Abstract
The recent discovery of glycine transporters in both the central nervous system and the periphery suggests that glycine transport may be critical to N-methyl-d-aspartate receptor (NMDAR) function by controlling glycine concentration at the NMDAR modulatory glycine site. Data obtained from whole-cell patch–clamp recordings of hippocampal pyramidal neurons, in vitro, demonstrated that exogenous glycine and glycine transporter type 1 (GLYT1) antagonist selectively enhanced the amplitude of the NMDA component of a glutamatergic excitatory postsynaptic current. The effect was blocked by 2-amino-5-phosphonovaleric acid and 7-chloro-kynurenic acid but not by strychnine. Thus, the glycine-binding site was not saturated under the control conditions. Furthermore, GLYT1 antagonist enhanced NMDAR function during perfusion with medium containing 10 μM glycine, a concentration similar to that in the cerebrospinal fluid in vivo, thereby supporting the hypothesis that the GLYT1 maintains subsaturating concentration of glycine at synaptically activated NMDAR. The enhancement of NMDAR function by specific GLYT1 antagonism may be a feasible target for therapeutic agents directed toward diseases related to hypofunction of NMDAR.
N-methyl-d-aspartate receptors (NMDAR) play a crucial role in several aspects of fast excitatory neurotransmission including the gating of an excitatory conductance with partial permeability to calcium (1) and, in some regions, long-term synaptic plasticity (2). Modulation of the NMDAR function occurs at a number of sites that are distinct from the glutamate-binding site (3). One of these sites is the strychnine-insensitive binding site where glycine acts to allosterically facilitate NMDAR function (4–6).
Glycine is a necessary coagonist (7), potentiating NMDAR function in a wide variety of preparations of cerebral cortex with an apparent dissociation constant in the range from about 100 to 300 nM (8). Glycine-dependent enhancement of iontophoretically applied NMDA and of the NMDA component of an evoked synaptic potential in cortical slices in vitro has been demonstrated (6); however, the effective concentration of the applied glycine could not be determined and its physiological role in NMDAR modulation remains unclear (9). This is in large part because the concentration of glycine in the cerebrospinal fluid is about 6 μM (10), which ought to saturate the NMDAR glycine site.
The recent molecular and biochemical characterization of a class of glycine transporter proteins (11–14) in brain suggests that the glycine concentration in microdomains may be regulated by these transporters. Of these, the glycine transporter type 1 (GLYT1) is expressed primarily in glia and neurons of the neocortex and archicortex in association with regions of high NMDA expression (15–17). We examined the role of glycine and GLYT1 on synaptic currents elicited by stimulation of the CA3-CA1 Schaffer collateral axons in hippocampal slices of the rat in vitro.
METHODS
Whole-cell recordings were obtained with a technique modified from Blanton et al. (18). Briefly, borosilicate glass electrodes (resistance, 4–6 MΩ) were filled with 120 mM potassium gluconate/10 mM KCl/3 mM MgCl2/10 mM Hepes/2 mM K2ATP/0.2 mM Na2GTP/0.25% biocytin. The pH was adjusted at 7.2 with KOH. During the experiments, the slices were perfused continuously with artificial cerebral fluid consisting of 124 mM NaCl/2 mM KCl/1.3 mM MgCl2/2.5 mM CaCl2/3 mM KH2PO4/10 mM glucose/26 mM NaHCO3. The osmolarity of the artificial cerebral fluid was 315 mosmol, the pH was adjusted at 7.35, and the temperature was maintained at 35°C. For the series of experiments with 6,7-dinitroquinoxaline-2,3-dione (DNQX) and bicuculline, a low-Mg2+ solution was used (0.1 mM MgCl2/3.7 mM CaCl2) to maintain same the extracellular cation concentration. Drugs were applied to the perfusion medium by using multiple perfusion lines that funneled into a single outlet near the recording area. Thus, any one of the multiple lines could be selected, allowing for fast (1–10 sec) switching between different media.
Postsynaptic currents (PSCs) were evoked with bipolar stimulation of the Schaffer collaterals. The stimulation intensity was adjusted to evoke PSC amplitude in the range of 75–100 pA at Vh = −60 mV. Data are expressed as the mean ± SE. For the experimental series with bicuculline and DNQX, the magnesium concentration was used to increase the amplitude of the NMDA-dependent current to a degree that could be observed easily (19). This was necessary, at least in part, because of the competitive antagonism of the glycine site by DNQX (20–22). For this series of experiments, we have measured the amplitude of the excitatory PSC (EPSC) at Vh = −55 mV.
The glycine derivative, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS; Fig. 1), is a selective and potent inhibitor of GLYT1 in the rat brain with a Ki of 5 nM and no effect on the activity of GLYT2. Similarly, NFPS had no measurable effect on the amino acid transport of either proline, glutamate, or γ-aminobutyric acid (GABA). At a concentration of 100 μM, this compound does not displace the binding of [H]3MDL-105,519 to the NMDA glycine site, [H]3AMPA, or [H]3kainate binding. Under conditions of high NMDA channel activity (10 μM glutamate/200 mM glycine), [H]3MK-801 binding also is not affected (J. F. McKelvey, L. A. Borden, and H. C. Fibiger, personal communication).
Figure 1.
The chemical structure for N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine.
RESULTS
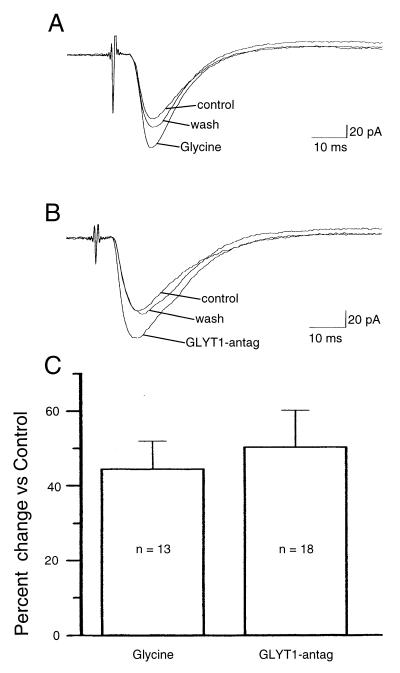
In agreement with a previous report (23), exogenous glycine (10 μM) applied to hippocampal slices from 8- to 12-week-old rats in vitro enhanced the amplitude of the Schaffer collateral-evoked PSC by 44.5 ± 7.5% (n = 13; P < 0.005, Wilcoxon signed-ranks test; Fig. 2A). This enhancement was blocked completely by the NMDAR antagonist 2-amino-5-phosphonopentanoic acid (APV; 50 μM; n = 5; data not shown).
Figure 2.
Glycine and GLYT1 antagonist enhanced the PSC evoked by Schaffer collateral stimulation. (A) Glycine (10 μM) increased the amplitude of the evoked PSCs. (B) GLYT1 antagonist (100 nM) also increased the amplitude of the evoked PSCs. Each trace is the average of 5 PSCs (Vh = −60 mV). (C) The histogram shows the percentage of change of the amplitude of the PSC induced by glycine (10 μM) or by GLYT1 antagonist (100 nM).The measurement of the amplitude of the PSC was done 4 msec after the beginning of the PSC.
The PSC elicited by Schaffer collateral stimulation is a compound EPSC–inhibitory PSC (IPSC). NMDAR can gate cationic channels that contribute to the EPSC (24, 25) and to the excitatory drive on interneurons responsible for the IPSC (26). The following analysis is concerned with the effects on the EPSC component measured at Vh = −60 mV that are most easily observed as a change in the PSC amplitude. The IPSC component contributes primarily the rate of the PSC decay.
The effect of a selective GLYT1 antagonist, NFPS, was assessed. GLYT1 antagonist (100 nM) enhanced the amplitude of the evoked PSC by 50.4 ± 9.8% (n = 18; P < 0.005; Wilcoxon signed-ranks test; Fig. 2B). The enhancement was dependent on NMDAR-mediated neurotransmission as it was blocked by APV (50 μM; n = 8; Fig. 3A). In the presence of an antagonist of the NMDAR glycine site (27), 7-chlorokynurenic acid (5 μM), GLYT1 antagonist had no effect (n = 10; Fig. 3B).
Figure 3.
When APV (A, 50 μM) and 7-Cl-Kyn (B, 5 μM) were added to the medium, the enhancing effect of GLYT1 antagonist was blocked completely, suggesting a selective effect on the NMDA component of the PSC. (C) However, the addition of strychnine (25 μM), an antagonist of the glycine-gated chloride channel, did not block the enhancing effect of GLYT1 antagonist (100 nM). Each trace is the average of five PSCs (Vh = −60 mV). (D) The histogram shows the percentage of change of the amplitude of the PSC induced by APV (50 μM) and APV (50 μM) plus GLYT1 antagonist (100 nM), and 7-Cl-Kyn (5 μM) and 7-Cl-Kyn (5 μM) plus GLYT1 antagonist (100 nM). The effect of strychnine (25 μM) and GLYT1 antagonist (100 nM) was compared with the effect of strychnine alone. The measurement of the amplitude of the PSC was done 4 msec after the beginning of the PSC.
Since glycine can gate a chloride conductance via activation of a strychnine-sensitive receptor, we examined the effect of strychnine (25 μM). By itself, strychnine enhanced the amplitude of the PSC (n = 3, Fig. 3D), suggesting either a stimulus-evoked glycine-mediated inhibition or an effect on GABA type A (GABAA) currents. The enhancement induced by GLYT1 antagonist (100 nM) was not modified by strychnine (55 ± 9.6%; n = 5; Fig. 3C).
We isolated the NMDAR component of the evoked PSC with the addition of a GABAA receptor antagonist, bicuculline (20 μM), and the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) antagonist, DNQX (20 μM), in a low-Mg2+-concentration solution so that a direct effect on NMDAR function could be assessed. However, the selective antagonists of AMPA receptors, CNQX and DNQX, also are competitive antagonists at the NMDAR glycine site (20–22). Thus, no conclusions regarding the concentration of endogenous glycine can be drawn based on the activity of this isolated NMDAR component since the concentration–response relationship is shifted to the right by the AMPA antagonists used for the isolation. Nevertheless, a direct effect on the NMDAR function was observed (Fig. 4A). Under these conditions, the GLYT1 antagonist (100 nM) reversibly enhanced the NMDA-mediated PSC by 135 ± 36% (Vh = −55 mV; n = 8; P < 0.01; Wilcoxon signed-ranks test). The effect was apparent at all membrane potentials examined (Vh from −85 to −45 mV) but more pronounced at depolarizing membrane potentials (Fig. 4A).
Figure 4.
GLYT1 antagonism enhanced the isolated NMDA component of the Schaffer collateral-induced synaptic current. (A, 1) Bicucilline (20 μM) and DNQX (20 μM) were added to the medium with a low-Mg2+ concentration to isolate the NMDA component of the EPSC. (A, 2) GLYT1 antagonist (100 nM) enhanced the amplitude of the EPSCs. The maximal effect, indicated by the arrow, was observed at more depolarizing membrane potentials. (A, 3) This trace shows the recovery. (B) The current–voltage curve of the amplitude of the isolated EPSC shows the voltage sensitivity of the EPSC enhancement by GLYT1 antagonism. The measurement of the amplitude of the EPSC was done 25 msec after the beginning of the EPSC.
Our initial investigation showed that glycine (1 μM) did not enhance the PSC at any membrane potential examined (Vh = −90 to −50 mV; n = 8). However, the Kd of the glycine-binding site at the NMDA receptor complex suggests that bath application of glycine (1 μM) should increase the NMDA-mediated current. It is possible that the glycine transporter has sufficient capacity to maintain synaptic glycine concentrations at low levels despite the addition of exogenous glycine. This is supported by the observation that simultaneous application of GLYT1 antagonist (100 nM) and glycine (1 μM) induced an enhancement of the amplitude of the evoked PSC by 85.5 ± 12.4% (n = 7; Fig. 5A). This effect is greater than the one obtained with GLYT1 antagonist alone (P < 0.01; Mann–Whitney U test; Fig. 5B). The earlier observation of a PSC enhancement by glycine (10 μM) indicates that this higher concentration can surpass the capacity of the glycine transport system to maintain an unaltered local glycine concentration.
Figure 5.
GLYT1 antagonism can alter glycine concentration proximal to the NMDAR. (A) Glycine (1 μM) in the bath caused no change on the amplitude of the PSC. However, the simultaneous application of glycine (1 μM) and GLYT1 antagonist (100 nM) to the bath induced an enhancement of the amplitude of the PSC. Each trace is the average of five PSCs (Vh = −60 mV). (B) The histogram illustrates the percentage of change of the amplitude of the PSC induced by glycine and/or GLYT1 antagonist compared with control. Glycine (1 μM) did not change the amplitude of the PSC compared with control. GLYT1 antagonist (100 nM) significantly enhanced the amplitude of the PSC. GLYT1 antagonist (100 nM) and glycine (1 μM) enhanced the amplitude of the PSC to a significantly greater extent. The measurement of the amplitude of the PSC was done 4 msec after the beginning of the PSC.
The high concentration of glycine in the cerebrospinal fluid raises a related question. Can the glycine transporter lower glycine concentration within the proximity of the NMDAR to a subsaturating level? We examined the effect of continuous bath perfusion with medium containing glycine (10 μM) for at least 5 min followed by the addition of GLYT1 antagonist (100 nM). Blockade of the transporter in the presence of glycine (10 μM) resulted in an additional enhancement of the PSC by 42.5 ± 10.5% (n = 3) over that induced by glycine (10 μM) alone (Fig. 6A). Compared with the baseline, GLYT1 antagonist (100 nM) plus glycine (10 μM) induced an increase of the PSC by 104.4 ± 20.1% (n = 3; P < 0.01; Mann–Whitney U test; Fig. 6B). This finding suggests a high transporter capacity and/or subsequent intracellular sequestration of glycine that is capable of reducing cerebrospinal fluid levels of glycine in the synapse to a range affecting NMDAR function.
Figure 6.
(A) Glycine (10 μM) induced an increase of the PSC. The simultaneous application of glycine (10 μM) and GLYT1 (100 nM) induced a further enhancement of the amplitude of the PSC over that induced by glycine (10 μM) alone. Each trace is the average of five PSCs (Vh = −60 mV). The measurement of the amplitude of the PSC was done 4 msec after the beginning of the PSC.
DISCUSSION
The activity of the NMDA channel is controlled by the functional occupancy of both glutamate- and glycine-binding sites of the NMDAR (7). On the basis of our results, we conclude that under physiological conditions, the glycine site on the NMDAR is not saturated. Increasing the glycine concentration augments synaptically mediated NMDAR function in the hippocampal slices observed as an increase in PSC amplitude of more than 40%. Thus, regulation of the local glycine concentration at the synapse could be an important means to modulate the NMDAR component of glutamatergic transmission.
The results are consistent with a selective action of the NFPS to antagonize glycine transport proximal to the NMDAR. The enhancement of the evoked EPSC was blocked completely by APV, an antagonist of the NMDAR, and by 7-chlorokynurenate, an antagonist to the glycine-binding site on the NMDAR at which glycine acts as a coagonist. Although unlikely, it is possible that NFPS acts as a glycine agonist at this glycine-binding site. However, the affinity for this site is negligible in that even at 100 μM, there was no evidence for displacement of the glycine-binding site ligand, [H]3MDL-105,519 (this ligand is displaced by glycine with an IC50 of 100 nM; M. De Vivo, J. F. McKelvey, L. A. Borden, and H. C. Fibiger, personal communication).
An antagonism of glycine acting at the strychnine-sensitive site that activates chloride channels conceivably might result in an apparent enhancement of the evoked EPSC. However, strychnine had only a slight enhancing effect by itself on the EPSC amplitude, and, when NFPS was added, an enhancement of the EPSC with an amplitude identical to that of control conditions was observed in every case. This is inconsistent with either an antagonistic or agonistic action at the strychnine-sensitive, glycine-mediated chloride current.
The source of the glycine that acts on the NMDAR is unknown, but its tetrodotoxin-sensitive release can be stimulated by either electrical or high-potassium stimuli (28). In the in vitro hippocampal slice, there is a sufficient spontaneous rate of glycine release to support NMDAR activity. However, the spontaneous release of glycine is insufficient to saturate the glycine modulatory site even when GLYT1 is antagonized. This is suggested by the additive effects of exogenous glycine and the GLYT1 antagonist compared with the antagonist alone.
The capacity of the transport and/or intracellular sequestration of glycine is orders of magnitude greater than the Kd of the glycine-binding site. We have observed that even during perfusion with medium containing glycine at 10 μM, the glycine transport is of sufficient capacity to maintain glycine concentration within the proximity of the NMDAR at a subsaturating concentration. If we assume that glycine transport has a similar capacity in vivo, then it is likely that the glycine site is not saturated and that NMDAR function is regulated in part by GLYT1.
Hypofunction of the NMDAR has been implicated in neuropsychiatric disorders including schizophrenia (29). The noncompetitive antagonists of the NMDAR, including phencyclidine and ketamine, can induce in normal subjects the positive, negative, and cognitive symptoms of the disorder and specifically exacerbate these symptoms in remitted schizophrenia (30, 31). Furthermore, postmortem studies reveal reductions in glutamate and decreased activity of glutamate carboxyl-peptidase II that degrades an endogenous antagonist for NMDAR (26), N-acetyl-aspartate-glutamate, in frontal and temporal cortices in schizophrenia (32). Accordingly, pharmacological enhancement of NMDAR function might reduce symptoms of schizophrenia. As direct agonists at NMDAR bear the risk of provoking seizures and excitatoxic neuronal degeneration (33), strategies that indirectly augment NMDAR function would be preferable. In this vein, recent controlled studies indicate that glycine or d-cycloserine, a partial agonist at the NMDAR glycine modulatory site that more readily crosses the blood–brain barrier, significantly reduces negative and cognitive symptoms in neuroleptic partially responsive schizophrenic patients (34, 35).
Given the results from our studies, treatment with GLYT1 antagonist might be a more effective way of pharmacologically increasing glycine modulatory-site occupancy and NMDAR function in disorders such as schizophrenia.
Acknowledgments
We thank Melissa Mudrick for technical support, Fran McNeil for secretarial assistance, and Allelix Neuroscience, Inc., for providing the drug GLYT1 antagonist. R.B. is supported by a fellowship of the Medical Research Council of Canada and by a grant of the Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD). T.M.M. is a medical student from Germany supported by the Biomedical Sciences Exchange Program. J.T.C. is supported by the NARSAD Hilton Senior Investigator Award. R.W.G. is supported by the Department of Veterans Affairs.
ABBREVIATIONS
- NMDAR
N-methyl-d-aspartate receptor
- PSC
postsynaptic current
- EPSC
excitatory PSC
- IPSC
inhibitory PSC
- GABA
γ-aminobutyric acid
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid
- NFPS
N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine
- APV
2-amino-5-phosphonopentanoic acid
- GLYT1 and GLYT2
glycine transporter type 1 and 2, respectively
References
- 1.MacDermott A J, Mayer M L, Westbrook G L, Smith S J, Barker J L. Nature (London) 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 2.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J W, Ascher P. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 5.Mayer M L, Vyklicky L, Jr, Clements J. Nature (London) 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- 6.Thomson A M, Walker V E, Flynn D M. Nature (London) 1989;338:422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- 7.Kleckner N W, Dingledine R. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto H, Simon J R, Aprison M H. J Neurochem. 1981;37:1015–1024. doi: 10.1111/j.1471-4159.1981.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher E J, Lodge D. Eur J Pharmacol. 1988;151:161–162. doi: 10.1016/0014-2999(88)90711-x. [DOI] [PubMed] [Google Scholar]
- 10.McGale E H F, Pye I F, Stonier C, Hutchinson E C, Aber G M. J Neurochem. 1977;29:291–297. doi: 10.1111/j.1471-4159.1977.tb09621.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith K E, Borden L A, Hartig P R, Branchek T, Weinshank R L. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q R, Nelson H, Mandiyan S, Lopez-Corcuera B, Nelson N. FEBS Lett. 1992;305:110–114. doi: 10.1016/0014-5793(92)80875-h. [DOI] [PubMed] [Google Scholar]
- 13.Guastella J, Brecha N, Weigmann C, Lester H A, Davidson N. Proc Natl Acad Sci USA. 1992;89:7189–7193. doi: 10.1073/pnas.89.15.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q R, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. J Biol Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- 15.Borowsky B, Mezey E, Hoffman B J. Neuron. 1993;10:851–863. doi: 10.1016/0896-6273(93)90201-2. [DOI] [PubMed] [Google Scholar]
- 16.Adams R H, Sato K, Shimada S, Tohyama M, Puschel A W, Betz H. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zafra F, Aragon C, Olivares L, Danbolt N C, Gimenez C, Storm-Mathisen J. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanton M G, LoTurco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 19.Mayer M L, Westbrook G L, Guthrie P B. Nature (London) 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 20.Birch P J, Grossman C J, Hayes A G. Eur J Pharmacol. 1988;156:177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- 21.Kleckner N W, Dingledine R. Mol Pharmacol. 1989;36:430–436. [PubMed] [Google Scholar]
- 22.Dingledine R, Kleckner N W, McBain C J. Adv Exp Med Biol. 1990;268:17–26. doi: 10.1007/978-1-4684-5769-8_3. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox K S, Fitzsimonds R M, Johnson B, Dichter M A. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- 24.Sah P, Hestrin S, Nicoll R A. J Physiol (London) 1990;430:605–616. doi: 10.1113/jphysiol.1990.sp018310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perouansky M, Yaari Y. J Physiol. 1993;465:223–244. doi: 10.1113/jphysiol.1993.sp019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunze H C R, Rainnie D G, Hasselmo M E, Barkai E, Hearn E F, McCarley R W, Greene R W. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp J A, Foster A C, Leeson P D, Priestley T, Iverson L L, Woodruff G N. Proc Natl Acad Sci USA. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klancnik J M, Cuenod M, Gaehwiler B H, Jiang Z P, Do K Q. Neuroscience. 1992;49:557–570. doi: 10.1016/0306-4522(92)90226-r. [DOI] [PubMed] [Google Scholar]
- 29.Olney J W, Farber N B. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 30.Javitt D C, Zukin S R. Am J Psychiatry. 1991;48:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 31.Krystal J H, Karper L P, Seibyl J P, Freeman G K, Delaney R, Bremmer I D, Heninger G R, Bowers M D, Charney D S. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 32.Tsai G, Passani L A, Slusher B S, Carter R, Kleinman J E, Coyle J T. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M, Coyle J T. FASEB J. 1987;1:446–455. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- 34.Javitt D C, Zylberman I, Zukin S R, Heresco-Levy U, Lindmayer O. Am J Psychiatry. 1994;151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 35.Goff D C, Tasi G, Monoach D S, Coyle J T. Am J Psychiatry. 1995;152:1213–1215. doi: 10.1176/ajp.152.8.1213. [DOI] [PubMed] [Google Scholar]