Abstract
Elimination of excess climbing fiber (CF)–Purkinje cell synapses during cerebellar development involves a signaling pathway that includes type 1 metabotropic glutamate receptor, Gαq, and the γ isoform of protein kinase C. To identify phospholipase C (PLC) isoforms involved in this process, we generated mice deficient in PLCβ4, one of two major isoforms expressed in Purkinje cells. PLCβ4 mutant mice are viable but exhibit locomotor ataxia. Their cerebellar histology, parallel fiber synapse formation, and basic electrophysiology appear normal. However, developmental elimination of multiple CF innervation clearly is impaired in the rostral portion of the cerebellar vermis, in which PLCβ4 mRNA is predominantly expressed. By contrast, CF synapse elimination is normal in the caudal cerebellum, in which low levels of PLCβ4 mRNA but reciprocally high levels of PLCβ3 mRNA are found. These results indicate that PLCβ4 transduces signals that are required for CF synapse elimination in the rostral cerebellum.
The mRNA for phospholipase C (PLC) β4 has been reported to be particularly enriched in cerebellar Purkinje cells (PCs) (1, 2, 3). PCs express high levels of type 1 metabotropic glutamate receptor (mGluR1) (4, 5), which stimulates PLC through the activation of heterotrimeric G-proteins of the Gαq/11 family (6, 7). PCs are also very rich in the γ isoform of protein kinase C (PKCγ) (8, 9, 10) and in inositol 1,4,5-trisphosphate receptors (11), both of which are activated after hydrolysis of phosphatidylinositol 4,5-bisphosphate by the PLCβ enzymes. Gαq has been shown to colocalize with mGluR1 in PC dendritic spines (S. Nakagawa, J. Tanaka, M.W., M.K., M.I.S., and Y.I., unpublished data). It is, therefore, likely that the signal transduction cascade from mGluR1 to PKCγ and inositol 1,4,5-trisphosphate receptor activation proceeds via Gαq and PLCβ4 and that this cascade specifically plays an important role in PC function.
PCs receive distinct types of excitatory inputs from parallel fibers (PFs) and climbing fibers (CFs) (12, 13). Each PF synapse is weak, but one PC receives inputs from many (≈105) PF synapses. In contrast, CFs originate from the inferior olive and form strong excitatory synapses on proximal dendrites of PCs. In an adult mouse, >85% of PCs are innervated by single CFs. Massive elimination of supernumerary CFs occurs during the second and third postnatal weeks until a one-to-one relation between CFs and PCs is attained at approximately postnatal day 21 (P21). This relationship then is maintained through adult life. It was reported previously that mutant mice deficient in PKCγ, mGluR1, or Gαq retain persistent multiple CFs into adulthood and display motor discoordination (14, 15, 16). These results suggest that Gαq mediates signals from mGluR1 that are necessary for regression of multiple CFs during cerebellar development.
To identify the isoform(s) of PLCβ that transduces these signals, we used mutant mice deficient in PLCβ4. These mice were used previously to investigate the physiological significance of PLCβ4 in visual functions (17). Those results suggested that PLCβ4 plays a role in a step in visual processing that occurs after the initial photocascade in the rod outer segment (17). As reported previously (18), the PLCβ4 mutant mice display typical signs of motor discoordination and locomotor ataxia that are relatively severe in the hindlimb. We therefore examined the cerebellar morphology and electrophysiology of the PLCβ4 mutant mice.
MATERIALS AND METHODS
Electron Microscopy and Morphometry.
For electron microscopy, three mutant and three wild-type mice were perfused transcardially with 0.5% glutaraldehyde and 4% paraformaldehyde in 0.1M sodium cacodylate buffer (pH 7.2). Parasagittal microslicer sections (400 μm in thickness) through the cerebellar midline were prepared and processed for Epon block as reported (15). Silver-gold ultrathin sections were prepared from the culmen (lobule 4 + 5) and were stained with 1% uranyl acetate and mixed lead solution. From each mouse, 10 electron micrographs of the molecular layer were randomly taken at an original magnification of ×4,000 and were printed at the final magnification of ×16,000. Quantitative measurement of synapse profile number was done as reported (15).
In Situ Hybridization.
Two nonoverlapping oligonucleotides antisense to mouse PLCβ1–4 cDNAs (3) were labeled with 32S-dATP by using terminal deoxyribonucleotidyl transferase (BRL) and were used for in situ hybridization. Under deep pentobarbital anesthesia, the cerebellum were freshly removed from adult C57BL mice and were frozen in powdered dry ice. Frozen cryostat sections mounted on glass slides were processed for fixation, prehybridization, hybridization, and washing as reported (3). Sections were exposed to nuclear track emulsion (NTB-2, Kodak) for 2 months. After development, some sections were lightly counterstained with toluidine blue.
Electrophysiology.
Sagittal cerebellar slices of 200- to 300-μm thickness were prepared from the wild-type and mutant mice as described (14, 15, 16, 19). Whole-cell recording was made from visually identified PCs by using a 40× water immersion objective attached to either an Olympus (New Hyde Park, NY) (BH-2) or a Zeiss (Axioskop) upright microscope (20, 21, 22, 23). Resistance of patch pipettes was 3–6 megaohms when filled with an intracellular solution composed of (in mM): 60 CsCl, 30 Cs d-gluconate, 20 tetraethylammonium-Cl, 20 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, 4 MgCl2, 4 ATP, and 30 Hepes, (pH 7.3, adjusted with CsOH). The composition of standard bathing solution was (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose, which was bubbled continuously with a mixture of 95% O2 and 5% CO2. Bicuculline (10 μM) was always present in the saline to block spontaneous inhibitory postsynaptic currents (21, 22, 23). Ionic currents were recorded with either Axopatch-1D (Axon Instruments, Foster City, CA) or EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) and were stored on a DAT data recorder (Sony, Tokyo) for later analysis (14, 15, 16, 19). Stimulation and on-line data acquisition were performed by using the pulse 7.5 program (HEKA) on a Macintosh computer. The signals were filtered at 3 kHz and were digitized at 20 kHz. Fitting of the decay phases of excitatory postsynaptic currents (EPSCs) was done with the pulsefit 7.5 program (HEKA). For stimulation of CFs and PFs, a glass pipette with 5- to 10-μm tip diameter filled with standard saline was used. Square pulses were applied for focal stimulation (duration, 0.1 ms; amplitude, 0–100 V for CF stimulation, 1–10 V for PF stimulation) (14, 19).
RESULTS
Generation of PLCβ4-Deficient Mice.
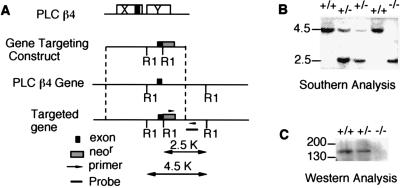
A mouse line that lacks PLCβ4 was established as described in Fig. 1A. The exon of the PLCβ4 gene, which encodes the catalytic domain responsible for PLC activity (24), was disrupted. The null mutation in the PLCβ4 gene was detected by PCR and was confirmed by Southern blot analysis (Fig. 1B). The lack of PLCβ4 protein in the cerebellum was demonstrated by Western blot analysis (Fig. 1C). The PLCβ4 mutant mice were viable and fertile. However, they exhibited various signs of ataxia, which became obvious ≈2–3 weeks after birth. These included general uncoordinated movements, body swaying while moving, ataxic gait, and intention tremors. The PLCβ4 mutant mice walked with shorter strides and could not walk for an extended time on a straight path. When placed on a thin rod, the PLCβ4 mutant mice could not coordinate the placement of their hind feet. These abnormalities were not caused by muscle weakness, because the mutant mice showed normal grasping responses. The differences in motor performance between the wild-type and the PLCβ4 mutant mice were obvious in the rotorod test. The duration of balancing on the rod of the PLCβ4 mutant was significantly shorter than that of wild-type mice (data not shown) and similar to the behavior of the homozygous Gαq deficient mice described (16).
Figure 1.
Generation of PLCβ4-null mutant mice. (A) The gene targeting construct and the homologous recombination process are depicted. (B) Southern blot analysis. Mouse tail DNA was isolated and digested with EcoRI and was separated by gel electrophoresis. After transfer to a nylon membrane, DNA was probed with the probe shown in A. (C) Western blot analysis. Cerebellar homogenates from the wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice were separated on a 7.5% polyacrylamide gel, were transferred to a nitrocellulose membrane, and were detected with a PLCβ4-specific antibody (Santa Cruz Biotechnology).
Normal Gross Cerebellar Anatomy and Parallel Fiber Synapse Formation.
The cerebellum of the PLCβ4 mutant mice showed similar size and foliation to that of wild-type mice at 2 months of age. No appreciable differences were found in the thickness of respective cortical layers and in granule cell density. In both strains of mice, cell bodies of PCs were aligned in a monolayer at similar intervals, and their dendritic structures were indistinguishable. The formation of PF–PC synapses was examined by electron microscopy at 2 months of age. In both strains of mice, the molecular layer contained numerous profiles of PC spines, round or oval swellings having a few fragments of smooth endoplasmic reticulum and well developed postsynaptic density. Most of the PC spines formed contacts with PF terminals, which contained a number of clear round synaptic vesicles. In both strains of mice, the PF–PC synapses were surrounded by cell processes of the Bergmann astroglia. When counting the PF–PC synapses on electron micrographs, the profile number per 100 μm2 was 21.3 ± 1.3 for the wild-type mice and 20.1 ± 1.7 for the mutant mice, showing no significant differences (n = 3; t test, P = 0.27).
Persistent Multiple Climbing Fiber Innervation of PLCβ4 Mutant Purkinje Cells in the Rostral Cerebellum.
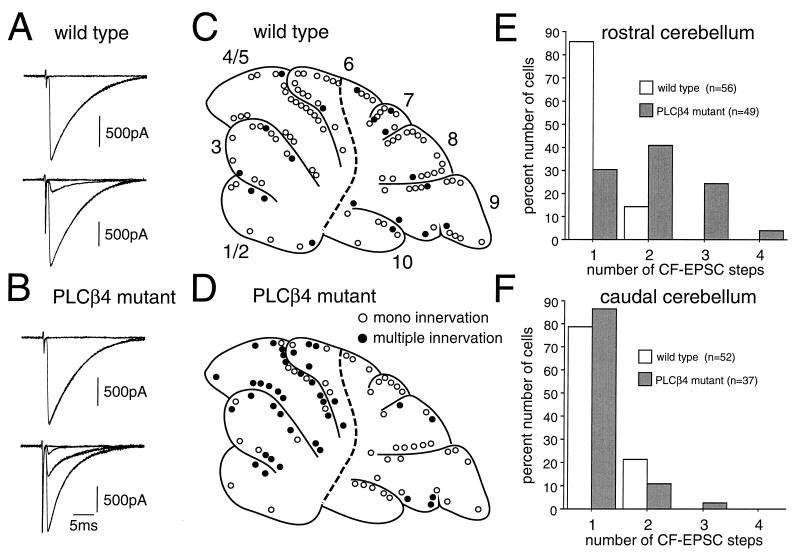
Midsagittal cerebellar slices were prepared from 22- to 109-day-old (P22-P109) wild-type mice or age-matched PLCβ4 mutant animals. CFs were stimulated in the granule cell layer, and evoked responses in single PCs were recorded by using patch-clamp techniques in the whole-cell configuration (14, 15, 16, 20, 21, 22). When a CF was stimulated, a clearly discernible EPSC was elicited in an all-or-none fashion (e.g., Fig. 2 A and B, upper traces). In some PCs, more than one discrete CF-EPSC was elicited at different stimulus sites or at one stimulus site with different stimulus thresholds (e.g., Fig. 2 A and B, lower traces). The number of CFs innervating the recorded PC was estimated by the number of discrete CF-EPSC steps elicited in that PC (14, 15, 16). We found that, in total, a significantly higher percentage of PCs remain multiply-innervated in PLCβ4 mutant mice (45.3%, 39 of 86) than in wild-type mice (17.6%, 19 of 108).
Figure 2.
Persistent multiple CF Innervation of PCs confined to the rostral cerebellum in mature PLCβ4 mutant mice. (A and B) EPSCs elicited by stimulation of CFs in the granule cell layer in wild-type (A) and PLCβ4 mutant (B) PCs. Records were taken from mice at P50 (A, upper), P80 (A, lower), P46 (B, upper), and p46 (B, lower), respectively. One or two traces are superimposed at each threshold intensity. Stimuli were applied at 0.2 Hz. Holding potential was −10 mV except for the lower trace in B that was taken at 0 mV. The number of CFs innervating the recorded PC was estimated by the number of discrete CF-EPSC steps. (C and D) Spatial distribution of mono-innervated (open circles) and multiply-innervated (filled circles) PCs of the wild-type (C) and PLCβ4 mutant (D) cerebella. Recording sites of PCs obtained from six wild-type (n = 108 PCs) and six PLCβ4 mutant (n = 86 PCs) cerebella were plotted on the standard midsagittal plane of the vermis. The broken line indicates the demarcation of the border of the rostral and caudal cerebellum that corresponds to the border of the two cerebellar regions in terms of PLCβ4 and PLCβ3 mRNA expression patterns (see Fig. 3). Note that multiply-innervated PCs were much more numerous in the rostral than in the caudal cerebellum in PLCβ4 mutant mice. (E and F) Summary histograms showing the number of discrete steps of CF-EPSCs of the wild-type (open columns) and PLCβ4 mutant (hatched-columns) PCs sampled in the rostral (E) and caudal (F) cerebellum. Data were obtained from mice at P22-P109. Note that the percentage of multiply-innervated PCs is significantly higher for the PLCβ4 mutant than the wild type mice (P < 0.0001, χ2 test for independent samples) in the rostral cerebellum. The wild-type and mutant PCs almost all were analyzed before knowledge of the mouse genotype was revealed.
The cerebellum is not necessarily homogeneous in terms of the distribution of various signaling molecules. We looked for possible interlobular differences in CF innervation by plotting the recording sites of PCs on the standard sagittal plane. In wild-type mice, PCs with mono CF innervation and those with multiple CF innervation were distributed evenly (Fig. 2C). By marked contrast, in PLCβ4 mutant mice, multiply-innervated PCs were much more numerous in the rostral half (lobule 1 to the rostral half of lobule 6) than in the caudal half (the caudal half of lobule 6 to lobule 10) of the cerebellum (Fig. 2D). The border does not coincide with the classical anatomical boundary, the primary fissure. It, rather, corresponds to the newly found cerebellar boundary in some mutant mice (25) or that determined by the staining patterns of biochemical markers, such as zebrins (26). The frequency distribution histogram constructed from data in the rostral cerebellum (Fig. 2E) clearly shows that the difference between the wild-type and PLCβ4 mutant mice was highly significant. By marked contrast, in the caudal cerebellum, the frequency distribution of mono-innervated PLCβ4 mutant PCs was similar to that of wild-type PCs (Fig. 2F). These results indicate that PLCβ4 mutant mice have a defect in CF synapse elimination that is manifested primarily in the rostral half of the cerebellar vermis.
Expressions of PLCβ3 and -β4 Are Reciprocally Regulated in PCs.
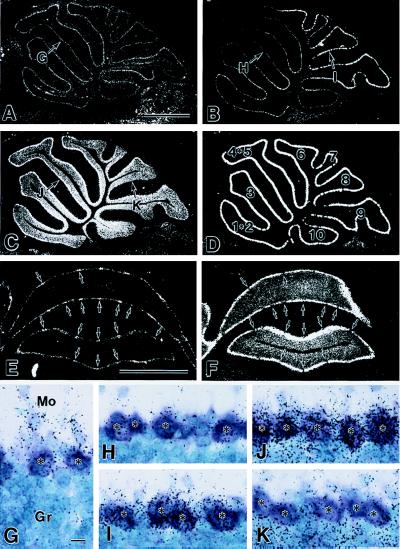
The overall distribution of PLCβ isoforms in rodent brains was studied, and PCs were reported to express PLCβ1, -β3, and -β4 (1, 2, 3). We examined regional differences in expression of these mRNAs along rostrocaudal and mediolateral axes of the cerebellum. After hybridization and emulsion autoradiography, PCs were identified by counterstaining with toluidine blue, which visualized the perikarya as a metachromatic purple color against the blue perikarya of granule cells (Fig. 3 G–K). The PLCβ1 mRNA was detected at low levels in the PC somata, showing no apparent regional differences (Fig. 3 A and G). Hybridization signals for PLCβ3 mRNA were restricted to PC somata (Fig. 3 B, E, H, and I), exhibiting stronger signals in PCs of the caudal cerebellum than those in the rostral cerebellum (Fig. 3B). The PLCβ4 mRNA was expressed in PCs and granule cells (Fig. 3 C, F, J, and K). In contrast to the PLCβ3 mRNA expression pattern, levels of PLCβ4 mRNA in PCs were stronger in the rostral than in the caudal cerebellum (Fig. 3C). The boundary for regions with PCs expressing high levels of PLCβ3 or PLCβ4 mRNA fell in the middle of lobule 6 (Fig. 3 B and C).
Figure 3.
PLCβ4 and PLCβ3 mRNAs are expressed reciprocally in PCs of the rostral and caudal cerebellum. In situ hybridization showing expressions of the PLCβ1 (A and G), PLCβ3 (B, E, H, and I), and PLCβ4 (C, F, J, and K) mRNAs in the mature wild-type mouse cerebellum. (A–D) Dark-field micrographs taken from adjacent sagittal cerebellar sections near the midline. As a reference, cell bodies of PCs were visualized by detecting calbindin mRNAs in D. The numbers in D represent the cerebellar lobules in the vermis. (E and F) Dark-field micrographs taken from adjacent coronal sections through the rostral cerebellum. Note that locations of PCs with high levels of PLCβ3 mRNA (arrows in E) correspond to those with low PLCβ4 mRNA levels in the adjacent section (arrows in F). (G–K) Bright-field micrographs taken from the sites indicated in cerebellar sections A–C. Asterisks indicate cell bodies of PCs that displayed metachromatic color changes by counterstaining with toluidine blue. Note that PCs only weakly express PLCβ3 mRNA (H) and strongly express PLCβ4 mRNA (J) in the rostral cerebellum and vice versa in the caudal cerebellum (I and K). [Bars = 1 mm (A and E) and 10 μm (G).]
Examination of coronal sections revealed a reciprocal expression pattern of PLCβ3 and PLCβ4 mRNA expression in the mediolateral cerebellar axis. In sections through the rostral cerebellum, the majority of PCs expressing high levels of PLCβ4 and low levels of PLCβ3 mRNAs formed thick parasagittal zones, which were interspersed periodically by one or two PCs with low PLCβ4 and high PLCβ3 mRNAs (Fig. 3 E and F). Identical results were obtained by using two nonoverlapping probes for in situ hybridization. These results indicate that PLCβ3 and PLCβ4 are the major isoforms in PCs and that the cerebellar regions in which PLCβ4 is predominantly expressed (Fig. 3C) correspond to those in which a significantly higher percentage of PCs were multiply-innervated by CFs in PLCβ4 mutant mice (Fig. 2C).
CF Innervation During Early Postnatal Days.
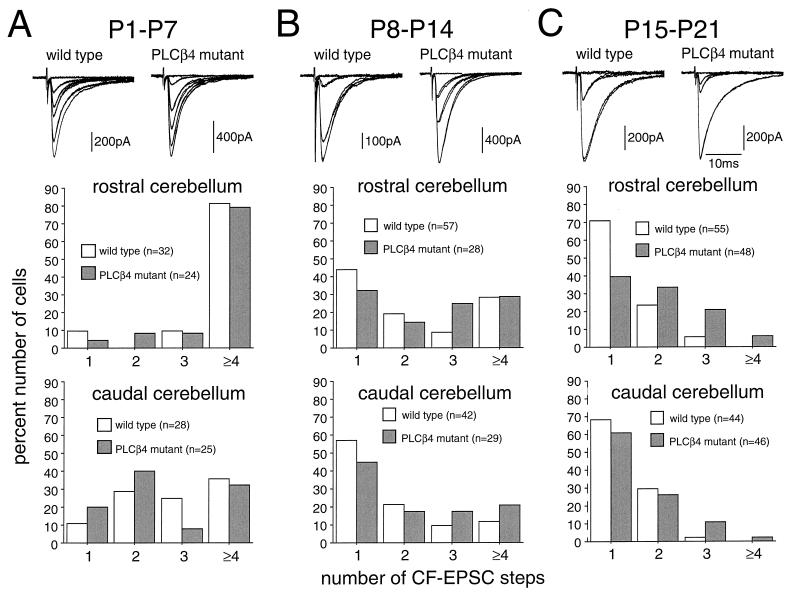
We followed the developmental course of CF innervation in the rostral and caudal cerebellum. Fig. 4 shows that, during the first (P1-P7, Fig. 4A) and second (P8-P14, Fig. 4B) postnatal weeks, there were no significant differences between the wild-type and PLCβ4 mutant mice in either rostral or caudal cerebellum. During the third postnatal week (P15-P21, Fig. 4C), however, clear differences between the two cerebellar regions became obvious. In the rostral cerebellum, the percentage of multiply-innervated PCs decreased markedly in the wild-type mice when compared with the percentage found during P8-P14. In contrast, the change in frequency distribution was much less prominent in PLCβ4 mutant mice (Fig. 4 B and C, middle). The frequency distribution histogram from data in the rostral cerebellum (Fig. 4C, middle) clearly shows that the difference between the wild-type and PLCβ4 mutant mice was highly significant. By contrast, in the caudal cerebellum, the reduction of multiply-innervated PCs proceeded to the same extent between the wild-type and PLCβ4 mutant mice (Fig. 4 B and C, lower). The frequency distributions were not significantly different between the two strains of mice (Fig. 4C, lower). These results suggest that the synapse elimination process that occurs during the third postnatal week is impaired specifically in the rostral cerebellum of the PLCβ4 mutant mice.
Figure 4.
Early postnatal development of CF innervation. (A, upper) Specimen records of CF-EPSCs of the wild-type (P7; holding potential, −30 mV) and PLCβ4 mutant (P6; holding potential, −60 mV) PCs that were sampled in the rostral cerebellum. One or two traces were superimposed at each threshold intensity. Stimuli were applied at 0.2 Hz (A, middle) Shown is a frequency distribution histogram for PCs in the rostral cerebellum from four wild-type and three PLCβ4 mutant mice at P1-P7. Twenty-five of 32 wild-type PCs in the rostral and 20 of 28 in the caudal cerebellum were studied blind to the mouse genotype. As for PLCβ4 mutant mice, all cells in the rostral and caudal cerebellum were studied blind to mouse genotype. (B) Similar to A, but for data from mice at P8-P14. Specimen records were taken from the wild-type (P10; holding potential, −20 mV) and PLCβ4 mutant (P10; holding potential −60 mV) PCs that were sampled in the rostral cerebellum. Data were obtained from six wild-type and four PLCβ4 mutant mice. All cells were studied blind to the mouse genotype. (C) Similar to A and B, but for data from mice at P15-P21. Specimen records were taken from the wild-type (P16; holding potential, 0 mV) and PLCβ4 mutant (P18; holding potential, 0 mV) PCs that were sampled in the rostral cerebellum. Data were obtained from six wild-type and seven PLCβ4 mutant mice. All cells were studied blind to the mouse genotype.
We found no significant differences in the rostral and caudal cerebellum, in basic electrophysiological properties of CF-EPSCs in the wild-type and PLCβ4 mutant mice. The 10–90% rise time or the decay time-constants were similar in the wild-type and PLCβ4 mutant mice. CF-EPSCs showed prominent paired-pulse depression in both mono-innervated and multiply-innervated PCs (14, 15, 19, 21) derived from the wild-type and PLCβ4 mutant mice at varying interpulse intervals (data not shown). The current–voltage relations were linear in both mono-innervated and multiply-innervated PCs derived from the two strains of mice (data not shown). CF-EPSCs in the wild-type and PLCβ4 mutant mice were not affected by an NMDA receptor blocker, dl-2-amino-5-phosphonopentanoate (100 μM), but were suppressed totally by an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor blocker, 6-cyano-7-nitroquinoxaline-2,3-dion (10 μM). These results suggest that, in both the rostral and caudal cerebellum, CF to PC synapses in the PLCβ4 mutant mice are functional and their electrophysiological properties are largely similar to the wild-type mice.
The nature of the EPSCs elicited by PF stimulation was examined in mice at P27-P50. In the rostral and caudal cerebellum, there was no significant difference between the wild-type and PLCβ4 mutant mice in the kinetics of PF-EPSCs. In both starins of mice, PF-EPSCs displayed paired-pulse facilitation at varying interpulse intervals from 10 to 300 ms (14, 19, 21). Furthermore, PF-EPSCs in the wild-type and PLCβ4 mutant mice were not affected by dl-2-amino-5-phosphonopentanoate (100 μM) but were totally suppressed by 6-cyano-7-nitroquinoxaline-2,3-dion (10 μM). Taken together, these results suggest that PF to PC transmission in the PLCβ4 mutant mice is normal in the rostral and caudal cerebellum.
DISCUSSION
Our results demonstrate that mice deficient in PLCβ4 display clear ataxia with typical signs of motor discoordination. The PLCβ4 mutant mice displayed impaired regression of multiple CF innervation of PCs that was primarily confined to the rostral cerebellar vermis (Fig. 2). Although the causal relationship is not fully understood, motor discoordination and impaired regression of multiple CFs also have been reported in several strains of mutant mice deficient in mGluR1 (15, 19, 27, 28), PKCγ (14, 29), glutamate receptor δ2 subunit (GluRδ2) (30), glutamate transporter GLAST (31), or Gαq (16). However, in these mutant mice, regional differences within the cerebellum in terms of persistent multiple CF innervation have not been reported. Therefore, ataxia and motor discoordination in PLCβ4 mutant mice suggest that the establishment of CF mono-innervation in the rostral vermis is important for coordinated locomotion in mice. In support of this notion, lesions to lobules 4 to 6 of the cerebellar vermis result in impaired locomotion in other mammals, including the cat and rat (32, 33).
Our results also indicate that the specific regression of multiple CF innervation that occurs in the second phase of development (P10-P20) is impaired in the rostral vermis of PLCβ4 mutant mice. The importance of functional PF-PC synapse formation in the developmental regression of multiple CF innervation has been revealed by previous studies with “agranular or hypogranular” cerebellar mutants, such as reeler, staggerer, and weaver mice (for review, see ref. 34) and with knockout mice with inactivated GluRδ2 gene (30, 35). By contrast, PLCβ4 mutant mice exhibit persistent multiple CF innervation without showing obvious defects in granule cells, PF synapse formation, or the basic electrophysiology of PF-mediated excitatory synaptic transmission. In this respect, PLCβ4 mutant mice share a common phenotype with previously reported mutant mice with inactivated mGluR1, Gαq, or PKCγ gene (14, 15, 16).
mGluR1, Gαq, and PKCγ all are expressed abundantly and homogeneously in adult wild-type PCs; mGluR1 is localized on the perisynaptic membrane of PC dendritic spines (4, 5), Gαq/11 is on the extra-junctional membrane of PC dendritic spines (S. Nakagawa, J. Tanaka, M.W., M.K., M.I.S., and Y.I., unpublished data), and PKCγ is in the cytoplasm of PC dendrites and somata (8, 9, 10). In the present study, we found that expression of PLCβ4 mRNA is heterogeneous in the rostrocaudal and mediolateral axes (Fig. 3), and the expression pattern appears to be similar to that seen when the cerebellum is stained for biochemical markers, such as zebrins (26). Reflecting this regional heterogeneity, PCs with persistent multiple CF innervation in PLCβ4 mutant mice emerge in cerebellar regions in which PLCβ4 mRNA is predominantly expressed. In the vermis, they preferentially occur in the rostral half (lobule 1 to the rostral half of the lobule 6). Based on these and previous results, we suggest that, in the rostral cerebellar vermis, activity at excitatory synapses of PCs modulates the mGluR1 signaling that acts through Gαq, PLCβ4, and PKCγ to facilitate the elimination of multiple CF innervation during the third postnatal week. Furthermore, our present results indicate that PLCβ1 does not compensate for the lack of PLCβ4 function in the rostral cerebellar vermis. This notion is supported by recent results that PLCβ1 deficient mice do not exhibit any signs of motor discoordination (18).
In the caudal half of the cerebellar vermis, the developmental elimination normally proceeds in PLCβ4 mutant mice (Figs. 2 and 4). Here, the expression of PLCβ4 mRNA is low, and that of PLCβ3 mRNA is high (Fig. 3). Biochemical data indicate that PLCβ3 and PLCβ4 are activated indistinguishably by Gαq and Gα11 (17, 36). Thus, it is conceivable that PLCβ3, presumably together with PLCβ1, compensates for the lack of PLCβ4 function in the caudal cerebellum.
The signal transduction cascade from mGluR1 to PKCγ via Gαq and PLCβ4 appears to be activated at PF–PC synapses rather than at CF–PC synapses. This notion is based on the following two findings. First, mGluR1, Gαq/11, and PLCβ4 are colocalized in the PC dendritic spines (refs. 4 and 5 and S. Nakagawa, J. Tanaka, M.W., M.K., M.I.S., and Y.I., unpublished data). Second, the CF elimination process requires activation of NMDA receptors (ref. 37 and S. Kakizawa, M.W., Y.I., and M.K., unpublished data), which do not contribute to either PF- or CF-EPSCs in PCs (14, 19) but do play significant roles in mossy fiber–granule cell excitatory transmission (38). Thus, signals generated in the PC dendritic spines activate PKCγ and then influence CF to PC synapses either directly or indirectly through further intracellular cascades.
Acknowledgments
We thank K. Matsumoto and V. Mancino for expert technical help and Dr. N. Kawai for continuous encouragement. This work has been supported by grants to M.K. from the Japanese Ministry of Education, Science, Sports and Culture, from the Human Frontier Science Program, and by Special Coordination Funds for promoting Science and Technology from the Science and Technology Agency of the Japanese Government. This work also has been supported by a grant to H.-S.S. from the Creative Research Initiatives of the Korean Government and by grants to D.W. and to M.I.S. from the National Institutes of Health. S.O. was a recipient of a fellowship from the Deutsche Forschungsgemeinschaft and the Guenther Foundation.
ABBREVIATIONS
- PC
Purkinje cell
- mGluR1
type 1 metabotropic glutamate receptor
- PLC
phospholipase C
- PKC
protein kinase C
- PF
parallel fiber
- CF
climbing fiber
- P21
postnatal day 21
- EPSC
excitatory postsynaptic current
Footnotes
Present address: Institut für Pharmakologie, Freie Universität Berlin, Thielallee 69-73 Berlin, Germany.
References
- 1.Tanaka O, Kondo H. Neurosci Lett. 1994;182:17–20. doi: 10.1016/0304-3940(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 2.Roustan P, Abitbol M, Nénini C, Ribeaudeau F, Gérard M, Vekemans M, Mallet J, Dufier J-L. NeuroReport. 1995;6:1837–1841. doi: 10.1097/00001756-199510020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Nakamura M, Sato K, Kano M, Simon M I, Inoue Y. Eur J Neurosci. 1998;10:2016–2025. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 4.Baude A, Nusser Z, Roberts J D, Mulvihill E, McIlhinney R A, Somogyi P. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 5.Nusser Z, Mulvihill E, Streit P, Somogyi P. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi S. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 7.Pin J-P, Duvoisin R. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 8.Hidaka H, Tanaka T, Onoda K, Hagiwara M, Watanabe M, Ohta H, Ito Y, Tsurudome M, Yoshida T. J Biol Chem. 1988;263:4523–4526. [PubMed] [Google Scholar]
- 9.Huang F L, Yoshida Y, Nakabayashi H, Young W S, Huang K-P. J Neurosci. 1988;8:4734–4744. doi: 10.1523/JNEUROSCI.08-12-04734.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito N, Kikkawa U, Nishizuka Y, Tanaka C. J Neurosci. 1988;8:369–382. doi: 10.1523/JNEUROSCI.08-02-00369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Nature (London) 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 12.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 13.Palay S L, Chan-Palay V. Cerebellar Cortex. Berlin: Springer; 1974. [Google Scholar]
- 14.Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 15.Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 16.Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson R F, Inoue Y, Kano M, Simon M I. Proc Natl Acad Sci USA. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon M I, Wu D. Proc Natl Acad Sci USA. 1996;93:14598–14601. doi: 10.1073/pnas.93.25.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Jun K S, Lee S B, Kang N-G, Min D S, Kim Y-H, Ryu S H, Suh P G, Shin H-S. Nature (London) 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 19.Aiba A, Kano M, Chen C, Stanton M E, Fox G D, Herrup K, Zwingman T A, Tonegawa S. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 20.Edwards F A, Konnerth A, Sakmann B, Takahashi T. Pflügers Arch Eur J Physiol. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 21.Konnerth A, Llano I, Armstrong C M. Proc Natl Acad Sci USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llano I, Marty A, Armstrong C M, Konnerth A. J Physiol (London) 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano M, Rexhausen U, Dreessen J, Konnerth A. Nature (London) 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee S B, Rhee S G. Curr Opin Cell Biol. 1995;7:183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 25.Napieralski J A, Eisenman L M. J Comp Neurol. 1996;364:718–728. doi: 10.1002/(SICI)1096-9861(19960122)364:4<718::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Hawkes R, Gravel C. Prog Neurobiol. 1991;36:309–327. doi: 10.1016/0301-0082(91)90004-k. [DOI] [PubMed] [Google Scholar]
- 27.Conquet F, Bashir Z I, Davies C H, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, et al. Nature (London) 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 28.Levenes C, Daniel H, Jaillard D, Conquet F, Crépel F. NeuroReport. 1997;8:571–574. doi: 10.1097/00001756-199701200-00038. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim J J, Hashimoto K, Thompson R F, Tonegawa S. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 30.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, et al. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 31.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S-I, Kawashima N, et al. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong D M. J Physiol (London) 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong D M. Arch Ital Biol. 1990;128:183–207. [PubMed] [Google Scholar]
- 34.Crépel F. Trends Neurosci. 1982;5:266–269. [Google Scholar]
- 35.Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hepler J R, Kozasa T, Smrcka A V, Simon M I, Rhee S G, Sternweis P C, Gilman A G. J Biol Chem. 1993;268:14367–14375. [PubMed] [Google Scholar]
- 37.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy S G, Sakimura K, Mishina M. J Neurosci. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]