Abstract
A total of 26% of the pneumococci isolated from an outpatient clinic in Nairobi, Kenya, during 1991 to 1992 had intermediate levels of penicillin resistance. Gene fingerprinting and DNA sequencing were used to distinguish the penicillin-binding protein (PBP) 1A, 2B, and 2X genes in 23 resistant isolates. Isolates were grouped into those that had identical forms of each of the three PBP genes (fingerprint groups) and those that had identical rRNA gene restriction patterns (ribotypes). Both methods divided the isolates into 11 groups. In a few cases, horizontal gene transfer appeared to have distributed an identical altered PBP gene into different pneumococcal lineages. Eight isolates were indistinguishable by ribotyping or multilocus enzyme electrophoresis and contained identical PBP 1A genes. Although these isolates were therefore members of the same clone, they were divided into two fingerprint groups which contained different PBP 2X and 2B genes. Presumably, members of this clone have acquired different altered PBP 2X and 2B genes on two separate occasions. One of these fingerprint groups contained isolates of serotype 14, whereas the other contained isolates of both serotypes 14 and 7. The identification of isolates in the latter group that are identical by all criteria, except serotype, implies the occurrence of a change in serotype. The predominant serotypes of the penicillin-resistant pneumococci from Nairobi were serotypes 14 and 19. In both cases, isolates of the same serotype which required the same MIC of penicillin were not members of a single clone, indicating that identity of serotype and MIC are not sufficient criteria for defining clones of resistant pneumococci even when the bacteria are isolated from a single clinic.
Full text
PDF
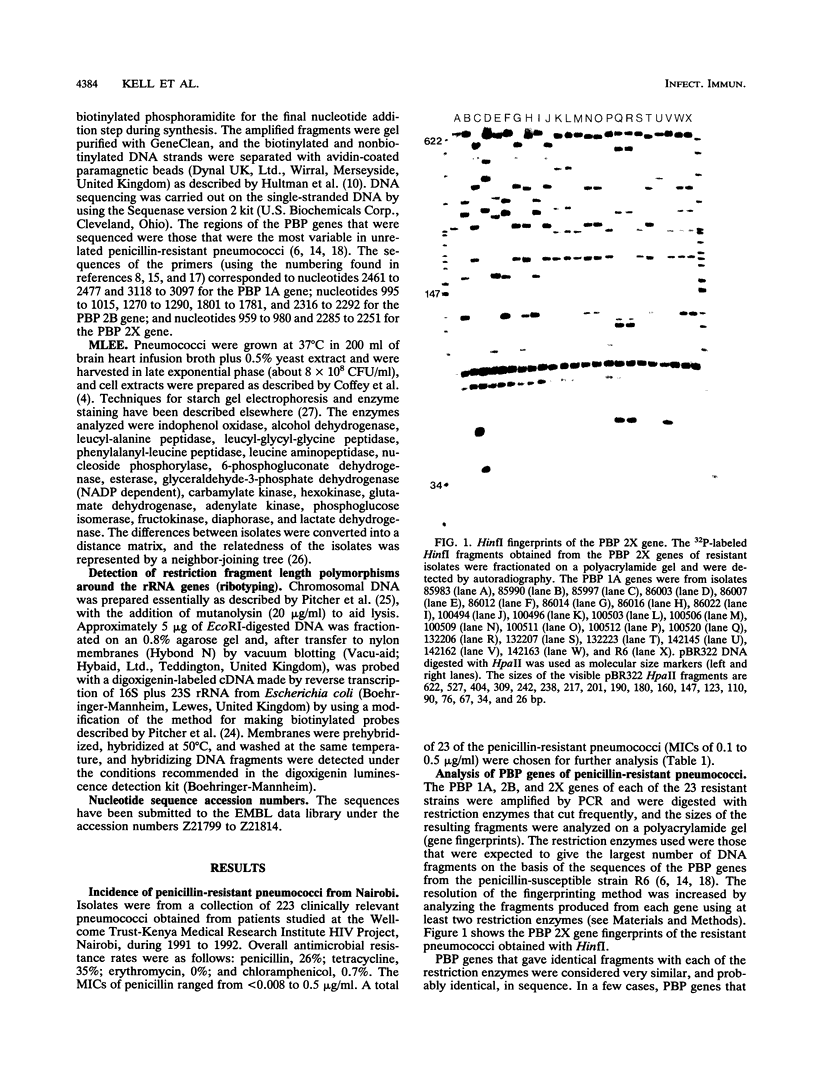







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992 Jul;15(1):77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- Coffey T. J., Dowson C. G., Daniels M., Zhou J., Martin C., Spratt B. G., Musser J. M. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991 Sep;5(9):2255–2260. doi: 10.1111/j.1365-2958.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Brannigan J. A., George R. C., Hansman D., Liñares J., Tomasz A., Smith J. M., Spratt B. G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Extensive re-modelling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989 Jan;3(1):95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 1989 Sep 25;17(18):7518–7518. doi: 10.1093/nar/17.18.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Ellerbrok H., Briese T., Handwerger S., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and -resistant pneumococci: immunological relatedness of altered proteins and changes in peptides carrying the beta-lactam binding site. Antimicrob Agents Chemother. 1986 Oct;30(4):553–558. doi: 10.1128/aac.30.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabes D., Nachman S., Tomasz A. Penicillin-binding protein families: evidence for the clonal nature of penicillin resistance in clinical isolates of pneumococci. J Infect Dis. 1989 Jan;159(1):16–25. doi: 10.1093/infdis/159.1.16. [DOI] [PubMed] [Google Scholar]
- Koornhof H. J., Wasas A., Klugman K. Antimicrobial resistance in Streptococcus pneumoniae: a South African perspective. Clin Infect Dis. 1992 Jul;15(1):84–94. doi: 10.1093/clinids/15.1.84. [DOI] [PubMed] [Google Scholar]
- Laible G., Hakenbeck R., Sicard M. A., Joris B., Ghuysen J. M. Nucleotide sequences of the pbpX genes encoding the penicillin-binding proteins 2x from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol Microbiol. 1989 Oct;3(10):1337–1348. doi: 10.1111/j.1365-2958.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Laible G., Spratt B. G., Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991 Aug;5(8):1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Liñares J., Pallares R., Alonso T., Perez J. L., Ayats J., Gudiol F., Viladrich P. F., Martin R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979-1990). Clin Infect Dis. 1992 Jul;15(1):99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- Martin C., Briese T., Hakenbeck R. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding proteins 1a and 1b. J Bacteriol. 1992 Jul;174(13):4517–4523. doi: 10.1128/jb.174.13.4517-4523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Sibold C., Hakenbeck R. Relatedness of penicillin-binding protein 1a genes from different clones of penicillin-resistant Streptococcus pneumoniae isolated in South Africa and Spain. EMBO J. 1992 Nov;11(11):3831–3836. doi: 10.1002/j.1460-2075.1992.tb05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton A. Pneumococcal antimicrobial resistance: the problem in Hungary. Clin Infect Dis. 1992 Jul;15(1):106–111. doi: 10.1093/clinids/15.1.106. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Facklam R., Reeves M., Hunter S., Swenson J. M., Hill B. C., Tenover F. C. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob Agents Chemother. 1992 Oct;36(10):2176–2184. doi: 10.1128/aac.36.10.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz R., Musser J. M., Crain M., Briles D. E., Marton A., Parkinson A. J., Sorensen U., Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992 Jul;15(1):112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- Muñoz R., Coffey T. J., Daniels M., Dowson C. G., Laible G., Casal J., Hakenbeck R., Jacobs M., Musser J. M., Spratt B. G. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991 Aug;164(2):302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- Muñoz R., Dowson C. G., Daniels M., Coffey T. J., Martin C., Hakenbeck R., Spratt B. G. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1992 Sep;6(17):2461–2465. doi: 10.1111/j.1365-2958.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibold C., Wang J., Henrichsen J., Hakenbeck R. Genetic relationships of penicillin-susceptible and -resistant Streptococcus pneumoniae strains isolated on different continents. Infect Immun. 1992 Oct;60(10):4119–4126. doi: 10.1128/iai.60.10.4119-4126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltman W. D., 2nd, Talkington D. F., Lipinski A. E., Crain M. J., Dixon J. M., Briles D. E. Evidence for a clonal origin of relative penicillin resistance among type 9L pneumococci in northwestern Canada. J Infect Dis. 1992 Apr;165(4):671–675. doi: 10.1093/infdis/165.4.671. [DOI] [PubMed] [Google Scholar]