Based on the results of the U.K. Prospective Diabetes Study (UKPDS), “… treatment of type 2 diabetes [should] include aggressive efforts to lower blood glucose levels as close to normal as possible. …” This was the recommendation the American Diabetes Association promulgated based on the results of the UKPDS when published (1). The suggestion was soon adopted by official guidelines in every region of the world (2). They are generally consistent in recommending an A1C goal of <7.0%. However, the results of the UKPDS remained inconclusive with respect to cardiovascular (CV) complications because of a risk reduction that was only close to statistical significance (−16%, P = 0.052). In support of the UKPDS results, however, a recent meta-analysis of randomized trials in type 2 diabetes (3) calculated a 19% reduction in the incidence of any type of macrovascular event associated with improved long-term glycemic control. Moreover, a strong association between glycemic control and micro- and macrovascular disease has been highlighted in type 1 diabetic patients (4,5).
Nevertheless, these results have been challenged by recent large-scale intervention trials. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial randomized >10,000 subjects with type 2 diabetes and vascular disease, or multiple CV risk factors, to an intensive treatment program targeting normal blood glucose values and A1C <6%, or a standard treatment program aiming at A1C between 7 and 7.9%. The intensive blood glucose arm was prematurely stopped because of excess mortality (hazard ratio [HR] 1.22, 95% CI 1.01–1.46; P = 0.04) and lack of significant reduction of primary outcome, i.e., a composite of nonfatal myocardial infarction, nonfatal stroke, or death from CV causes (HR 0.90, 0.78–1.04; P = 0.16) (6). After a median 5-year follow-up, the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), the largest-ever study of strategy of intensive glucose control, involving 11,140 high-risk type 2 diabetic patients, provided no evidence of major CV event reduction (HR 0.94, 0.84–1.06; P = 0.32) when A1C was lowered to 6.5% (7). Similar results have been achieved by the Veterans Administration Diabetes Trial (8). It is noteworthy that all three trials comprised patients with diabetes of longstanding duration, and in two of the studies, subjects with previous CV events were included, leaving unaltered (or at least unsettled) the importance of achieving and maintaining good glycemic control from the time of diabetes diagnosis. This view is supported by the results of the 10-year follow-up of the UKPDS (9). Despite early loss of glycemic differences, significant reduction in the risk for microvascular complications, myocardial infarction, and all-cause mortality was observed in patients originally in the intensive treatment group.
The number of newly diagnosed type 2 diabetic patients achieving, and even more importantly, maintaining glycemic target is limited. No specific data are available, but in the UKPDS, which comprised only newly diagnosed type 2 diabetic patients with no previous CV events, average A1C was ∼7%. This threshold value, however, was not maintained for >4 years (1), and more recent data indicated that <50% of this patient population is at target (10). Therefore, effective treatment for type 2 diabetes would require an early and effective intervention, though flexible enough to ensure longstanding metabolic control. To set new paradigms of treatment, strategies should be elaborated that identify and overcome current limitations. Many hurdles may hamper the possibility of a greater number of patients achieving target, and a tentative list of such factors is given in Table 1 and discussed in this article.
Table 1.
Barriers in achieving and maintaining glycemic control in type 2 diabetic patients
|
INEFFECTIVE DIET AND EXERCISE INITIATIVES
Diet and exercise remain the cornerstone of treatment of type 2 diabetes, as recently confirmed by the European Association for the Study of Diabetes/American Diabetes Association treatment algorithm (11), but for lifestyle modification to be effective, it requires long-term adherence. Look AHEAD (Action for Health in Diabetes) (12) is the first large clinical trial investigating long-term health impact of intensive lifestyle intervention in 5,145 overweight or obese adults with type 2 diabetes. Follow-up is ongoing and is planned to continue for 11.5 years to assess whether CV morbidity and mortality can be reduced by long-term weight reduction achieved by diet, physical activity, and behavior modification. At 1 year, body weight decreased by 8.6% and the percentage of participants with A1C <7% increased from 46 to 73% with a doubling (from 10 to 23.6%) of those achieving guideline goals for glycemic, blood pressure, and lipid control (12). Whether these initial favorable effects will be maintained over time and whether they will be transformed into a CV benefit will be verified in the ensuing follow-up.
More apparent in the interim is the impact of lifestyle modifications for prevention of type 2 diabetes, as indicated by the 58% reduction in the conversion rate to overt diabetes in the high-risk populations of the Diabetes Prevention Program (13) and Diabetes Prevention Study (14). Nonetheless, despite these striking results, implementation of similar programs in the general population remains problematic and, at least in the short term, expensive enough to require dedicated political decisions.
LIMITED PHARMACOLOGIC ARMAMENTARIUM
The ideal antidiabetic agent should normalize plasma glucose profiles, minimize side effects, and prevent development of micro- and macrovascular complications. Obviously no such agent is available, nor is it likely to be available in the near or medium-term future. Table 2 clearly shows there is no shortage of antidiabetic drugs, with even more to come (15). Each of these agents has pros and cons, but even more importantly, none of them is likely to ensure sustained good glycemic control over time. Although the UKPDS experience is limited to traditional antidiabetic agents, it has disseminated a valuable lesson. Regardless of the agent initially prescribed to the patient, ultimately, glycemic control trespassed on the target threshold. The question may be rephrased as to whether there is treatment that provides more durable efficacy. This issue has been recently dealt with in A Diabetes Outcome Progression Trial (ADOPT). In this study, the cumulative incidence of monotherapy failure at 5 years was highest with glyburide (34%; P < 0.001), intermediate with metformin (21%), and lowest with rosiglitazone (15%, P < 0.001) (16). The longer durability of rosiglitazone has been interpreted on the basis of simultaneous improvement of the two main pathogenetic mechanisms of type 2 diabetes, i.e., insulin resistance and β-cell function. Loss of β-cell function is the main reason for deterioration of glucose tolerance and glycemic control (17), and glitazones have been claimed to preserve β-cell function (18). From this perspective, vast interest has generated the availability of glucagon-like peptide (GLP)-1 analogs and dipeptidyl peptidase-4 inhibitors based on preclinical studies suggesting maintenance of β-cell function and mass with these drugs (19). These intriguing but preliminary findings require clinical confirmation, so that for the moment, it would be unwise to anticipate the “golden” treatment; rather, it would be preferable to learn how to better use the available pharmacologic tools.
Table 2.
Available antidiabetic agents for treatment of type 2 diabetes
| Sulfonylureas |
| First generation |
| Second generation |
| Third generation |
| Modified release |
| Glinides |
| Nonsulfonylureic |
| Amino acid derivatives |
| Biguanides |
| Metformin |
| Thiazolidinediones |
| Rosiglitazone |
| Pioglitazone |
| Fixed-dose oral hypoglycemic agents combinations |
| α-Glucosidase inhibitors |
| Acarbose |
| Voglibose |
| Insulin |
| Regular |
| Long-acting |
| Pre-mixed |
| Insulin analogs |
| Rapid-acting |
| Long-acting |
| Inhaled |
| GLP-1 analogs |
| Dipeptidyl peptidase-4 inhibitors |
| Amylin analog |
CONSERVATIVE MANAGEMENT
The “stepwise approach”' is usually adopted to manage glycemic control in type 2 diabetes. On diagnosis, lifestyle modification is initiated, followed by treatment with a single oral antidiabetic agent, which is often up-titrated to maximal recommended doses before combination therapy is introduced. However, this conservative approach has a number of drawbacks. On the contrary, a proactive approach and therapy tailored to the individual by methodical selection among available agents can optimize patient care (20).
Several clinical trials have demonstrated the effectiveness of diet and exercise in preventing diabetes and reducing disease progression (13,14), but, as aforementioned, such regimens are difficult to implement and maintain, and glycemic control is rarely achieved. Hence, in conjunction with lifestyle intervention, pharmacologic approaches become the key component of diabetes management to the point that the recent American Diabetes Association/European Association for the Study of Diabetes consensus suggested nutritional therapy should be initiated together with metformin (11). The latter is almost unanimously recognized as the drug of choice, but failure is expected to occur. In the UKPDS, after 9-year monotherapy, cumulative incidence of failure was 87% in obese patients randomized to metformin (21). In ADOPT, the 5-year cumulative incidence of metformin failure was 21% (16), and in two large retrospective analyses (22,23), the rate of metformin secondary failure was 35.5 and 38% at 4 and 5.7 years, respectively. Although different definitions for failure were used in these studies, all suggested that unacceptable therapeutic inertia occurs in clinical practice. Analysis of the 1994–2002 Kaiser Permanente Northwest database revealed that the average time between achieving A1C action point of 8% and switching to, or adding a second oral antidiabetic agent for patients on metformin, or sulfonylurea monotherapy, was 14.5 or 20.5 months, respectively (24). The authors of this analysis concluded that, “Clinicians should change glucose-lowering treatments in type 2 diabetes much sooner or use treatments that are less likely to fail” (24). This view has been reinforced by the Global Partnership of Effective Diabetes Management (20) and by the American Association of Clinical Endocrinologists (25). Taking the same approach is the more recent American Diabetes Association/European Association for the Study of Diabetes consensus statement advocating individualized therapeutic choices to be considered as soon as A1C overcomes a 7.0% threshold (11), supporting more intensive and earlier use of combination therapy and introduction of insulin therapy if glycemic control is not achieved. Several studies have shown how early use of submaximal combination doses of agents can improve glycemic control without significantly increasing side effects (26,27). The use of multiple drugs should be considered not simply on the basis of greater efficacy, but also in terms of a rational therapeutic approach of the multiple pathogenetic mechanisms underlying hyperglycemia and its progression. In particular, the primary role of progressive loss of β-cell function should be taken into full consideration as discussed hereunder.
ADVERSE EVENTS
In adopting a more intensive early intervention, the possibility of incurring adverse events may be higher compared with more relaxed treatment strategies. Moreover, side effects of the antihyperglycemic therapy may affect patient compliance. The profile of a drug is best described by its efficacy-to-safety ratio, but this ratio may vary as a function of the dose used. A typical example is provided by metformin (28). A progressive reduction in A1C is observed by increasing the dose from 500 up to 2,000 mg/day, with no further improvement in glycemic control above such dosage. On the contrary, progressive increase in metformin dose is associated with increased prevalence of patients experiencing gastrointestinal distress. As aforementioned, early combination therapy allows use of a submaximal dose of hypoglycemic agents, thus reducing the risk of adverse events. In the EMPIRE study (26), patients were randomized to either 2,000 mg/day metformin or the combination of 1,000 mg metformin plus 8 mg/day rosiglitazone. Although there was no significant difference in A1C after 4 months of treatment, the number of patients who discontinued because of gastrointestinal-related adverse events was significantly lower with combination therapy (all gastrointestinal events 3.1 vs. 6.8%; diarrhea 1.6 vs. 4.2%; abdominal pain 1.0 vs. 2.3%). Conversely, incidence of edema and body weight gain is lower when glitazones are associated with metformin compared with association with sulfonylureas and insulin. The GLP-1 analogs and dipeptidyl peptidase-4 inhibitors possess an interesting safety profile associated with body weight loss or neutrality (18), but the risk of hypoglycemia, which is almost nonexistent with monotherapy, becomes a factor when these agents are combined with sulfonylureas. Hypoglycemia is indeed the main concern in the context of intensive treatment initiated at the time of diagnosis of type 2 diabetes. The incidence of hypoglycemia in these patients has been recently analyzed by the U.K. Hypoglycemia Study Group (29), which showed that even with early insulin use in this condition, the frequency of hypoglycemia was generally equivalent to that observed in patients treated with sulfonylureas and considerably lower than during the first 5 years of treatment in type 1 diabetes. This low rate of hypoglycemia in type 2 diabetes may be further reduced by accurate selection of treatment. Thus, insulin sensitizers are not associated with hypoglycemia, its incidence is very low with incretin-based therapy, and use of both fast- and long-acting insulin analogs have been reported to be associated with less hypoglycemic events (30).
POOR COMPLIANCE
Unpredicted adverse events and inadequacy to cope with them may undermine a patient's self-reliability and adherence to treatment. Lack of compliance is often perceived with a sense of frustration by physicians. However, adherence is subjective and difficult to assess in a reliable manner. Moreover, patients, particularly those with mild alteration of their metabolic control, may not perceive the seriousness of their disease because of the absence of symptoms and/or they may lack confidence in the immediate or future benefits of medication. It is important for physicians to strive to attain compliance from their patients. Understanding the severity of the disease and the importance of adherence to prescribed treatment would require more time devoted to patient education and education reinforcement. In the survey by Browne at al (31), only 35% of patients recalled receiving advice about their medication, no more than 10% of patients using sulfonylureas appreciated the risk of hypoglycemia, and only 20% of those taking metformin were aware of potential gastrointestinal side effects. Physicians, nurses, and pharmacists also had gaps in their knowledge. Approximately 50% answered questions correctly on the timing, mechanism of action, and side effects of oral antidiabetic agents (31). These results emphasize the importance of continued education and consistency of information from members of primary and secondary teams but may also underlie medical inertia. As it has been recently suggested (32), failure to appreciate long-term benefits of treatment intensification may represent a common mechanism underlying both patient nonadherence and physician clinical inertia, i.e., “clinical myopia.”
Polypharmacy may represent another burden to the patient, particularly in light of multifactorial intervention required. An increasing number of fixed-combination tablets of oral agents is becoming available, and data in the literature indicate how adherence to therapy may be more beneficial with these combinations compared with the use of a single drug tablet (33).
UNDERLYING PHYSIOPATHOLOGY
Type 2 diabetes is a complex disease where several pathogenetic mechanisms coexist, in particular, insulin resistance and reduced β-cell function (34). Insulin resistance occurs in >85% of patients and is associated with impaired insulin-mediated glucose uptake in insulin-dependent tissues (mainly skeletal muscle) and ineffective suppression of hepatic glucose production. The latter is the main cause for increased fasting plasma glucose levels due to inappropriate acceleration of gluconeogenesis. Insulin resistance is also closely interlinked with numerous risk factors for CV disease (35), as well as being an independent risk factor for CV disease (36). In individuals predisposed to type 2 diabetes, the early alteration of insulin sensitivity is already associated with marked impairment of the β-cell. Modest alteration of glucose tolerance, even within nondiagnostic boundaries, is associated with marked reduction of β-cell mass and function (37). Moreover, it is the progressive loss of β-cell mass and function that sets the pace for transition from normal glucose tolerance to diabetes. Therefore, treatments designed to correct pathogenetic abnormalities that are already present in the pre-diabetic condition may ensure prolonged glycemic control. Based on this pathophysiological background, DeFronzo in his Banting Medal Lecture (38) proposed that triple therapy should be initiated as early as possible, rather than adopting a stepwise approach simply based on A1C targeting. According to this proposal, the effects of which will be tested in a randomized trial, metformin will be used to improve insulin action on the liver, pioglitazone to ameliorate peripheral insulin action, and GLP-1 analogs (or dipeptidyl peptidase-4 inhibitors) to improve β-cell function, and, possibly, preserve β-cell mass. It is of interest that the treatment appears to be safe enough to be used with confidence in the early stage of the disease, since none of the three drugs conveys risk for hypoglycemia. Moreover, the anti-obesity effect of GLP-1 analogs and metformin may prevent glitazone-mediated body weight gain. Although rationale and fascinating, this proposal need to be assessed with proper clinical trials and no implementation should be considered without preliminary confirmation of efficacy and safety.
In summary, modern pharmacopeia facilitates a rational therapeutic approach aiming at reversal of the alterations that account for progressive deterioration of glucose homeostasis, enabling, at least in theory, maintenance of long-term glycemic control.
SUBOPTIMAL HEALTH CARE SYSTEM
Effective and sustained glycemic control in type 2 diabetes is unlikely to depend only on rational treatment. Education, motivation, prevention, and development of micro- and macrovascular complications, and comorbidities, are indications for a structured multidisciplinary approach. Ideally, the patients should be supported by a multidisciplinary team comprising primary care physicians, diabetologists, diabetes education nurses, dietitians, pharmacists, podiatrists, and various other specialists. This multidisciplinary team should be designed to promptly react to any new educational, diagnostic, and therapeutic need. Data are available to support the notion that with such an approach, glycemic control, hospitalization, and patients' quality-of-life are all significantly improved (39). Continuous education is also integral to diabetes management, since it has been repeatedly shown that it not only improves metabolic control, but also contributes to more cost-effective intervention (40). While there may be challenges in applying these approaches due to economical constraints, the relevance of involving the patient within the diabetes care team must be acknowledged as pivotal to improving the proportion of individuals achieving good glycemic control. All components of the diabetes team should recognize their crucial role in enabling and empowering patients to control their medical condition.
CHANGING THE PARADIGM
The growing number of individuals with type 2 diabetes, the still limited therapeutic success, and the burden of micro- and macrovascular complications necessitate a change in treatment of diabetes. Such change can only occur by overcoming the multiple limitations hampering our ability to ensure adequate longstanding glycemic control to as many patients as possible. In previous sections, we tried to outline some of these limitations. Many others may be added, but our list may suffice deliberation.
Obesity is the most common phenotypic trait of type 2 diabetes and it directly affects the possibility of achieving sustained glycemic control. Unfortunately, effective anti-obesity drugs are still lacking, particularly after the use of endocannabinoid receptor antagonists has been halted (41). An even more salient point is that obesity is the main driving force for the current epidemic of type 2 diabetes; thus, fighting obesity represents a major task in the attempt to prevent this disease. Unfortunately, this tactic is unlikely to be solved at the individual level. Rather, as outlined by Simpson et al. (42), a more comprehensive approach not limited to high-risk individuals should be adopted by implementing strategies of lifestyle modification directed at the community, addressing young generations by introducing formal and structured educational programs into the school curricula, and by exposing youngsters to the disaster and mayhem of states of ill health. All this requires societal and political decisions, such as treating metabolic poisoning, i.e., high caloric fat content in food with the same tax penalty approach used for other health-menacing factors, namely cigarettes, alcohol, and carbon emission.
Diabetes is diagnosed as soon as fasting plasma glucose exceeds 125 mg/dl. Merely crossing that line does not mean we are facing a “mild diabetic condition.” There is no “mild diabetes”; it is diabetes with the full array of risks for developing complications that are definitely a threat to the quality of life and life expectancy of patients. Therefore, prompt restoration and maintenance of glycemic control as close as, and for as long as possible, to normal levels, is mandatory.
To achieve this goal, advantages and disadvantages of available therapeutic tools should be mastered by the diabetologist. This is necessary to optimize these methods and to combine them in a logical manner. In doing so, a proactive approach should be adopted from the first day of diagnosis, an approach stigmatized in the recent American Association of Clinical Endocrinologists consensus: “adopt an uncompromising insistence on treating to target” (25). Adverse events may be a matter of concern when such insistence is implemented, but again, conscious use of agents in combination can reduce such a risk.
There is no chance of maintaining good results without a close partnership between the health care providers and the diabetic patient. Both sides should undergo a continuous, reciprocal educational program with verification and updating of information, and all efforts should be made to ensure efficacious communication. For this purpose, establishing a diabetes team appears to play an important role. Financial constraints may limit the size, but although it may be small, it is crucial that the medical personnel aim at well-defined goals and follow definite and shared management protocols.
However, three main “innovations” are seminal in the change in treatment paradigm. The first is that we now have the tools to aim treatment at reversal of the mechanisms responsible for evolution of the disease. Recognizing that alterations are already present in individuals with minor disturbance of glucose tolerance and that allowing hyperglycemia to develop can only worsen those mechanisms sets the rationale for early intensive combination treatment. Therefore, at the time of diagnosis, even if glucose parameter is only slightly above diagnostic thresholds, insulin action in peripheral tissue should be ameliorated, suppression of hepatic glucose production improved, and β-cell function supported. Such an approach is mainly, but not totally, focused on glycemic control. Thus, amelioration of insulin resistance can be expected to result in a better profile of CV risk.
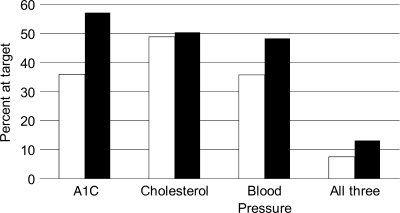
Prevention of CV morbidity and mortality remains a major task in the management of type 2 diabetes. A holistic approach is then necessary, as suggested by the results of the Steno-2 studies (43,44). Intensified multifactorial intervention, with tight glucose regulation and the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents, and behavior modification have sustained beneficial effects with respect to vascular complications and a lower risk of death from CV causes (43,44). Although this approach may be quite effective, it may not be that simple to implement in the diabetic community. It has been proven that control of blood glucose levels, blood pressure, and cholesterol levels reduces the risk of vascular disease among type 2 diabetic patients; however, the current state of control of these risk factors among individuals is uncertain. Analysis of the U.S. National Health and Nutrition Examination Survey (NHANES) database indicated that the number of people achieving target values for all the aforementioned risk factors remains unsatisfactory and does not change dramatically over time (45). These data are summarized in Fig. 1. It can be appreciated that although a positive trend may be apparent, no more than 13.2% of patients in NHANES 1999–2004 attained recommended goals of A1C level <7%, blood pressure <130/80 mmHg, and total cholesterol level <200 mg/dl (5.18 mmol/l). Therefore, while further public health efforts are needed to control CV risk factors among diabetic individuals, other solutions should be sought.
Figure 1.
Percentage of type 2 diabetic patients achieving treatment targets for glucose, total cholesterol, and blood pressure in NHANES 1999–2004 (44). □, 1999; ■, 2004.
Diabetes has been defined as a CV risk factor equivalent (46); thus, one potential solution may simply be prevention of diabetes. Several trials have shown that lifestyle modification is effective in preventing incident type 2 diabetes in high-risk groups (47). Whether diabetes prevention strategies ultimately will prevent the development of diabetic vascular complications is unknown, but CV risk factors are all favorably affected.
Finally, a major cultural and practical effort must be made to face the increasing health demand of an ever-increasing number of type 2 diabetic patients. A shift in the paradigm of treatment is needed and should aim at definite objectives, as already dictated by the diabetes community: “Diabetes must be prevented sooner and diagnosed earlier (48).” “…And once diagnosed, all types of diabetes must then be managed much more aggressively” (49).
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. U.K. Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 2. International Diabetes Federation Global guidelines for type 2 diabetes. Available at http://www.idf.org/home, 2005
- 3. Stettler C, Allemann S, Jüni P, Cull CA, Holman RR, Egger M, Krähenbühl S, Diem P: Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 2006; 152: 27– 38 [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643– 2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560– 2572 [DOI] [PubMed] [Google Scholar]
- 8. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD: VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129– 139 [DOI] [PubMed] [Google Scholar]
- 9. Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR: Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008; 359: 1565– 1576 [DOI] [PubMed] [Google Scholar]
- 10. Harris SB, Ekoé JM, Zdanowicz Y, Webster-Bogaert S: Glycemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study). Diabetes Res Clin Pract 2005; 70: 90– 97 [DOI] [PubMed] [Google Scholar]
- 11. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008; 31: 173– 152 [DOI] [PubMed] [Google Scholar]
- 12. Look AHEAD Research Group: Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30: 1374– 1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Unsitupa M: Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343– 1350 [DOI] [PubMed] [Google Scholar]
- 14. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bailey CJ: Potential new treatments for type 2 diabetes. Trends Pharmacol Sci 2001; 21: 259– 265 [DOI] [PubMed] [Google Scholar]
- 16. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 [DOI] [PubMed] [Google Scholar]
- 17. Del Prato S, Marchetti P, Bonadonna RC: Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2002; 51( Suppl. 1): S109– S116 [DOI] [PubMed] [Google Scholar]
- 18. Del Prato S: Beta-cell function and anti- diabetic pharmacotherapy. Diabete Metab Res Rev 2007; 23: 518– 527 [DOI] [PubMed] [Google Scholar]
- 19. Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696– 1705 [DOI] [PubMed] [Google Scholar]
- 20. Del Prato S, Felton A-M, Munro N, Nesto R, Zimmet P, Zinman B: on behalf of the Global Partnership For Effective Diabetes Management Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract 2005; 59: 1345– 1355 [DOI] [PubMed] [Google Scholar]
- 21. Turner RC, Cull CA, Frighi V, Holman RR: Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49): UK Prospective Diabetes Study (UKPDS) Group JAMA 1999; 281: 2005– 2012 [DOI] [PubMed] [Google Scholar]
- 22. Eurich DT, Simpson SH, Majumdar SR, Johnson JA: Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy 2005; 25: 810– 816 [DOI] [PubMed] [Google Scholar]
- 23. Riedel AA, Heien H, Wogen J, Plauschinat CA: Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy 2007; 27: 1102– 1110 [DOI] [PubMed] [Google Scholar]
- 24. Brown JB, Nichols GA, Perry A: The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27: 1535– 1540 [DOI] [PubMed] [Google Scholar]
- 25. Lebovitz HE, Austin MM, Blonde L, Davidson JA, Del Prato S, Gavin JR, 3rd, Handelsman Y, Jellinger PS, Levy P, Riddle M, Roberts VL, Siminerio LM: ACE/AACE Diabetes recommendations Implementation Writing Committee ACE/AACE consensus conference on the implementation of outpatients management of diabetes mellitus: consensus conference recommendations. Endocr Pract 2006; 12( Suppl. 1): 6– 12 [DOI] [PubMed] [Google Scholar]
- 26. Weissman P, Goldstein BJ, Rosenstock J, Waterhouse B, Cobits AR, Wooddell MJ, Strow LJ: Effects of rosiglitazone added to submaximal doses of metformin compared with dose escalation of metformin in type 2 diabetes: the EMPIRE Study. Curr Med Res Opin 2005; 21: 2029– 2035 [DOI] [PubMed] [Google Scholar]
- 27. Garber AJ, Larsen J, Schneider SH, Piper BBA, Henry D: Glyburide/Metformin Initial Therapy Study Group Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab 2002; 4: 201– 208 [DOI] [PubMed] [Google Scholar]
- 28. Garber AJ: Using dose-response characteristics of therapeutic agents for treatment decisions in type 2 diabetes. Diabetes Obes Metab 2000; 2: 139– 147 [DOI] [PubMed] [Google Scholar]
- 29. U.K. Hypoglycemia Study Group Risk of hypoglycemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50: 1140– 1147 [DOI] [PubMed] [Google Scholar]
- 30. Rolla A: Pharmacokinetic and pharmacodynamic advantages of insulin analogues and premixed insulin analogues over human insulins: impact on efficacy and safety. Am J Med 2008; 121( Suppl.): S9– S19 [DOI] [PubMed] [Google Scholar]
- 31. Browne DL, Avery L, Turner BC, Kerr D, Cavan DA: What do patients with diabetes know about their tablets? Diabet Med 2000; 17: 528– 531 [DOI] [PubMed] [Google Scholar]
- 32. Reach G: Patient non-adherence and healthcare-provider inertia are clinical myopia. Diabete Metab 2008; 34: 382– 385 [DOI] [PubMed] [Google Scholar]
- 33. Guillausseau PJ: Impact of compliance with oral antihyperglycemic agents on health outcomes in type 2 diabetes mellitus: a focus on frequency of administration. Treat Endocrinol 2005; 4: 167– 175 [DOI] [PubMed] [Google Scholar]
- 34. DeFronzo RA: Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004; 88: 787– 835 [DOI] [PubMed] [Google Scholar]
- 35. Del Prato S: Pathophysiology of insulin action in humans. In The Metabolic Syndrome at the Beginning of the XXIst Century. Serrano Rios M, Caro JF, Carraro R, Gutierrez Fuentes JA: Eds. Madrid, Spain, Elsevier España S.A., 2005, p. 180– 197 [Google Scholar]
- 36. Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M: HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002; 25: 1135– 1141 [DOI] [PubMed] [Google Scholar]
- 37. Lencioni C, Lupi R, Del Prato S: Beta-cell failure in type 2 diabetes. Curr Diab Rep 2008; 8: 179– 184 [DOI] [PubMed] [Google Scholar]
- 38. DeFronzo RA: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes 2009; 58: 773– 795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sadur CN, Moline N, Costa M, Michalik D, Mendlowitz D, Roller S, Watson R, Swain BE, Selby JV, Javorski WC: Diabetes management in a health maintenance organization: efficacy of care management using cluster visits. Diabetes Care 1999; 22: 2011– 2017 [DOI] [PubMed] [Google Scholar]
- 40. Gagliardino JJ, Etchegoyen G: for the PEDNID-LA Research Group A model educational program for people with type 2 diabetes. Diabetes Care 2001; 24: 1001– 1007 [DOI] [PubMed] [Google Scholar]
- 41. The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia [Internet Press Release], 23 October 2008. London, England, European Medicines Agency. Available from http://www.emea.europa.eu/humandocs/PDFs/EPAR/acomplia/53777708en.pdf Accessed on 17 November 2008
- 42. Simpson RW, Shaw JE, Zimmet PZ: The prevention of type 2 diabetes: lifestyle or pharmacotherapy? A challenge for the 21st century. Diabetes Res Clin Pract 2003; 59: 165– 180 [DOI] [PubMed] [Google Scholar]
- 43. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383– 393 [DOI] [PubMed] [Google Scholar]
- 44. Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580– 591 [DOI] [PubMed] [Google Scholar]
- 45. Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS: Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18: 222– 229 [DOI] [PubMed] [Google Scholar]
- 46. ATPIII final report. II: Rationale for intervention. Circulation 2002; 106: 3163– 3223 [Google Scholar]
- 47. Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton JA, Hsu RT: Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007; 334: 299– 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Canadian Diabetes Association Clinical Practice Guidelines 2003. Available at http://www.diabetes.ca/cpg
- 49. American Diabetes Association: Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2003; 26: S28– S32 [DOI] [PubMed] [Google Scholar]