [DOI] [PubMed] [Google Scholar]