Abstract
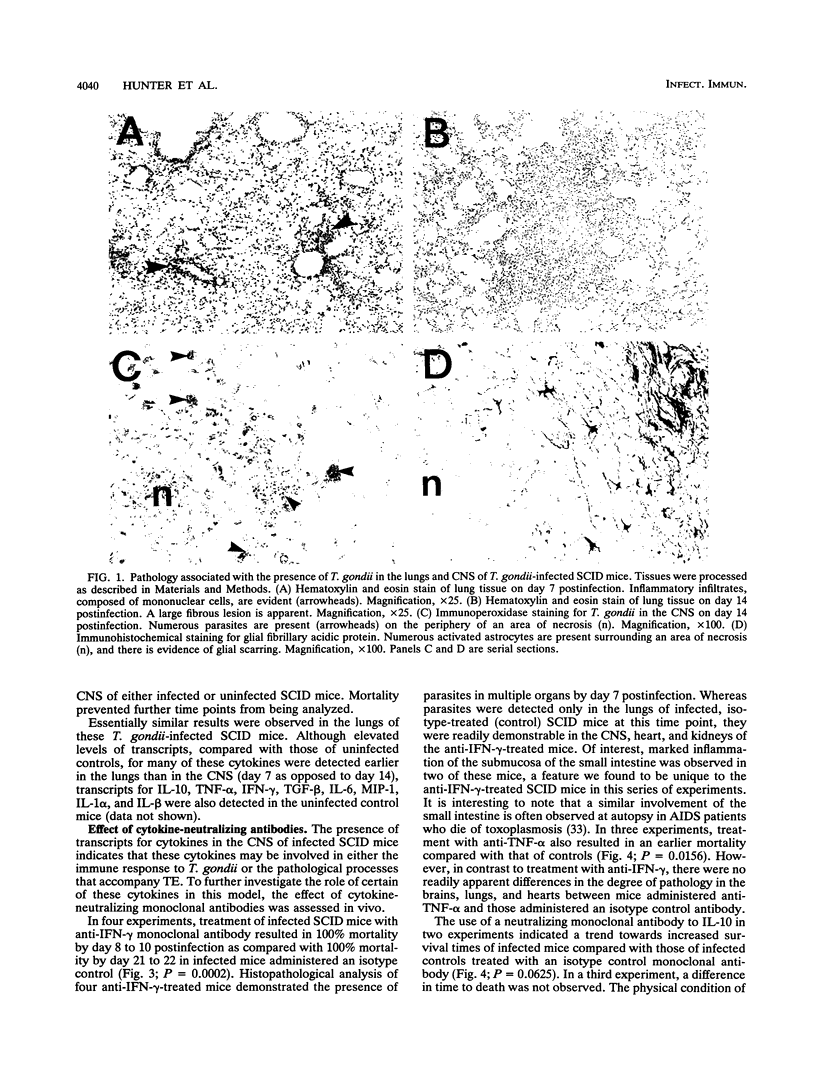
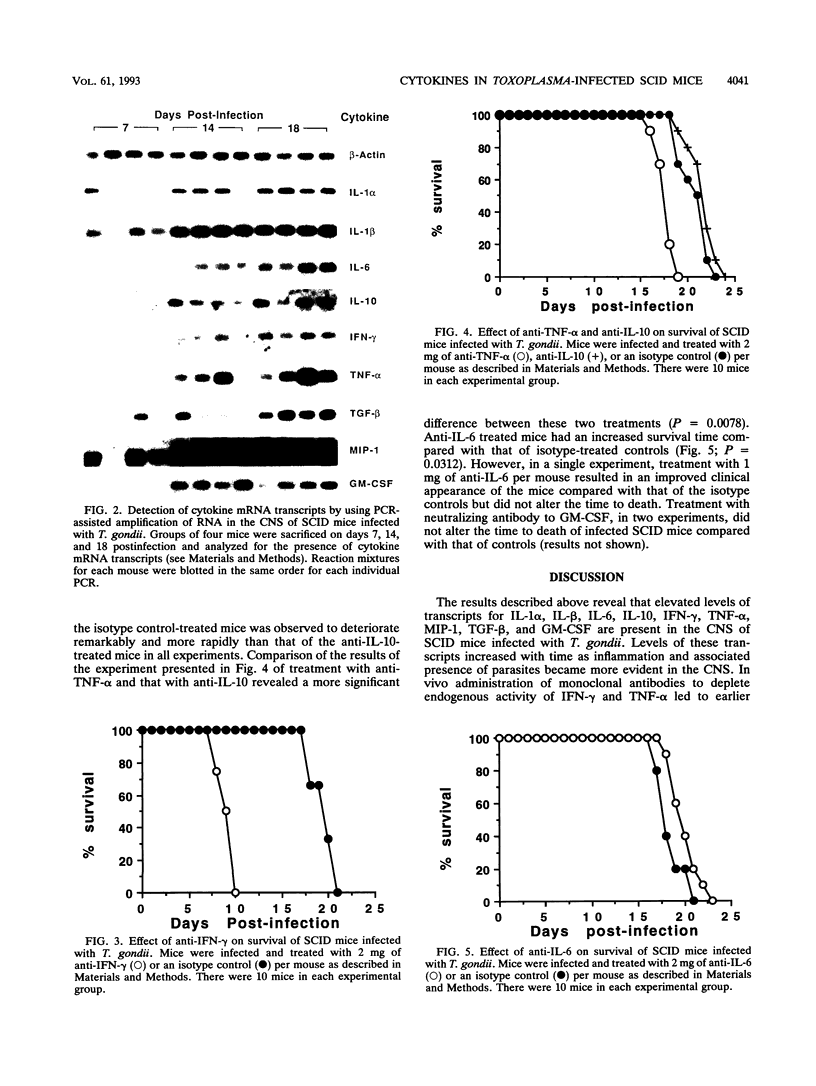
Levels of cytokine mRNA were studied in the central nervous system (CNS) of SCID mice infected with Toxoplasma gondii. This infection led to 100% mortality by day 23 postinfection. Inflammation was observed in the lungs on day 7 and in the heart, liver, and kidneys on days 14 and 18 of infection. In the CNS, necrotic, acellular lesions that contained numerous parasites, accompanied by a localized astrocyte activation, were evident on day 14. Polymerase chain reaction-assisted amplification of RNA revealed that, although transcripts for interleukin-1 alpha (IL-1 alpha) and IL-1 beta were present in the brains of uninfected mice, increased levels of these transcripts were detected on day 7 of infection. Transcripts for macrophage inflammatory protein 1 and transforming growth factor beta were also detected in brains of infected mice at this time point. On days 14 and 18, levels of these transcripts had increased and transcripts for IL-6, IL-10, gamma interferon (IFN-gamma), tumor necrosis factor alpha (TNF-alpha), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were also detected. Transcripts for IL-2 or IL-4 were not detected at any of the time points. Detection of locally produced cytokine transcripts may reflect involvement of the cytokines in the immunopathogenesis of this infection or involvement in mediating antitoxoplasma activity. To assess the possible role of endogenous IFN-gamma, TNF-alpha, IL-10, IL-6, and GM-CSF, cytokine-neutralizing monoclonal antibodies were administered to infected SCID mice. Neutralization of IFN-gamma or TNF-alpha led to earlier mortality than that in controls. In contrast, treatment with antibody to IL-10 and IL-6 increased survival time. Treatment with anti-GM-CSF did not alter the time to death. These results indicate that TNF-alpha and IFN-gamma are both involved in T-cell-independent mechanisms of resistance to T. gondii in SCID mice and that IL-10 and IL-6 may downregulate the immune response to this pathogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Araujo F. G. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991 May;59(5):1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Benveniste E. N. Lymphokines and monokines in the neuroendocrine system. Prog Allergy. 1988;43:84–120. [PubMed] [Google Scholar]
- Black C. M., Israelski D. M., Suzuki Y., Remington J. S. Effect of recombinant tumour necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989 Dec;68(4):570–574. [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brown C. R., McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990 Nov 15;145(10):3438–3441. [PubMed] [Google Scholar]
- Chang H. R., Grau G. E., Pechère J. C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990 Jan;69(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conley F. K., Jenkins K. A. Immunohistological study of the anatomic relationship of toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981 Mar;31(3):1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Devergie A., Auber P., Gluckman E., Beauvais B., Garin Y. J., Lariviere M. Toxoplasmosis in bone marrow-transplant recipients: report of seven cases and review. Clin Infect Dis. 1992 Aug;15(2):267–270. doi: 10.1093/clinids/15.2.267. [DOI] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Frei K., Piani D., Malipiero U. V., Van Meir E., de Tribolet N., Fontana A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992 May 15;148(10):3140–3146. [PubMed] [Google Scholar]
- Freund Y. R., Sgarlato G., Jacob C. O., Suzuki Y., Remington J. S. Polymorphisms in the tumor necrosis factor alpha (TNF-alpha) gene correlate with murine resistance to development of toxoplasmic encephalitis and with levels of TNF-alpha mRNA in infected brain tissue. J Exp Med. 1992 Mar 1;175(3):683–688. doi: 10.1084/jem.175.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992 Jul 1;149(1):175–180. [PubMed] [Google Scholar]
- Giulian D., Baker T. J., Shih L. C., Lachman L. B. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986 Aug 1;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., O'Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992 Jul;12(4):239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Hughes H. P., Speer C. A., Kyle J. E., Dubey J. P. Activation of murine macrophages and a bovine monocyte cell line by bovine lymphokines to kill the intracellular pathogens Eimeria bovis and Toxoplasma gondii. Infect Immun. 1987 Mar;55(3):784–791. doi: 10.1128/iai.55.3.784-791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. A., Gow J. W., Kennedy P. G., Jennings F. W., Murray M. Immunopathology of experimental African sleeping sickness: detection of cytokine mRNA in the brains of Trypanosoma brucei brucei-infected mice. Infect Immun. 1991 Dec;59(12):4636–4640. doi: 10.1128/iai.59.12.4636-4640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. A., Jennings F. W., Kennedy P. G., Murray M. Astrocyte activation correlates with cytokine production in central nervous system of Trypanosoma brucei brucei-infected mice. Lab Invest. 1992 Nov;67(5):635–642. [PubMed] [Google Scholar]
- Hunter C. A., Roberts C. W., Alexander J. Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol. 1992 Sep;22(9):2317–2322. doi: 10.1002/eji.1830220921. [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Roberts C. W., Murray M., Alexander J. Detection of cytokine mRNA in the brains of mice with toxoplasmic encephalitis. Parasite Immunol. 1992 Jul;14(4):405–413. doi: 10.1111/j.1365-3024.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Huskinson-Mark J., Araujo F. G., Remington J. S. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 1991 Jul;164(1):170–171. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- Ishida H., Hastings R., Kearney J., Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992 May 1;175(5):1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992 May;60(5):1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Lieberman A. P., Pitha P. M., Shin H. S., Shin M. L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988 Jan;157(1):1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- McCabe R., Remington J. S. Toxoplasmosis: the time has come. N Engl J Med. 1988 Feb 4;318(5):313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. J., Roberts C. W., Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991 May;84(2):207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol. 1991 Feb;3(1):49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter D., Deckert-Schlüter M., Schwendemann G., Brunner H., Hof H. Expression of major histocompatibility complex class II antigens and levels of interferon-gamma, tumour necrosis factor, and interleukin-6 in cerebrospinal fluid and serum in Toxoplasma gondii-infected SCID and immunocompetent C.B-17 mice. Immunology. 1993 Mar;78(3):430–435. [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Suzuki Y., Joh K., Orellana M. A., Conley F. K., Remington J. S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991 Dec;74(4):732–739. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988 Jun 1;140(11):3943–3946. [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. The involvement of TNF, IL-1 and IL-6 in the immune response to protozoan parasites. Immunol Today. 1991 Mar;12(3):A13–A16. doi: 10.1016/S0167-5699(05)80005-2. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]