Abstract
Background
There is little information regarding the effects of strength training on intermuscular fat (IMF). This study examines changes in IMF in response to strength training in carriers of the adrenergic receptor (ADR) β2Glu27 polymorphism versus noncarriers and between carriers of ADRα2b Glu9 polymorphism versus noncarriers.
Methods
Midthigh IMF and muscle area were measured by computed tomography (CT) before and after 10 weeks of single-leg strength training in healthy, sedentary middle-aged and older (50–83 years) men (n = 46) and women (n = 52) in both their trained and untrained (control) legs.
Results
The strength training program resulted in a substantial increase in one-repetition maximum strength (p < .001) and muscle area (p < .001), but no significant changes in IMF in the whole group. However, IMF was significantly reduced with strength training in participants carrying ADRβ2 Glu27 (−2. 3 ± 1.0 cm2, p = .028), but no significant change was observed with ADRβ2 Glu27 noncarriers. The decrease in IMF in ADRα2b Glu9 carriers (−1.9 ± 1.0 cm2, p = .066) was significantly different (−2.9 ± 1.5 cm2, p = .043) from a nonsignificant increase in ADRα2b Glu9 noncarriers. ADRβ2 Glu27 carriers who also carried ADRα2b Glu9 significantly lost IMF with strength training (−3.8 ± 1.5 cm2, p = .018).
Conclusion
ADR genotype influences IMF response to strength training.
Sarcopenia is the age-associated loss of muscle mass. The loss is associated with a decrease in muscle strength, deterioration in health status, and adverse effects on functional abilities in the elderly population (1). Aging adversely affects the quantity, as well as the composition, of skeletal muscle. For example, fat infiltration of muscle increases with age (2,3), leading to the accumulation of intermuscular fat (IMF). Elevated levels of thigh IMF has been linked to insulin resistance in muscle and to the development of type 2 diabetes (4,5). In addition, fat infiltration is associated with lower muscle strength (6), with poorer leg function (7), and with greater incidence of mobility limitations in elderly persons (8).
Although strength training is thought to be the intervention of choice for delaying the adverse consequences of sarcopenia (9), little or no information is available on the effects of strength training on limb IMF. In this regard, Sipila and Suominen (10) reported a reduced percentage of thigh IMF in response to strength training, but no information on absolute IMF change was provided. The reduced percentage of fat may have been due to the increase in thigh muscle mass alone, which would lower the percentage of fat tissue, even in the absence of changes in total fat mass. Estimated fat infiltration is reduced as a result of a combination of aerobic exercise training and strength training (11), and strength training combined with a low-calorie diet reduces thigh IMF (12). However, no information is available on the independent effects of strength training on IMF.
Strength training increases sympathetic nerve activity (13,14), and norepinephrine derived from sympathetic nerves regulates lipolysis by binding to stimulatory (β1, 2, or 3), primarily β2 in skeletal muscle (15), and inhibitory adrenergic receptors (ADRα2b). The balance between ADRβ2 and ADRα2b can at least partially determine the relative efficacy of norepinephrine (16). ADRβ2 Gln27Glu polymorphisms have been associated with fat reduction induced by exercise training in some (17,18) but not all studies (19). The combined effects of ADRβ2 Gln27Glu and ADRα2b Glu12/Glu9 polymorphisms on the body fat reduction induced by aerobic exercise training have been reported (18). Although one cross-sectional study has examined the association of genotype, reported physical activity levels, and thigh IMF (20), no studies have reported genotype influences on strength training effects on IMF. Thus, we hypothesized that strength training will significantly reduce IMF and that ADRβ2 Glu27 carriers and ADRα2b Glu9 gene noncarriers will experience significantly more IMF reduction than will ADRβ2 Glu27 noncarriers and ADRα2b Glu9 carriers, respectively.
Methods
Participants
Relatively healthy, sedentary, Caucasian (n = 67) and African American (n = 31) men (n = 46) and women (n = 52) aged 50–83 years served as participants in this study. They were nonsmokers and were free of significant cardiovascular, metabolic, or musculoskeletal disorders. Those who were already taking medications for > 3 weeks prior to the start of the study were permitted into the study as long as medications and dosages were not changed. After all procedures were explained, participants read and signed a consent form, which was approved by the Institutional Review Board of the University of Maryland, College Park. All participants maintained stable body weight and were asked to maintain their regular physical activity levels and dietary habits.
Genotyping
Genomic DNA was prepared from EDTA-anticoagulated whole-blood samples by standard salting-out procedures (Puregene DNA Extraction; Gentra Systems, Inc., Minneapolis, MN). A restriction fragment length polymorphism (RFLP) procedure (21) was used to genotype for ADRβ2 Gln27Glu polymorphisms using the Fnu4H restriction enzyme. ADRα2b polymorphisms were analyzed directly on 3% agarose gel for 3 hours at 100 V for maximum separation and clarity. The genotype procedures were validated by direct sequencing of a random collection of samples.
Body Composition Assessment
Body composition was estimated by dual-energy x-ray absorptiometry (DXA) using fan-beam technology (model QDR 4500A; Hologic, Waltham, MA) and procedures we described previously (22).
Computed Topography of the Midthigh
An axial 10 mm-thick computed tomography (CT) scan of the trained and untrained leg was obtained (GE Lightspeed Qxi; General Electric, Milwaukee, WI) at the midpoint of the most distal point of the ischial tuberosity to the most proximal part of the patella, while participants were in a supine position. CT scans were analyzed using Medical Image Processing, Analysis, and Visualization (MIPAV) software (National Institutes of Health, Bethesda, MD). Briefly, IMF was segmented from the subcutaneous fat by manual drawing of a line along the deep fascial plane surrounding the thigh muscles with the exclusion of bone marrow fat (5). The IMF was distinguished from normal density muscle based on the range of Hounsfield Units (HU), that is, IMF = −190 to −30 vs 31 to 100 for normal density muscle, as previously described (5,23). Coefficient of variation of repeated measurements was <5% for both.
Strength Testing
One-repetition maximum strength tests were assessed for the knee extensors before and after the strength training program using air-powered resistance knee-extension machines (Keiser Co. Inc., Fresno, CA), using standardized procedures as we described previously (22). Two familiarization training sessions were performed prior to the baseline 1-repetition maximum test and to the training program (19). This procedure helps to control for inflated increases in strength due to initial changes in motor unit recruitment pattern (i.e., skill acquisition) and reduces risk of injury during testing and training.
Training Program
The training program consisted of unilateral (one-leg) training of the knee extensors of the right leg, three times per week, for ∼10 weeks. Training was performed on a Keiser A-300 air-powered leg extension machine. The untrained control leg was kept in a relaxed position throughout the training program. Following the two familiarization training sessions previously described, the training consisted of five sets of knee extension exercise for those aged <75 years and four sets for those aged ≥75 years, as described by Delmonico and colleagues (22). We chose not to require participants aged ≥75 years to perform the last set because of the concern that overtraining in this age group might result in a reduction in strength gains with training (24).
Statistical Analysis
ADR genotypic distributions were evaluated for conformity with Hardy–Weinberg equilibrium using the chi-square test with two degrees of freedom. Differences between pre- and posttraining physical characteristics were tested by paired t test. The influences of genotype on the effects of strength training on IMF and muscle area were determined by analysis of covariance (ANCOVA). The change in IMF and muscle area was calculated by subtracting the difference between the changes (pretraining to posttraining) of the untrained leg from those of the trained leg. The ADRβ2 genotype was categorized as ADRβ2Glu27 carriers (Gln27Glu and Glu27Glu) and Glu27 noncarriers (Gln27Gln). The ADRα2b genotype was categorized as ADRα2b Glu9 carriers (Glu12/Glu9 and Glu9/Glu9) and Glu9 noncarriers (Glu12/Glu12). The initial linear model for IMF included the two genotype groups, ethnicity, and sex hormone replacement as class variables, while age, change in body fat, and baseline values were covaried. Muscle area was covaried for age and baseline values only. Interactions were tested and found to be nonsignificant. Means were weighted for sex-hormone replacement, ethnicity and genotype distributions. The significance was set as p < .05.
Results
Participant Characteristics
The physical characteristics of participants at baseline and after training are shown in Table 1. Men significantly increased their fat-free mass and total body mass, whereas women showed no significant change in fat-free mass or total body mass in response to strength training. There was no significant change in body fat or percent body fat in either men or women with strength training. The 1-repetition maximum strength values increased significantly by 28.4% in men (+9.1 kg) and 27.8% in women (+5.2 kg).
Table 1.
Physical Characteristics at Baseline and After Strength Training
| Participant Characteristics | Baseline | After Strength Training |
|---|---|---|
| Men (n = 46) | ||
| Age, y | 64.4 ± 1.2 | |
| Height, cm | 173.8 ± 1.0 | — |
| Total body mass, kg | 84.0 ± 1.8 | 84.5 ± 1.9* |
| Body fat, kg | 23.4 ± 1.0 | 23.3 ± 1.0 |
| Percent body fat, % | 27.4 ± 0.8 | 27.2 ± 0.7 |
| FFM, kg | 60.6 ± 1.1 | 61.2 ± 1.1† |
| 1-repetition maximum, kg‡ | 32.4 ± 1.2 | 41.5 ± 1.6† |
| Women (n = 52) | ||
| Age, y | 62.7 ± 1.2 | |
| Height, cm | 162.5 ± 0.8 | — |
| Total body mass, kg | 73.2 ± 1.7 | 73.3 ± 1.8 |
| Body fat, kg | 28.9 ± 1.1 | 28.7 ± 1.1 |
| Percent body fat, % | 38.8 ± 0.7 | 38.5 ± 0.7 |
| FFM, kg | 44.3 ± 0.7 | 44.6 ± 0.8 |
| 1-repetition maximum, kg‡ | 18.7 ± 1.0 | 23.9 ± 1.0† |
Notes: Values are mean ± standard error.
p < .05.
p < .01.
There were two women and one man who had missing 1-repetition maximum data.
FFM = fat-free mass.
Genotype
The ADR genotype frequencies as shown in Table 2 were comparable to those reported previously (18), and fit the expectation of Hardy–Weinberg equilibrium for each polymorphism (ADRβ2 Gln27Glu, χ2 = 0. 068, p = .967; ADRα2b Glu12/Glu9, χ2 = 0. 357, p = .836). This was also true within African American (ADRβ2 Gln27Glu, χ2 = 1.977, p = .372; ADRα2b Glu12/Glu9, χ2 = 0. 285, p = .867) and Caucasian groups (ADRβ2 Gln27Glu, χ2 = 0.524, p = .770; ADRα2b Glu12/Glu9, χ2 = 0. 349, p = .840).
Table 2.
Adrenergic Receptor (ADR) Gene Polymorphisms: Alleles, Genotype Frequencies, and Sample Sizes
| ADR Gene Polymorphisms Alleles and Genotypes |
Frequency | Sample Size |
|---|---|---|
| ADRα2b Glu12 | 0.73 | 0 |
| ADRα2b Glu9 | 0.27 | 0 |
| ADRα2b Glu12/Glu12 | 0.52 | 51 |
| Glu12/Glu9 | 0.42 | 41 |
| Glu9/Glu9 | 0.06 | 6 |
| ADRβ2 Gln27 | 0.72 | 0 |
| ADRβ2 Glu27 | 0.28 | 0 |
| ADRβ2 Gln27/Gln27 | 0.52 | 51 |
| Gln27/Glu27 | 0.41 | 40 |
| Glu27/Glu27 | 0.07 | 7 |
IMF and Muscle Area
IMF and normal density muscle (NDM) areas before and after training are shown in Table 3. IMF was not significantly changed with strength training in the group as a whole (0.35 ± 0.68 cm2, p = .611). However, there were significant training-induced reductions in IMF in the ADRβ2 Glu27 carriers (−2. 3 ± 1.0 cm2, p = .028; Figure 1), which were significantly different (−3. 8 ± 1.5 cm2, p = .014) from the increased nonsignificant values observed in the ADRβ2 Glu27 noncarriers. The ADRβ2 Gln27Glu polymorphism explained 5.6% of the variation in change in IMF. The training-induced decrease in IMF in the ADRα2b Glu9 carriers approached significance (−1.9 ± 1.0 cm2, p = .066; Figure 2), and was significantly different (−3.0 ± 1.4 cm2, p = .043) from the increased nonsignificant values in ADRα2b Glu9 noncarriers. The ADRα2b Glu12/Glu9 polymorphism explained 3.7% of the variation in change in IMF.
Table 3.
Intermuscular Fat (IMF) and Normal Density Muscle (NDM) at Baseline and After Strength Training
| Baseline | After Strength Training | |||
|---|---|---|---|---|
| Trained Leg | Untrained Leg | Trained Leg | Untrained Leg | |
| IMF, cm2 | 48.33 ± 1.8 | 49.26 ± 1.7 | 47.39 ± 2.3 | 48.14 ± 2.1 |
| NDM, cm2 | 344.01 ± 8.7 | 334.96 ± 8.5 | 368.49 ± 9.0* | 340.10 ± 8.3 |
Note: Values are mean ± standard error.
p < .01.
Figure 1.
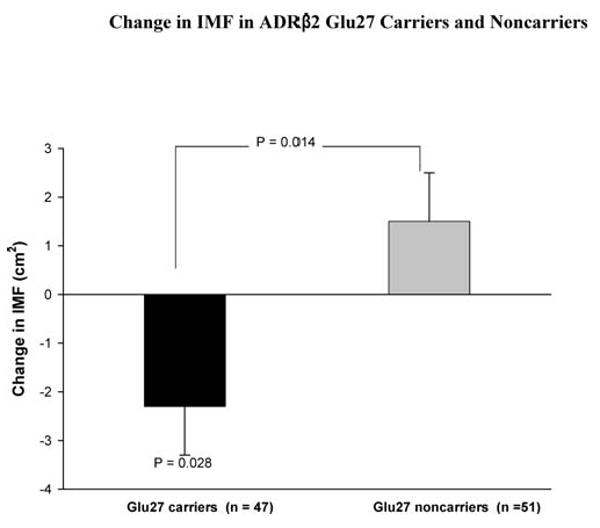
Change of intermuscular fat (IMF) with strength training in adrenergic receptor (ADR) β2 Glu27 carriers and noncarriers. Values of p connecting two bars represent tests of differences between genotypes, and those associated with a single bar represent change due to training effects in the designated genotype group. All other differences and changes were nonsignificant.
Figure 2.
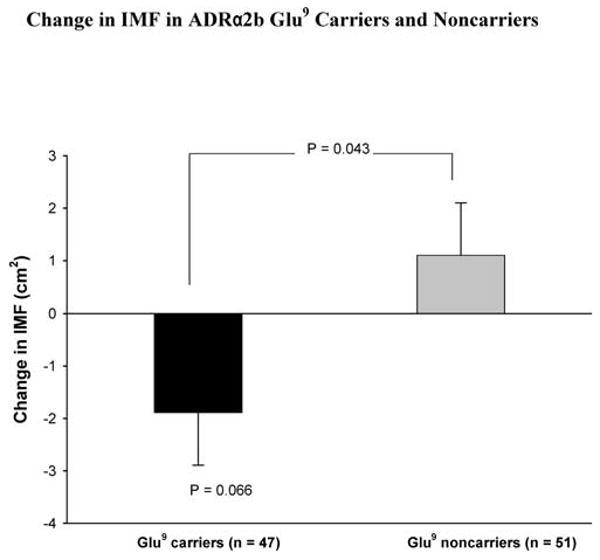
Change of intermuscular fat (IMF) with strength training in adrenergic receptor (ADR) α2b Glu9 carriers and noncarriers. Values of p connecting two bars represent tests of differences between genotype groups, and those associated with a single bar represent changes due to training effects in the designated genotype group. All other differences and changes were nonsignificant.
Carriers of ADRβ2 Glu27 who also carried ADRα2b Glu9 alleles (Glu27+/Glu9+) showed a significant decrease in IMF with strength training (−3.8 ± 1.6 cm2, p = .018; Figure 3), which was significantly different (−6.8 ± 2.3 cm2, p = .004) from a significant increase in IMF (3.0 ± 1.5 cm2, p = .046) in participants who did not carry either of these two alleles (ADRβ2 Glu27−/ADRα2b Glu9−). The ADRβ2 and ADRα2b genotypes combined explained 7.4% of the variation in the change in IMF.
Figure 3.
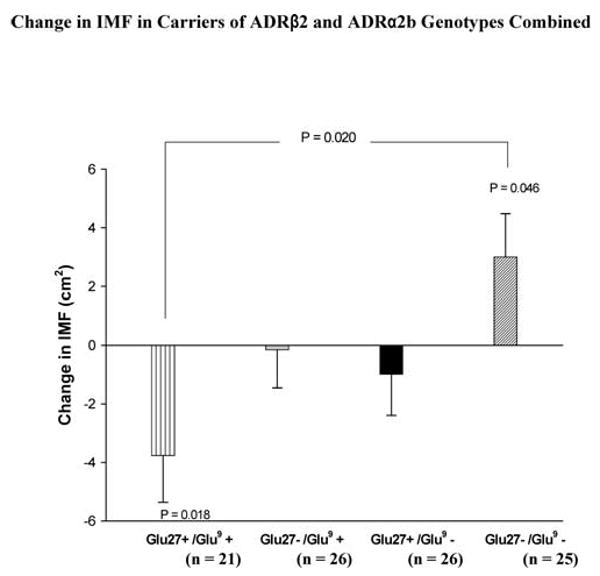
Change of intermuscular fat (IMF) with strength training in carriers of adrenergic receptor (ADR) β2 Gln27Glu and ADRα2b Glu12/Glu9 polymorphisms combined. Values of p connecting two bars represent tests of differences between designated genotype groups, and those associated with a single bar represent changes due to training effects for the designated genotype group. All other differences and changes were nonsignificant.
The significant increase in midthigh muscle area (19.3 ± 2.2 cm2, p < .001) was consistent for all genotypes with strength training.
Discussion
To our knowledge, this is the first study to examine the effects of strength training and the influence of ADR genotypes on IMF. These results support our hypothesis that ADR genotypes influence the responses of IMF to strength training, but do not support our hypothesis that strength training reduces IMF in the sample group as a whole, independent of genotype. The data demonstrate that strength training reduces IMF in those who are ADRβ2 Glu27 carriers and Glu27 carriers who also carry the ADRα2b Glu9 allele, but not in other genotype carriers.
It has been reported that strength training decreases both total and regional fat (25,26). ADRs, especially ADRβ2, have been shown to play a major role in lipolysis of skeletal muscle (15). Moreover, ADR genetic variants have been associated with adipose tissue deposition and catabolism (27,28). In this context, ADR genotypes may influence exercise training–induced fat reductions. For example, Phares and colleagues (18) reported a greater loss in total percent fat and in trunk fat in ADRβ2 Glu27 carriers than in noncarriers, in response to aerobic exercise training. In addition, ADRα2b Glu9 noncarriers who also carried ADRβ2 Glu27 lost greater fat mass than did noncarriers of either variant in their study. The results of the current study extend the results of Phares and colleagues (18) to strength training, by showing that strength training–induced IMF reduction is influenced by the ADRβ2 and ADRα2b gene polymorphisms.
Maintaining a low level of muscle fat could be important for elderly persons because of its association with metabolic disorders and functional disabilities (5–8). Nevertheless, it cannot be determined whether the magnitude of IMF loss with strength training in the present study is enough to result in improved functions or metabolic state. However, these results do suggest that reversing some of the age-related muscle loss is not the only potential value of strength training as an intervention for sarcopenia, at least for those of a specific genotype (e.g., carriers of ADRβ2 Glu27 allele alone or together with ADRα2b Glu9).
It is unclear why the strength training program did not result in a significant reduction in IMF in the entire group, independent of genotypes. A possible explanation may be that the energy expenditure of our training program was likely too low to account for a significant loss in IMF in the nonresponder genotype group. Only a single exercise that incorporates a single muscle group was used. Although multiple sets of this exercise were performed, the total exercise time (excluding rest periods) was < 5 minutes per training session, which constitutes a low level of energy expenditure. In addition, this type of heavy resistance, short duration exercise requires anaerobic metabolism as the primary energy pathways (29). Although increased intramuscular lipid oxidation has been observed in electrically induced contraction of isolated skeletal muscle (30) and after 5 hours of continuous knee extensor exercise (31), these studies used a very different experimental stimulus than was used in the present investigation.
However, despite the low energy expenditure of the training program, we found significant strength training– induced reductions in midthigh IMF in those participants who carry either the ADRβ2 Glu27 allele alone or both the ADRβ2 Glu27 and ADRα2b Glu9 alleles. In this regard, Green and colleagues (32) reported that the presence of the ADRβ2 Glu27 allele is protective against the agonist-induced decreases in ADRβ2 expression, although other studies claimed the opposite (33). On the basis of the Green and colleagues (32) study, we postulated that ADRβ2 Glu27 allele carriers may have more ADRβ2 receptors than ADRβ2 Glu27 allele noncarriers in response to strength training–induced catecholamine stimulation, thereby resulting in more hydrolysis of triglycerides in IMF. Small and colleagues (34) reported that the presence of ADRα2b Glu9 resulted in a decreased inhibition of adenyl cyclase by ADRα2b. Thus, we postulated that the presence of ADRα2b Glu9 favors lipolysis stimulated by ADRβ2, resulting in a greater likelihood of significant reductions in IMF in persons who carry both ADRβ2Glu27 and ADRα2b Glu9 alleles. Nevertheless, lipolysis is not a perfect predictor of fat loss because free fatty acids released from lipolysis can be re-esterified back to triglyceride, if not oxidized by muscle. Thus, it is the total amount of fatty acids actually oxidized, not just the level of lipolysis stimulated, that becomes the important biochemical step for explaining exercise training–induced fat loss. This issue was not addressed in this or any previous studies and will require further study.
There were several limitations to the present study. Although our sample size is considered relatively large when compared to previously published strength-training studies, it is small for genotype comparisons. Therefore, we limited our comparisons to ADRβ2 Glu27 carriers versus Glu27 noncarriers, instead of comparing all genotype groups. Another possible limitation is that the identified genotype effect in this study could be due to the linkage disequilibrium with other genes. For example, a linkage equilibrium effect may take place between the ADRβ2 Glu27 and ADRα2b Glu9 and any other nonfunctional polymorphism, such as ADRβ3 Tryp64Arg (19) or potentially functional alleles, such as ADRβ2Gly16Arg (35). In addition, the sample size of this investigation precludes sufficient statistical power for a multilocus approach. Also, the age range of participants in this study was quite large (50–83 years). However, we did covary age in our analysis to account for this heterogeneous age group. Although the inclusion of seven women who were taking hormone replacement medication in this study should be considered a limitation, we included these women as a separate group in the statistical analysis, and there were no significant differences in IMF response to strength training between the seven women who were taking medication and those who were not. Finally, our IMF assessments did not separate IMF changes in the quadriceps from those in the hamstrings.
Conclusion
To our knowledge, this is the first study to report that ADR genotype can influence the effects of strength training on IMF in middle-aged and older adults. The data indicate that persons who carry the ADRβ2 Glu27 allele alone or with the ADRα2b Glu9 allele experience a reduction in IMF as a result of strength training. The results of the present study also provide support for new hypotheses to investigate other gene polymorphisms, such as ADRβ3. This should be done using larger scale investigations with a focus on examining functional or metabolic improvements associated with the strength training-induced reductions in IMF.
Acknowledgments
This work was supported by grants AG-021500 and AG022791 from the National Institutes of Health/National Institute on Aging.
References
- 1.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 2.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Ross R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging. 2005;9:408–419. [PubMed] [Google Scholar]
- 4.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60A:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Roth SM, Ferrell RE, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging. 2000;4:143–155. [PubMed] [Google Scholar]
- 10.Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–340. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 11.Cuff DJ, Ignaszewski A, Meneilly GS, Tildesley HD, Martin A, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 12.Janssen I, Hudson R, Fortier A, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 13.Pratley R, Nicklas B, Rubin M, et al. Strength training increase resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;76:133–137. doi: 10.1152/jappl.1994.76.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Hurley BF, Seals DR, Allen WK, et al. The effects of high intensity strength training on cardiovascular function. Med Sci Sports Exerc. 1984;16:483–488. doi: 10.1249/00005768-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hagstrom-Toft E, Enoksson S, Moberg E, Bolinder J, Arner P. Beta-adrenergic regulation of lipolysis and blood flow in human skeletal muscle in vivo. Am J Physiol Endocrinol Metab. 1998;275:e909–e916. doi: 10.1152/ajpendo.1998.275.6.E909. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz J. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab. 2003;14:386–392. doi: 10.1016/s1043-2760(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 17.Macho-Azcarate T, Marti A, Gonzalez A, Martinez J, Ibanez J. Gln27Glu polymorphism in the beta2 adrenergic receptor gene and lipid metabolism during exercise in obese women. Int J Obes. 2002;26:1434–1441. doi: 10.1038/sj.ijo.0802129. [DOI] [PubMed] [Google Scholar]
- 18.Phares DA, Halverstadt AA, Shuldiner AR, et al. Association between body fat response to exercise training and multilocus ADR genotypes. Obes Res. 2004;12:807–815. doi: 10.1038/oby.2004.97. [DOI] [PubMed] [Google Scholar]
- 19.Garenc C, Perusse L, Rankinen T, et al. The trp64Arg polymorphism of the beta3-adrenergic receptor gene is not associated with training-induced changes in body composition: The HERITAGE Family Study. Obes Res. 2001;9:337–341. doi: 10.1038/oby.2001.43. [DOI] [PubMed] [Google Scholar]
- 20.Kritchevsky S, Nicklas B, Visser M, et al. Angiotensin-converting enzyme insertion/deletion genotype, exercise, and physical decline. JAMA. 2006;294:691–698. doi: 10.1001/jama.294.6.691. [DOI] [PubMed] [Google Scholar]
- 21.Avise JC, Lansman RA, Shade RO. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations I. Population structure and evolution in the genus peromyscus. Genetics. 1979;92:279–295. doi: 10.1093/genetics/92.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delmonico MJ, Kostek MC, Doldo NA, et al. Effects of moderate velocity strength training on peak muscle power and movement velocity: do women respond differently than men? J Appl Physiol. 2005;99:1712–1718. doi: 10.1152/japplphysiol.01204.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 24.Pearson D, Faigenbaum A, Conley M, Kraemer WJ. National strength and conditioning association's basic guidelines for the resistance training of athletes. J Strength Cond Res. 2000;22:14–27. [Google Scholar]
- 25.Treuth MS, Ryan AS, Fratley RE, et al. Effects of strength training on total and regional body composition in older men. J Appl Physiol. 1994;77:614–620. doi: 10.1152/jappl.1994.77.2.614. [DOI] [PubMed] [Google Scholar]
- 26.Treuth M, Hunter G, Kekes-Szabo T, Weinsier R, Goran M, Berland L. Reduction in intra-abdominal adipose tissue after strength training in older women. J Appl Physiol. 1995;78:1425–1431. doi: 10.1152/jappl.1995.78.4.1425. [DOI] [PubMed] [Google Scholar]
- 27.Lange LA, Norris JM, Langefeld CD, et al. Association of adipose tissue deposition and beta-2 adrenergic receptor variants: the IRAS family study. Int J Obes. 2005;29:449–457. doi: 10.1038/sj.ijo.0802883. [DOI] [PubMed] [Google Scholar]
- 28.Garenc C, Perusse L, Chagnon YC, et al. Effects of beta2-adrenergic receptor gene variants on adiposity: the HERITAGE Family Study. Obes Res. 2003;11:612–618. doi: 10.1038/oby.2003.88. [DOI] [PubMed] [Google Scholar]
- 29.Saltin B. Metabolic fundamentals in exercise. Med Sci Sport. 1973;5:137. [PubMed] [Google Scholar]
- 30.Dyck DJ, Bonen A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. Am J Physiol. 1998;275:E888–E896. doi: 10.1152/ajpendo.1998.275.5.E888. [DOI] [PubMed] [Google Scholar]
- 31.Sacchetti M, Saltin B, Osada T, Van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green SA, Turki J, Innis M, Liggett SE. Amino-terminal polymorphisms of the human beta2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 33.Moor P, Laporte JD, Abraham JH, et al. Polymorphism of the beta2-adrenergic receptor gene and desensitization in human airway smooth muscle. Am J Respir Crit Care Med. 2000;162:2117–2124. doi: 10.1164/ajrccm.162.6.9909046. [DOI] [PubMed] [Google Scholar]
- 34.Small K, Brown K, Forbes S, Liggett S. Polymorphic deletion of three intracellular acidic residues of the alpha2b-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- 35.Large V, Hellstrom L, Reynisdottir S, et al. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest. 1997;100:3005–3013. doi: 10.1172/JCI119854. [DOI] [PMC free article] [PubMed] [Google Scholar]


