Abstract
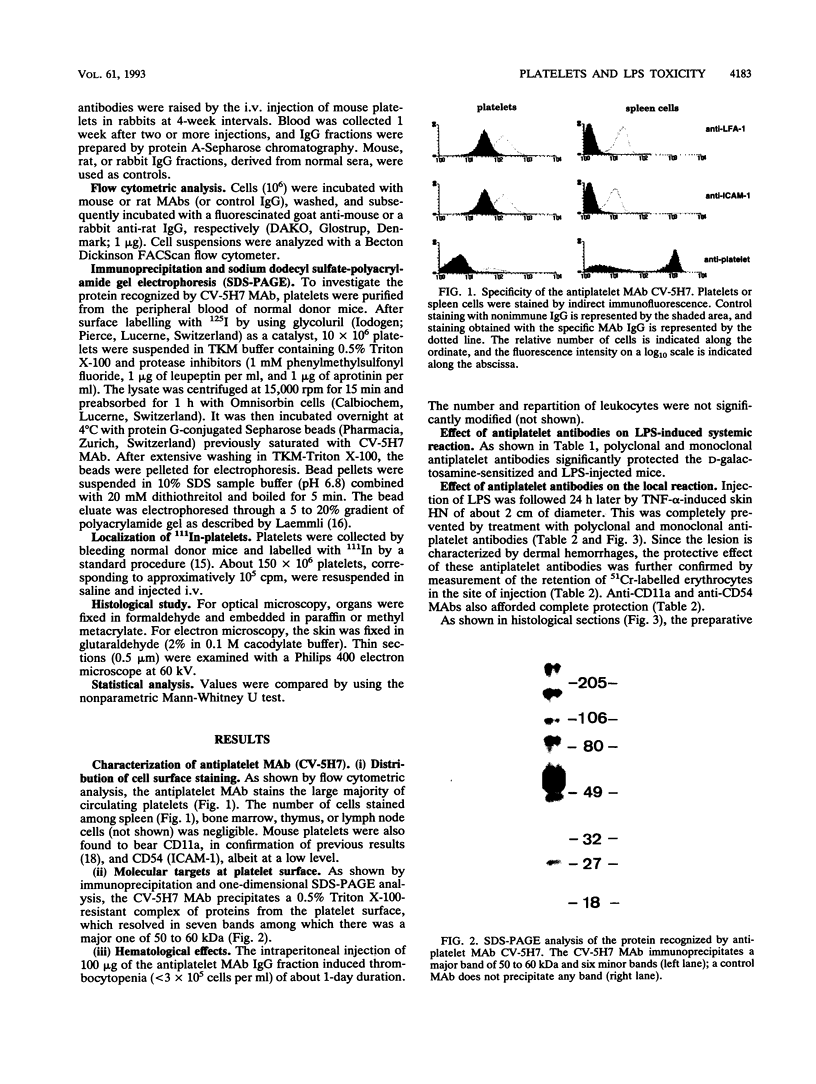
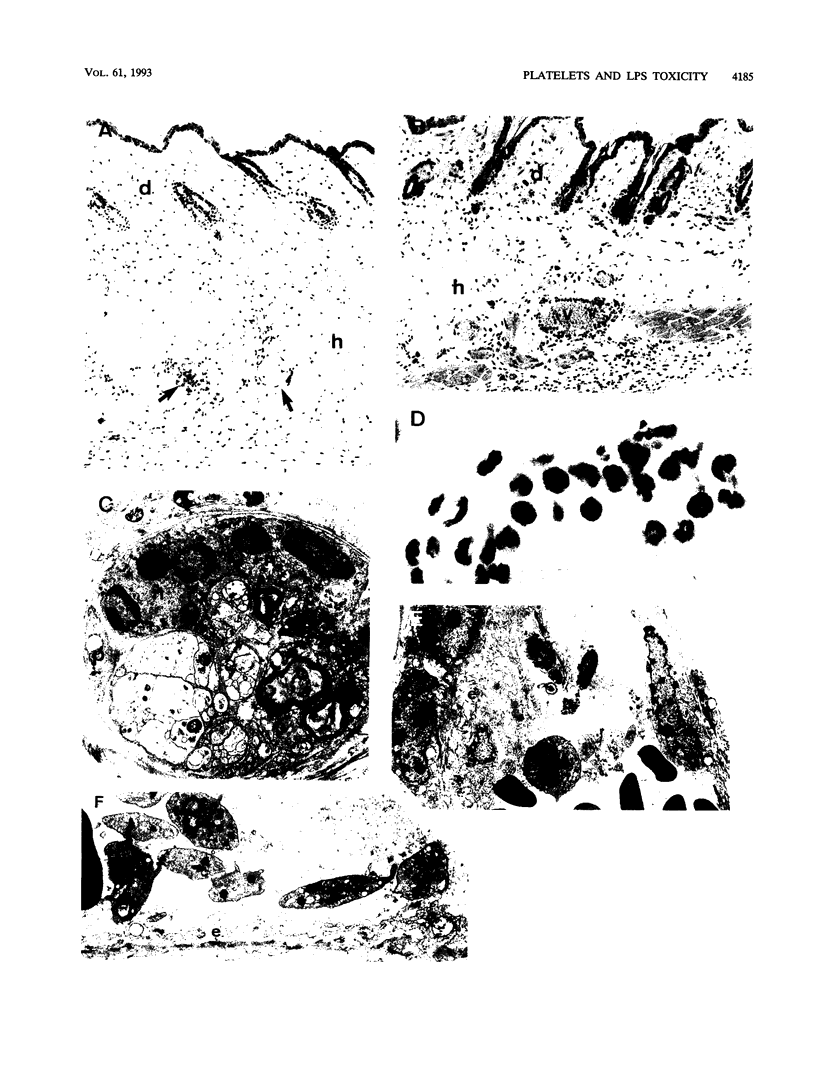
The role of platelets was investigated in two models of lipopolysaccharide (LPS)-induced toxicity in mice: the systemic reaction, provoked by intravenous LPS injection in D-galactosamine-sensitized recipients, which results in host death, and the local reaction, elicited in the skin by sequential injections of LPS and tumor necrosis factor alpha at 24-h intervals, which results in hemorrhagic necrosis. In both models, the depletion of platelets with a rabbit polyclonal or a mouse monoclonal antiplatelet immunoglobulin G afforded significant protection. In the local reaction, studies of the distribution of 111In-labelled platelets as well as optical and electron microscopy showed that platelets are localized in the dermal venules before hemorrhage occurs. Anti-CD11a (LFA-1) and anti-CD54 (ICAM-1) monoclonal antibodies prevented both platelet localization and hemorrhagic necrosis, and these determinants were detected on mouse platelets by immunofluorescence. The antiplatelet monoclonal antibody did not reduce the localization of polymorphonuclear leukocytes in the dermal venules, as shown by histological sections. Thus, in the local reaction, the stimulation with LPS and tumor necrosis factor alpha leads to a binding of platelets to the endothelium of venules by their beta 2 integrins, which seems necessary for the development of the hemorrhagic necrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. R., Doherty G. M., Buresh C. M., Venzon D. J., Norton J. A. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med. 1991 Apr 1;173(4):1029–1032. doi: 10.1084/jem.173.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenbright L. W., Barton R. W. Interactions of leukocyte integrins with intercellular adhesion molecule 1 in the production of inflammatory vascular injury in vivo. The Shwartzman reaction revisited. J Clin Invest. 1992 Jan;89(1):259–272. doi: 10.1172/JCI115570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The common mediator of shock, cachexia, and tumor necrosis. Adv Immunol. 1988;42:213–231. doi: 10.1016/s0065-2776(08)60846-9. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bout D., Joseph M., Pontet M., Vorng H., Deslée D., Capron A. Rat resistance to schistosomiasis: platelet-mediated cytotoxicity induced by C-reactive protein. Science. 1986 Jan 10;231(4734):153–156. doi: 10.1126/science.3079916. [DOI] [PubMed] [Google Scholar]
- Chang H. R., Vesin C., Grau G. E., Pointaire P., Arsenijevic D., Strath M., Pechère J. C., Piguet P. F. Respective role of polymorphonuclear leukocytes and their integrins (CD-11/18) in the local or systemic toxicity of lipopolysaccharide. J Leukoc Biol. 1993 Jun;53(6):636–639. doi: 10.1002/jlb.53.6.636. [DOI] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Fong J. S., Good R. A. Prevention of the localized and generalized Shwartzman reactions by an anticomplementary agent, cobra venom factor. J Exp Med. 1971 Sep 1;134(3 Pt 1):642–655. doi: 10.1084/jem.134.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Gretener D., Vesin C., Lambert P. H. Immunopathology of thrombocytopenia in experimental malaria. Immunology. 1988 Dec;65(4):501–506. [PMC free article] [PubMed] [Google Scholar]
- Heffner J. E., Sahn S. A., Repine J. E. The role of platelets in the adult respiratory distress syndrome. Culprits or bystanders? Am Rev Respir Dis. 1987 Feb;135(2):482–492. doi: 10.1164/arrd.1987.135.2.482. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Klonizakis I., Peters A. M., Fitzpatrick M. L., Kensett M. J., Lewis S. M., Lavender J. P. Radionuclide distribution following injection of 111Indium-labelled platelets. Br J Haematol. 1980 Dec;46(4):595–602. doi: 10.1111/j.1365-2141.1980.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery P. J., Berridge M. V. Expression of the leukocyte functional molecule (LFA-1) on mouse platelets. Blood. 1986 Jun;67(6):1757–1764. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Price T. H., Beatty P. G., Corpuz S. R. In vivo inhibition of neutrophil function in the rabbit using monoclonal antibody to CD18. J Immunol. 1987 Dec 15;139(12):4174–4177. [PubMed] [Google Scholar]
- Renesto P., Chignard M. Tumor necrosis factor-alpha enhances platelet activation via cathepsin G released from neutrophils. J Immunol. 1991 Apr 1;146(7):2305–2309. [PubMed] [Google Scholar]
- Rosen H., Milon G., Gordon S. Antibody to the murine type 3 complement receptor inhibits T lymphocyte-dependent recruitment of myelomonocytic cells in vivo. J Exp Med. 1989 Feb 1;169(2):535–548. doi: 10.1084/jem.169.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa T., Kodama T., Tominaga A., Okada M. Human platelets effectively kill K-562 cells, a chronic myelogenic leukemia cell line, in vitro. Jpn J Cancer Res. 1990 May;81(5):449–453. doi: 10.1111/j.1349-7006.1990.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Halgunset J., Haugen O. A., Aarset H., Aarden L., Waage A., Matsushima K., Kvithyll H., Boraschi D., Lamvik J. Cytokine-associated tissue injury and lethality in mice: a comparative study. Clin Immunol Immunopathol. 1991 Oct;61(1):69–82. doi: 10.1016/s0090-1229(06)80008-5. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Fritsche T. R., Henderson W. R., Jr Human platelet-mediated cytotoxicity against Toxoplasma gondii: role of thromboxane. J Exp Med. 1991 Jan 1;173(1):65–78. doi: 10.1084/jem.173.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]