Our study results suggest that there may be a large group of women at elevated risk of breast cancer for whom MR imaging would not be acceptable; for these women, supplemental screening with US combined with mammography could be considered.
Abstract
Purpose:
To determine reasons for nonparticipation in a trial of supplemental screening with magnetic resonance (MR) imaging after mammography and ultrasonography (US).
Materials and Methods:
Women(n = 2809) at elevated risk of breast cancer were enrolled in the American College of Radiology Imaging Network 6666 US Screening Protocol at 21 institutions. Fourteen institutions met technical and experience requirements for this institutional review board–approved, HIPAA-compliant substudy of supplemental screening with MR imaging. Those women who had completed 0-, 12-, and 24-month screenings with mammography combined with US were considered for a single contrast material–enhanced MR examination within 8 weeks after completing the 24-month mammography-US screening. A total of 1593 women had complete MR substudy registration data: 378 of them were ineligible for the study, and 1215 had analyzable data. Reasons for nonparticipation were determined. Demographic data were compared between study participants and nonparticipants.
Results:
Of 1215 women with analyzable data, 703 (57.9%), with a mean age of 54.8 years, were enrolled in the MR substudy and 512 (42.1%) declined participation. Women with a 25% or greater lifetime risk of breast cancer were more likely to participate (odds ratio, 1.53; 95% confidence interval: 1.10, 2.12). Of 512 nonparticipants, 130 (25.4%) refused owing to claustrophobia; 93 (18.2%), owing to time constraints; 62 (12.1%), owing to financial concerns; 47 (9.2%), because their physician would not provide a referral and/or did not believe MR imaging was indicated; 40 (7.8%), because they were not interested; 39 (7.6%), because they were medically intolerant to MR imaging; 29 (5.7%), because they did not want to undergo intravenous injection; 27 (5.3%), owing to additional biopsy or other procedures that might be required subsequently; 21 (4.1%), owing to MR imaging scheduling constraints; 11 (2.2%), because of the travel required; seven (1.4%), owing to gadolinium-related risks or allergies; and six (1.2%), for unknown reasons.
Conclusion:
Of 1215 women with elevated breast cancer risk who could, according to protocol guidelines, undergo breast MR imaging, only 57.9% agreed to participate.
Introduction
Contrast material–enhanced magnetic resonance (MR) imaging can depict many node-negative invasive breast cancers and ductal carcinomas in situ that are not seen at mammography or ultrasonography (US) (1). Although mammography remains the mainstay examination for breast cancer screening, it has reduced sensitivity when the breast parenchyma is dense (2). Women who are at high risk for breast cancer because they are known or suspected to have BRCA1 or BRCA2 mutations of known penetrance often begin undergoing screening when they are aged 25–30 years (3), when the breast tissue is more often dense. Mammography is particularly ineffective in depicting early breast cancer in BRCA1 carriers (4). In 2007, the American Cancer Society (ACS) recommended annual supplemental screening with MR imaging in addition to mammography in high-risk women (3).
In women at intermediate breast cancer risk, supplemental screening with MR imaging may or may not be warranted (3). This group includes women with a personal history of breast cancer, women with a history of previous lobular carcinoma in situ or atypical hyperplasia, women with a family history of intermediate risk (lifetime risk of 15%–20%), and those with dense breasts (3). The use of supplemental screening US has been advocated among women who are at high risk but are unable to tolerate MR imaging and those who are at intermediate risk (1).
The American College of Radiology Imaging Network (ACRIN) 6666 Protocol was designed for the evaluation of supplemental breast US screening in women with elevated risk of breast cancer and at least heterogeneously dense breasts (5). A total of 2809 women were enrolled in the main study, and all of them met the criteria for at least intermediate risk (6), although the definitions of risk were slightly different from those of the ACS (3). In the third round of annual screening in ACRIN 6666, eligible participants were offered a single contrast-enhanced breast MR imaging screening examination. We were surprised at the relatively low rates of participation in this MR imaging substudy that we observed initially. Thus, the purpose of this study was to determine the reasons for nonparticipation in a trial of supplemental screening with MR imaging after mammography combined with US.
Materials and Methods
Study sites were required to meet technical and experience requirements for the MR imaging substudy (7). Women invited to undergo MR imaging screening were actively enrolled participants in the original ACRIN 6666 Protocol (7) and needed to have completed three rounds (at 0, 12, and 24 months) of annual screening with mammography and US before the date of MR imaging. Women were approached for participation in the MR imaging substudy shortly before, at the time of, or shortly after they had completed the third round (24-month) of annual screening with mammography and US. The original ACRIN 6666 Protocol and the MR imaging substudy had been approved by the Cancer Therapy Experimental Protocols Committee of the National Cancer Institute, ACRIN, and the site institutional review boards. Study and patient consent protocols were Health Insurance Portability and Accountability Act compliant. Women were approached for participation from August 1, 2006, through April 21, 2008.
At most participating centers, women were told that their insurance provider would be billed (if their physician agreed to provide a referral) and that ACRIN study funding would cover any costs for MR imaging that were not covered by insurance. For the Canadian and Argentine sites, at which there was neither insurance nor governmental coverage for MR imaging screening, ACRIN contracted to pay $500 for each MR screening examination. For two American sites, hospital or university guidelines prohibited insurance billing for any examination that was part of a research protocol, and ACRIN contracted to pay these sites a maximum of $500 per MR screening examination. The amount of $500 was derived from actual costs and had been used in prior ACRIN protocols. Participants were required to agree specifically to undergo follow-up MR imaging at 6 months; undergo MR imaging–guided vacuum-assisted biopsy or US-guided core-needle biopsy (billed to insurance), if needed, on the basis of the MR results; and provide clinical follow-up information 11–14 months after completing the MR screening examination. The MR examination was to be scheduled, when possible, for 7–14 days after the onset of menses in premenopausal women and to be completed within 8 weeks—and in no case more than 91 days—after the 24-month screening mammographic and US examinations.
If a woman had any contraindications to MR imaging, this was recorded as her reason for not participating. These contraindications included pacemaker, aneurysm clip, or other implanted metallic device; lack of intravenous access; weight of more than 300 pounds; physical inability to tolerate positioning in the MR imaging unit; and impaired renal function as indicated by an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and/or a dialysis regimen (indicating a risk for nephrogenic systemic fibrosis [NSF]). Midway through the study recruitment period, on May 23, 2007, the Food and Drug Administration requested manufacturers to include a black box warning for all gadolinium-based contrast agents. This information, including disclosure of the risk of death, was immediately added to the consent forms for this study. Claustrophobia that could not be controlled with sedative or anxiolytic premedication under the physician’s orders was recorded separately.
Statistical Analyses
If the patient was eligible for but did not participate in the MR imaging substudy, the primary reason for nonparticipation was recorded after the patient was interviewed by the site research assistant and summarized on a form listing the possible reasons. “Other” comments were used if the response did not fit any existing category; these comments were later reviewed, and additional categories were created as needed. The demographic data on the MR substudy participants were compared with those of participants in the original ACRIN 6666 Protocol. Our statistical methods were primarily descriptive. Two-sample t tests and χ2 tests of association (Stata, version 10, Stata, College Station, Tex; SAS 9.1.3, SAS Institute, Cary, NC) were used to evaluate differences in various characteristics between the groups when necessary. However, to avoid reporting a large number of univariate comparisons that may or may not have been generalizable (because of confounding or effect modification), we modeled the probability of participation as a function of these characteristics in a logistic regression model. The following specific characteristics were evaluated: ethnicity, lifetime risk of breast cancer according to Gail et al (8) or Claus et al (9) model, 5-year risk of breast cancer according to Gail et al model, personal history of breast cancer, and prior mammography within 14 months of study entry. We used a random-effects model for site to adjust for institution-to-institution variability. We evaluated the fits of these logistic models by examining the likelihood and using the C-statistic, and a best model was selected. P ≤.05 was considered to indicate significance. We examined patient participation rates over time, including the periods before and after the March 28, 2007, publication of the ACS guidelines recommending MR imaging (3) and the May 23, 2007, Food and Drug Administration announcement regarding the risk of NSF.
Results
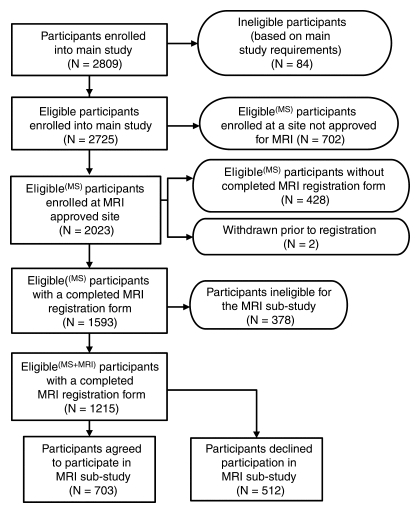
Of 21 sites included in the original ACRIN 6666 Protocol, 14 met technical and experience requirements for the MR imaging substudy. Of 2725 eligible participants enrolled in the main ACRIN 6666 Protocol, 2023 (74.2%) were at a site that was approved for MR imaging according to protocol guidelines. Some sites did not immediately meet all requirements, including institutional review board approval; thus, 428 women could not be registered for the MR imaging substudy. In addition, two women withdrew their consent prior to study registration. Thus, the remaining 1593 women could be approached for participation in the substudy (Figure). Three hundred seventy-eight women were ineligible; nearly half of these patients were not eligible because they did not undergo the 24-month screening mammography and US examinations by the cutoff date for entry into the MR imaging substudy. Of the remaining 1215 women, 703 (57.9%) agreed to participate and 512 (42.1%) did not.
Flowchart shows overall ACRIN 6666 study population, including the women who agreed and those who declined to undergo a single contrast-enhanced MR screening examination. MS = mammographic screening.
Demographic information on the women who did and did not agree to participate in the MR substudy and on the overall population of women who were eligible for the main ACRIN 6666 Protocol are detailed in Table 1. The median age of the patients in all three groups was the same, 55 years, with ages ranging from 25 to 91 years among the women who enrolled in the main protocol 2 years earlier and ages ranging from 26 to 87 years among the women who declined to enroll in the MR substudy. The results of the random-effects logistic regression model are detailed in Table 2. The Hispanic or Latino women enrolled in the main ACRIN 6666 Protocol tended to be less likely to participate: 96 agreed to participate, and 94 refused, representing 13.7% of the 703 substudy participants and 18.4% of the 512 nonparticipants (odds ratio, 0.51 [95% confidence interval: 0.24, 1.10]). Women with the greatest lifetime risk (≥25% risk according to Gail et al [8] or Claus et al [9] model) were more likely to participate in the MR substudy: 155 agreed to participate, and 78 declined, representing 22.0% of those who agreed to participate versus 15.2% of those who did not (odds ratio, 1.53 [95% confidence interval: 1.10, 2.12]). The reasons the 512 women declined to participate in the MR substudy are detailed in Table 3. The most common reasons were claustrophobia, with 130 (25.4%) women citing this reason for declining, and time constraints or other priorities, with 93 (18.2%) women stating that they declined for this reason.
Table 1.
Comparison of Demographic Data among Women in Main ACRIN 6666 Protocol and Those Who Agreed and Declined to Undergo Breast MR Screening
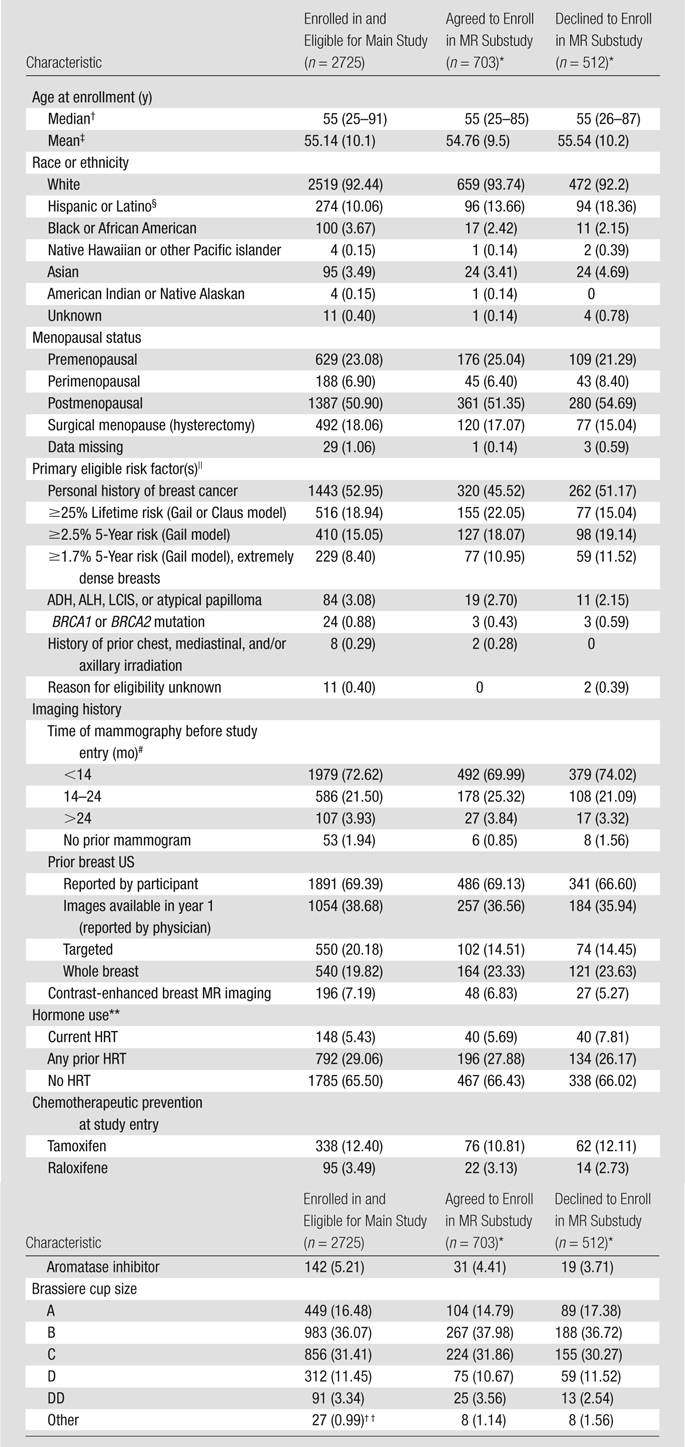
Note.—Unless otherwise noted, data are numbers of patients, with percentages in parentheses.
As detailed in the Figure, registration for the MR imaging substudy was completed for a total of 1215 women, 703 of whom agreed to participate and 512 of whom declined to participate.
Numbers in parentheses are ranges.
Numbers in parentheses are standard deviations.
The 86 Hispanic or Latino participants who agreed to participate in the MR substudy and the 79 Hispanic or Latino participants who declined to participate were enrolled at Centro de Estudios Radiológicos Integrales de la Mamá, Buenos Aires, Argentina.
Some data listed for primary eligible risk factor vary slightly from those originally reported (6) because the Gail model risk had been calculated erroneously and was included as an eligible risk factor for a few patients younger than 35 years or with a history of lobular carcinoma in situ (LCIS). ADH = atypical ductal hyperplasia, ALH = atypical lobular hyperplasia.
Number of months before study entry at which mammography was performed.
HRT = hormone replacement therapy.
Includes seven cases with missing brassiere cup size.
Table 2.
Results of Random-Effects Logistic Regression Analysis to Predict Enrollmentamong Women Approached for Breast MR Screening
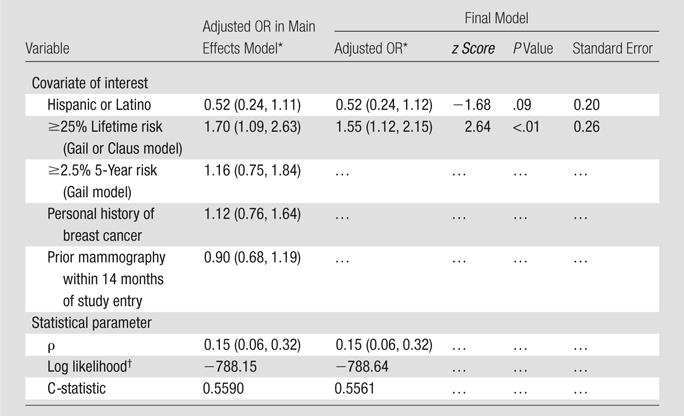
Note.—Results pertain to 1215 women approached for breast MR imaging screening. OR = odds ratio.
Numbers in parentheses are 95% confidence intervals.
P = .87 for comparison of two models at log likelihood ratio testing.
Table 3.
Reasons for Declining or Not Completing Contrast-enhanced Breast MR Screening among Women with Elevated Breast Cancer Risk
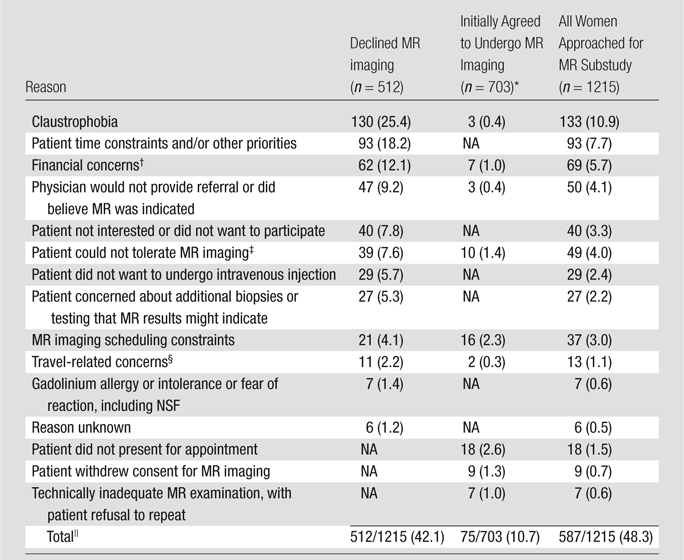
Note.—Data are numbers of women. NA = not applicable in given group of women.
Only a subset of responses was available for recording reasons for not completing MR imaging among the women who agreed to participate in the MR imaging substudy.
Financial concerns included those related to insurance coverage and/or the deductible.
Patient could not tolerate MR imaging owing to pacemaker or other implant, body habitus, and/or frail medical condition.
Patient was concerned about the distance required to travel for MR examination and/or the related inconvenience, or patient moved.
One participant underwent MR imaging outside the time frame specified for the study protocol. Thus, a total of 627 participants successfully completed the MR screening examination according to the protocol.
Of the 703 women who initially agreed to participate in the MR substudy, nine withdrew their consent before undergoing the MR examination. Another 46 women did not actually undergo MR imaging because they changed their mind or did not present for the appointment (n = 18), they encountered problems with scheduling the MR examination (n = 16), they had financial concerns (n = 7), their physician would not provide a referral (n = 3), or the distance to travel for the MR examination was prohibitive (n = 2). Among the remaining 648 participants who were actually placed in the MR unit, 13 (2.0%) were unable to tolerate the examination, and for three of these women, this was owing to claustrophobia (Table 3). For another seven participants, the resultant MR images were deemed unreadable and the patient refused to repeat the examination. For one patient, MR imaging was completed more than 91 days after the 24-month mammography and US examinations. As such, 627 women—51.6% of the 1215 eligible women asked to participate—successfully completed the MR screening examination within the specified period. Participation rates varied by site and ranged from 29% to 81% among the sites that were active for more than 1 year. At one site, which became qualified to offer MR imaging during the last 2 months of study enrollment, only two (8%) of 24 women asked to participate were able to enroll in the study.
Among the 627 women who completed the MR imaging examination, the examinations for 238 (38%) of them were paid for completely by ACRIN without billing insurance. For the other 389 women, insurance was initially billed, resulting in no (n = 63) or partial (n = 124) payment.
When we evaluated the reasons for refusing to participate in the MR substudy cited before and after publication of the ACS recommendations for MR imaging screening—and the surrounding publicity—among the women at high risk for breast cancer (3), we noted a decrease in the rate of physicians not providing referrals or not believing MR imaging was indicated: from 20 (13.7%) of 146 cases before the ACS recommendations to 27 (7.4%) of 366 cases after March 28, 2007 (P = .03). However, the rates of reported claustrophobia increased over time: from 28 (19.2%) of 146 cases to 102 (27.9%) of 366 cases (P = .04). Other rates did not change significantly over time. Revision of the consent form in late May 2007 to include information regarding the risk of death from NSF did not significantly affect the rate of refusals due to concerns about gadolinium reactions (three of 199 cases on or before May 23, 2007, and four of 313 cases after this date [P = .83]).
Discussion
For a screening test to be practical, it must be widespread, well tolerated, and cost-effective. Breast MR imaging screening is increasingly becoming available and being used in the United States and Western Europe but is less available in Canada and much less available in South America and Asia. In the United States, breast MR imaging was offered at 64% of responding practices among surveyed members of the Society of Breast Imaging, but 31% of practices with MR imaging screening services did not perform MR imaging–guided breast interventional procedures (10). In our protocol, only 14 (70%) of 20 active ACRIN 6666 sites met the technical and experience requirements to perform breast MR imaging, and participation was postponed at two sites until at least three MR imaging–guided biopsies had been completed at each facility.
Although recent guidelines (3,11) recommend annual contrast-enhanced MR imaging in addition to mammography in certain groups of high-risk women, little has been written about patient acceptance of breast MR imaging. The data that do exist are based on prematurely terminated examinations or those that yielded poor image quality: Prior studies have not addressed those women who refuse to even attempt to undergo the MR examination.
Several factors are known to reduce patient acceptance of breast MR imaging and include claustrophobia and the requirement for intravenous contrast material. Rates of claustrophobia and anxiety severe enough to cause premature termination of the MR examination vary according to the type of MR examination, with the highest rates seen in association with brain, head and neck, and upper extremity MR imaging (12). In our series, 13 (2.0%) of 648 women who presented for breast MR imaging could not complete the examination, and three of these cases—0.5% of the women who were actually placed in the MR unit—were due to claustrophobia, similar to the 1.3% rate reported by Eshed et al (12).
Our study provided us with the unusual opportunity to record information regarding women who decline to undergo breast MR examinations. The rate of women reporting claustrophobia as the reason for declining (130 [25.4%] of 512) was very high in our series. In a Netherlands MR imaging screening study (13), lying in the magnet bore was the aspect of breast MR imaging most frequently reported (by 21% of those surveyed) as rather to extremely uncomfortable. Despite this, 44% of women in the Netherlands cohort preferred MR imaging for screening compared with 14% who preferred mammography. This was due in part to the higher percentage of women who reported that they would feel “completely reassured” by a favorable MR imaging result (64.4%) compared with the percentage of women who reportedly would be completely reassured by a favorable mammographic result (40.1%).
Higher rates of claustrophobia have been reported among middle-aged women compared with the rates reported among other demographic groups (14), and a correlation with higher education levels and higher socioeconomic status has been reported (15). Although hypnosis (16) and anxiolytic agents such as lorazepam or diazepam can be used effectively to reduce claustrophobia, they may not be generally accepted for screening. The rates of sedative or anxiolytic medication use were not recorded in this study; however, Murphy and Brunberg (17) reported that 14.3% of consecutive patients required sedation to complete MR imaging. In one study (18), 91% of surveyed respondents who underwent breast MR imaging for local staging or screening reported mild or no discomfort during the examination; however, the patients overestimated the potential benefits of MR imaging, and this probably increased their tolerance.
Open-bore MR imaging causes less patient anxiety and claustrophobia than does closed-bore MR imaging (19–21), but the lower field strength with open-bore MR imaging (typically 0.2–1.0 T) often precludes optimal breast MR imaging owing to the reduced signal-to-noise ratio and the lower spatial and temporal resolution. In one recent report (22), 18 (4.5%) of 397 consecutive patients referred for breast MR imaging were unable to undergo the examination owing to claustrophobia (n = 15) or body habitus (n = 3). These women underwent open-bore MR imaging at 0.2 T, and 86% sensitivity was observed (22). Motion-induced artifacts that result in a nondiagnostic breast MR imaging examination are more common among claustrophobic patients. In our series, the images generated from seven (1.1%) of the 648 attempted examinations were deemed unreadable because of motion artifact or failed contrast material injection, and the seven women refused to repeat the examination.
NSF is a rare complication associated with gadolinium-based contrast agents. Midway through our patient accrual period, on May 23, 2007, the Food and Drug Administration requested manufacturers to include a black box warning with all gadolinium-based contrast agents, and the potential risks, including the risk of death, were immediately added to the consent forms for this study. We required participants to meet accepted standards for renal function, with glomerular filtration rate testing performed in the women older than 60 years and in those suspected of having decreased renal function. Although the publicity surrounding the Food and Drug Administration announcement could have discouraged participation in this protocol, after the announcement we observed no increase in reports of concern about gadolinium reactions or NSF as the reason for declining participation. No women who participated in the protocol developed NSF.
A good screening test has few false-positive results: Healthy women should not be harmed. Rates of false-positive results influence both the costs and the tolerance for a screening test. With mammographic screening, a recall for additional imaging occurs for an average of 6%–13% of women, with biopsy performed in 3%–6% of women screened and 19%–32% of biopsies revealing cancer (23). The effects of false-positive results on screening compliance vary, with increased compliance or intent to comply in American women (24,25), no difference in compliance among European women, and decreased compliance in Canadian women (24).
In terms of breast MR imaging, recall rates of 11%–12% have been reported with the first screening and have decreased to as low as 2%–7% with subsequent screenings (26,27). In the very high-risk populations studied, biopsy has been recommended for 7%–18% of women on the basis of MR imaging results, and overall, 40% of these biopsies have revealed cancer (1). In our current series, 27 (5.3%) refusals—representing 2.2% of the 1215 women approached for participation—were due to concern about additional biopsies or testing that might be indicated at MR imaging. This concern was probably highlighted by the informed consent process. Essink-Bot et al (13) reported that 5.1% of participants in the Netherlands MR imaging screening cohort were “seriously worried about the scan result”; this worry probably reflected the participants’ fear that cancer might be present and, to a lesser degree, their concern about additional procedures or testing that might be required.
The ACS guidelines recommending supplemental MR imaging screening in women at high risk for breast cancer (3) were widely publicized in March 2007. Anecdotally, we noted improved participation rates after that announcement, and participation in our protocol was more likely among those women who had a very high lifetime risk of breast cancer and met the ACS criteria for MR imaging screening. Use of models to estimate the risk of genetic mutations that predispose one to breast cancer remains largely the domain of genetic counselors. Primary care physicians have less experience with such calculations and therefore may be reluctant to recommend MR imaging unless it is recommended by a breast specialist. For 47 (9.2%) of the 512 women who declined to participate in our MR substudy, the reason cited was that her primary care provider would not provide a referral or did not believe MR imaging screening was indicated. However, the frequency of this reason decreased after the ACS guidelines were publicized. Three (0.4%) of the 703 women who initially agreed to participate cancelled for the same reason.
Not all insurers cover contrast-enhanced breast MR imaging, even for women at high risk. In our population of 627 women at intermediate and high risk who completed an MR imaging examination, 62% of the examinations were performed at sites that initially billed the insurance company. For 48% of examinations initially billed to insurance, the site received no payment or only partial payment (less than $500) from insurance, and ACRIN paid the balance (up to $500) to the site. Even when the insurance company and/or ACRIN covered payment for the initial MR imaging examination, additional costs to the health care system and the patient could result from follow-up MR imaging or other testing, including biopsy. Concerns about costs prompted the refusal of 12.1% of the women who declined to participate in our substudy. Uninsured women can be billed well over $2000 for MR imaging, which is clearly prohibitive for a screening test.
For more than half (52.95%) of the participants in the main ACRIN 6666 Protocol, a personal history of breast cancer was the main risk factor (6) and 3.01% of women in the main protocol had a history of atypical ductal or lobular hyperplasia, atypical papilloma, or lobular carcinoma in situ. Per the ACS guidelines, there remains insufficient evidence in favor of or against supplemental screening with MR imaging in women at intermediate risk of breast cancer (3). This guideline may have influenced referring physicians and even insurance companies. Overall, 19.01% of the main ACRIN 6666 population had a 25% or greater lifetime risk of breast cancer as the main risk factor according to the Gail et al (8) or Claus et al (9) model and thereby met ACS guidelines (3) for MR screening. Another 0.88% had a history of BRCA mutation as the main risk factor, and 0.29% had prior chest, mediastinal, or axillary radiation therapy by age 30 years and at least 8 years earlier as the main risk factor also meeting ACS guidelines (3) for MR imaging. Thus, just over 20% of women in the main ACRIN 6666 protocol met ACS guidelines for high-risk MR imaging screening (3), with the remaining participants meeting the criteria for the intermediate risk category.
Because the lifetime risk of breast cancer decreases with increasing age, we allowed other definitions of risk in the ACRIN 6666 Protocol. A total of 15.08% of the participants in the main ACRIN 6666 Protocol had a 5-year breast cancer risk of at least 2.5% according to the Gail et al model (8); another 8.44% of the women had a 5-year risk of at least 1.7% according to this model and extremely dense breasts. These definitions are not specifically addressed in the current ACS guidelines. Although women with a lifetime risk of breast cancer of at least 20% according to the Gail et al model (8) meet the ACS guidelines, the ACS recommends against using this model to calculate risk because it does not include paternal family history or patient age at diagnosis and cannot be used to predict risk of BRCA mutation. (See online supplement to ACS guidelines [3].) The Claus et al model (9) is among the models recommended by the ACS. (See online supplement to ACS guidelines [3].) Other accepted models used to determine risk for the purposes of MR imaging include the BRCAPRO (28), Tyrer et al (29), and BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) (30,31) models. However, these models were not widely available at the time the original ACRIN 6666 Protocol was designed, and each of these models can yield underestimations of risk in small families that have few relatives with breast or ovarian cancer.
Inconvenience (related to time constraints in 93 cases and to the required travel in 11 cases) was a factor in the refusal to participate for 104 (20.3%) of 512 women. MR imaging scheduling constraints were problematic for another 21 (4.1%) women who declined participation and for 16 (2.3%) of 703 women who initially agreed to undergo MR imaging. During the patient accrual period, many sites began to contact women in advance of their scheduled 24-month mammography and US examinations and to offer scheduling the MR examination for the same day as the 24-month procedures when possible. Despite these efforts, scheduling MR imaging for days 7–14 after the onset of menses can be challenging for perimenopausal women with irregular periods. Women in the main ACRIN 6666 Protocol had already completed three annual screenings with mammography and US and were probably more motivated to participate in the substudy than are many other women who undergo screening. Some women in the main protocol, however, may have been tired of participating in research protocols.
No set dialogue was used to discuss the MR examination with potential participants, although researchers were encouraged to provide summary data from trials that had included both MR imaging and US examinations, in which 93% of cancers had been seen with combined screening with MR imaging and mammography compared to 54% with combined screening with mammography and US (27,32–35). Despite these results, many participants in the main ACRIN 6666 Protocol may have felt secure with their mammography and US results alone or believed that they were “doing enough.”
In summary, in a population of 1215 women at elevated risk of breast cancer who were offered a contrast-enhanced MR imaging screening examination, only 703 (57.9%) agreed. Seventy-five women—representing 6.2% of those asked to participate and 10.7% of those who agreed to participate—did not successfully complete the MR examination, and one did not complete MR imaging within the specified time of 91 days after mammography and US. Thus, only 627 (51.6%) of the women approached for the substudy successfully completed MR imaging screening according to the protocol. The modest rate of participation was due in part to high rates of claustrophobia (over 25%) among the women who declined participation and a lack of perceived benefit on the part of the patient and/or referring physician. Our study results suggest that there may be a large group of women at elevated risk of breast cancer for whom MR imaging would not be acceptable; for these women, supplemental screening with combined US and mammography could be considered, provided the woman is informed of the risk of false-positive results with US screening (6) and the reduced sensitivity for cancer detection compared with the risks of false-positive results and the sensitivity of MR imaging (1).
Advances in Knowledge.
Of 1215 women at elevated risk of breast cancer who were offered contrast-enhanced MR imaging screening, 703 (57.9%) agreed, and 627 (51.6%) of the women asked to participate actually completed the screening according to the study protocol.
Claustrophobia was the most common reason (in 130 [25.4%] of 512 women) given for nonparticipation.
Implications for Patient Care.
In prior research to evaluate tolerance for breast MR imaging examinations, patient acceptance of this examination was probably overestimated, as the data of only those women who underwent MR imaging were evaluated; rates of claustrophobia in particular have probably been underestimated in prior reports.
Our results may help to inform public health agencies that recommend breast MR imaging screening in subgroups of women at increased risk of breast cancer, as there appear to be large groups of women in whom alternative screening strategies should be considered.
Acknowledgments
The authors thank the other site investigators and research associates who participated in the MR imaging substudy of ACRIN 6666, specifically A. Thomas Stavros, MD, Radiology Imaging Associates, Denver, Colo; Gary Whitman, MD, University of Texas M. D. Anderson Cancer Center, Houston, Tex; Anne Hoyt, MD, David Geffen School of Medicine at University of California Los Angeles Medical Center, Los Angeles, Calif; William A. Poller, MD, Allegheny Singer Research Institute, Pittsburgh, Pa; and Marcela Böhm-Vélez, MD, Weinstein Imaging Associates, Pittsburgh, Pa. The authors also thank R. Edward Hendrick, PhD, University of Colorado, Denver, Colo, for assistance with MR image review and site quality control; Mark D. Schleinitz, MD, Brown University, Providence, RI, and Barbara K. LeStage, MHP, for thoughtful review and comments; Cynthia B. Olson, MBA, MHS, for administrative assistance; and Jean B. Cormack, PhD, Center for Statistical Sciences, Brown University, Providence, RI, for many helpful discussions. W.A.B. is a consultant to Naviscan and has received equipment support from Siemens and MediPattern. R.A.J. has a research collaboration with GE Medical Systems. R.G.B. is on the ultrasonography advisory boards of and has received equipment support from Siemens and Philips Ultrasound. The laboratory with which E.D.P. is affiliated receives research support from GE Medical Systems, Sectra, Konica Minolta, and Hologic. W.P.E. is on the scientific advisory board of Hologic. M.C.M. is a consultant to Johnson and Johnson and SenoRx and is on the scientific advisory board of Hologic. E.B.M. is on the scientific advisory boards of Hologic, MediPattern, and Siemens; has received equipment support from Philips Ultrasound, Siemens, and Toshiba Ultrasound and research support from SuperSonic Imagine and Siemens; and is a consultant to Philips Ultrasound. The remaining authors have no financial disclosures.
Received May 30, 2009; revision requested July 2; revision received July 28; accepted August 4; final version accepted August 5.
Supported by grants from the Avon Foundation.
Funding: This research was supported by the National Cancer Institute (grants U01 CA079778, U01 CA89008).
See Materials and Methods for pertinent disclosures.
Abbreviations:
- ACRIN
- American College of Radiology Imaging Network
- ACS
- American Cancer Society
- NSF
- nephrogenic systemic fibrosis
References
- 1.Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009;192:390–399 [DOI] [PubMed] [Google Scholar]
- 2.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92:1081–1087 [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89 [DOI] [PubMed] [Google Scholar]
- 4.Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001;19:924–930 [DOI] [PubMed] [Google Scholar]
- 5.Berg WA. Rationale for a trial of screening breast ultrasound: American College of Radiology Imaging Network (ACRIN) 6666. AJR Am J Roentgenol 2003;180:1225–1228 [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299:2151–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACRIN Protocol 666. American College of Radiology Imaging Network Web site. http://acrin.org/Portals/0/Protocols/6666/Protocol-ACRIN_6666%20Admin%20Update%2011.30.07.pdf. http://acrin.org/Portals/0/Protocols/6666/Protocol-ACRIN_6666%20Admin%20Update%2011.30.07.pdf Accessed May 30, 2009.
- 8.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879–1886 [DOI] [PubMed] [Google Scholar]
- 9.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994;73:643–651 [DOI] [PubMed] [Google Scholar]
- 10.Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J Roentgenol 2008;191:332–339 [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network Web site. http://www.nccn.org/professionals/physician_gls/PDF/breast-screening.pdf. http://www.nccn.org/professionals/physician_gls/PDF/breast-screening.pdf Accessed May 30, 2009.
- 12.Eshed I, Althoff CE, Hamm B, Hermann KG. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging 2007;26:401–404 [DOI] [PubMed] [Google Scholar]
- 13.Essink-Bot ML, Rijnsburger AJ, van Dooren S, de Koning HJ, Seynaeve C. Women’s acceptance of MRI in breast cancer surveillance because of a familial or genetic predisposition. Breast 2006;15:673–676 [DOI] [PubMed] [Google Scholar]
- 14.Dewey M, Schink T, Dewey CF. Claustrophobia during magnetic resonance imaging: cohort study in over 55,000 patients. J Magn Reson Imaging 2007;26:1322–1327 [DOI] [PubMed] [Google Scholar]
- 15.Sarji SA, Abdullah BJ, Kumar G, Tan AH, Narayanan P. Failed magnetic resonance imaging examinations due to claustrophobia. Australas Radiol 1998;42:293–295 [DOI] [PubMed] [Google Scholar]
- 16.Simon EP. Improving tolerance of MR imaging with medical hypnosis. AJR Am J Roentgenol 1999;172:1694–1695 [DOI] [PubMed] [Google Scholar]
- 17.Murphy KJ, Brunberg JA. Adult claustrophobia, anxiety and sedation in MRI. Magn Reson Imaging 1997;15:51–54 [DOI] [PubMed] [Google Scholar]
- 18.Zakaria S, Brandt KR, Degnim AC, Thomsen KM. Patients’ perceptions of breast MRI: a single-center study. AJR Am J Roentgenol 2009;192:1149–1154 [DOI] [PubMed] [Google Scholar]
- 19.Bangard C, Paszek J, Berg F, et al. MR imaging of claustrophobic patients in an open 1.0T scanner: motion artifacts and patient acceptability compared with closed bore magnets. Eur J Radiol 2007;64:152–157 [DOI] [PubMed] [Google Scholar]
- 20.Michel SC, Rake A, Götzmann L, et al. Pelvimetry and patient acceptability compared between open 0.5-T and closed 1.5-T MR systems. Eur Radiol 2002;12:2898–2905 [DOI] [PubMed] [Google Scholar]
- 21.Savnik A, Malmskov H, Thomsen HS, et al. MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high-field MRI (1.5 T). Eur Radiol 2001;11:1030–1038 [DOI] [PubMed] [Google Scholar]
- 22.Calabrese M, Brizzi D, Carbonaro L, Chiaramondia M, Kirchin MA, Sardanelli F. Contrast-enhanced breast MR imaging of claustrophobic or oversized patients using an open low-field magnet. Radiol Med 2009;114:267–285 [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006;241:55–66 [DOI] [PubMed] [Google Scholar]
- 24.Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med 2007;146:502–510 [DOI] [PubMed] [Google Scholar]
- 25.Pisano ED, Earp J, Schell M, Vokaty K, Denham A. Screening behavior of women after a false-positive mammogram. Radiology 1998;208:245–249 [DOI] [PubMed] [Google Scholar]
- 26.Kriege M, Brekelmans CT, Boetes C, et al. Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition. Cancer 2006;106:2318–2326 [DOI] [PubMed] [Google Scholar]
- 27.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004;292:1317–1325 [DOI] [PubMed] [Google Scholar]
- 28.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol 2002;20:2701–2712 [DOI] [PubMed] [Google Scholar]
- 29.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111–1130 [DOI] [PubMed] [Google Scholar]
- 30.Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98:1457–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antoniou AC, Durocher F, Smith P, Simard J, Easton DF. INHERIT BRCAs program members BRCA1 and BRCA2 mutation predictions using the BOADICEA and BRCAPRO models and penetrance estimation in high-risk French-Canadian families. Breast Cancer Res 2006;8:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427–437 [DOI] [PubMed] [Google Scholar]
- 33.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23:8469–8476 [DOI] [PubMed] [Google Scholar]
- 34.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 2007;244:381–388 [DOI] [PubMed] [Google Scholar]
- 35.Sardanelli F, Podo F, D’Agnolo G, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology 2007;242:698–715 [DOI] [PubMed] [Google Scholar]