Abstract
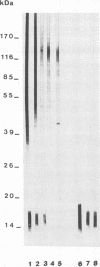
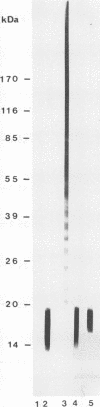
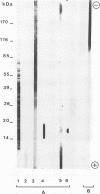
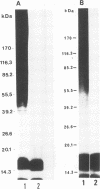
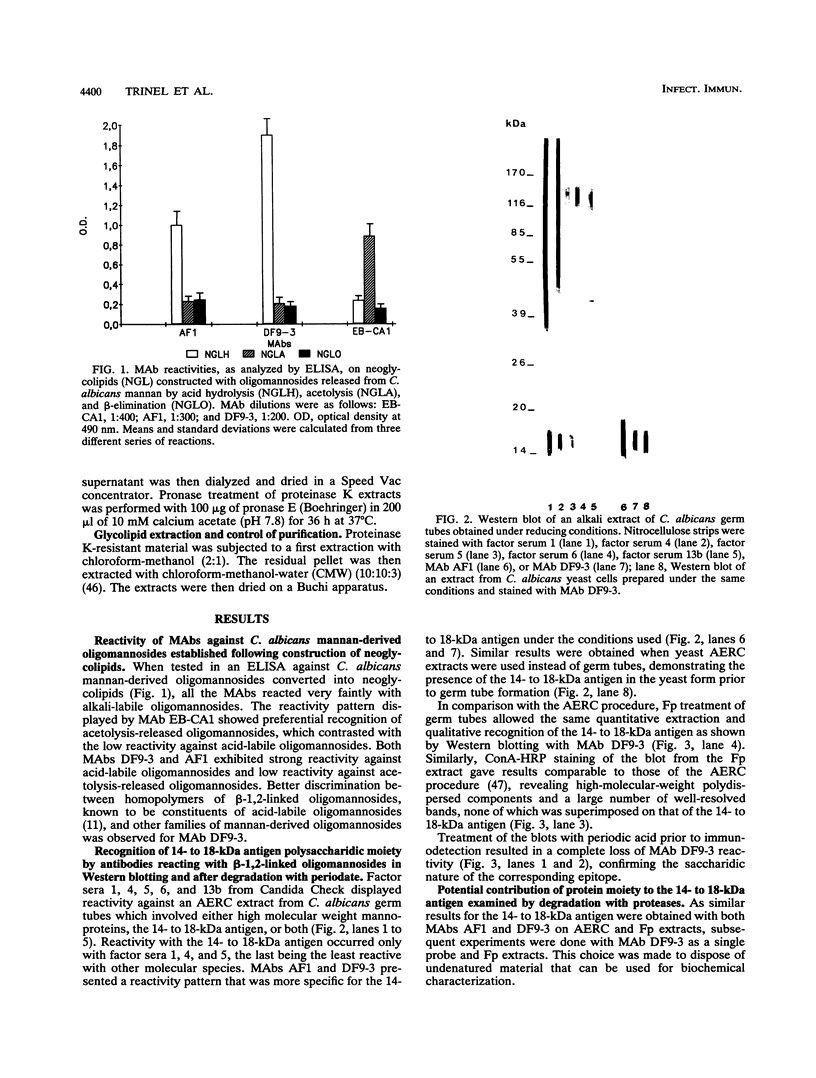
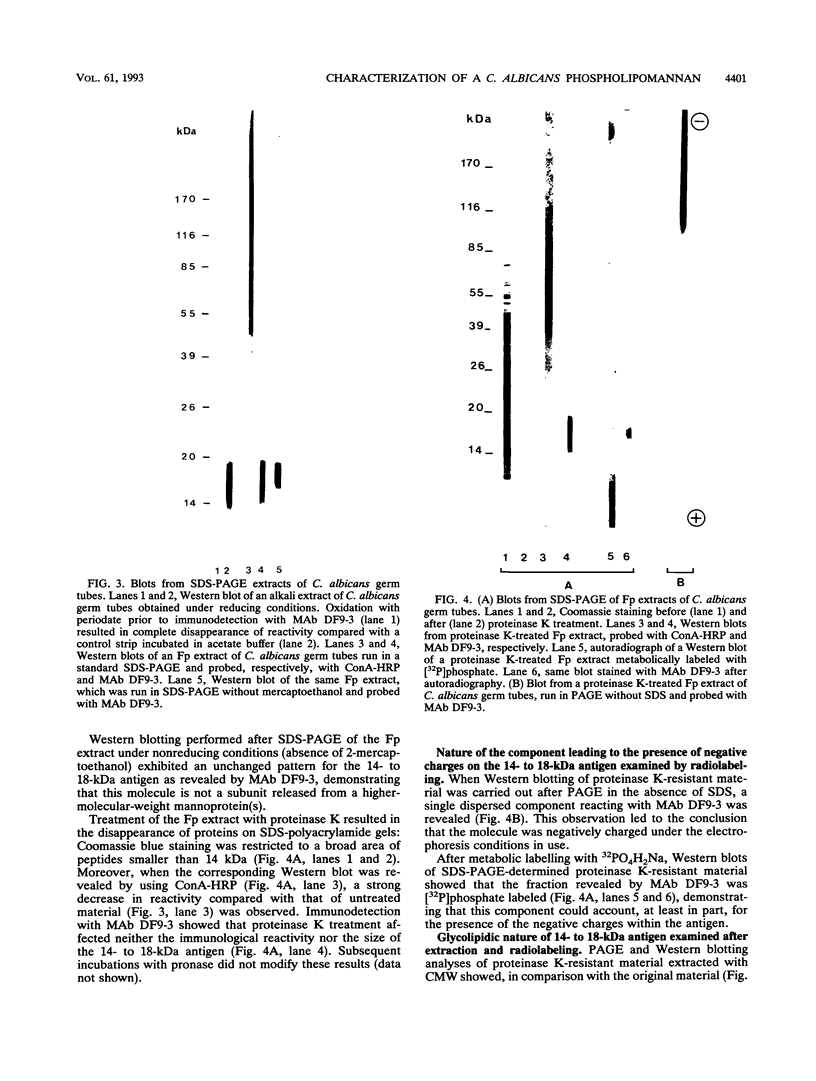
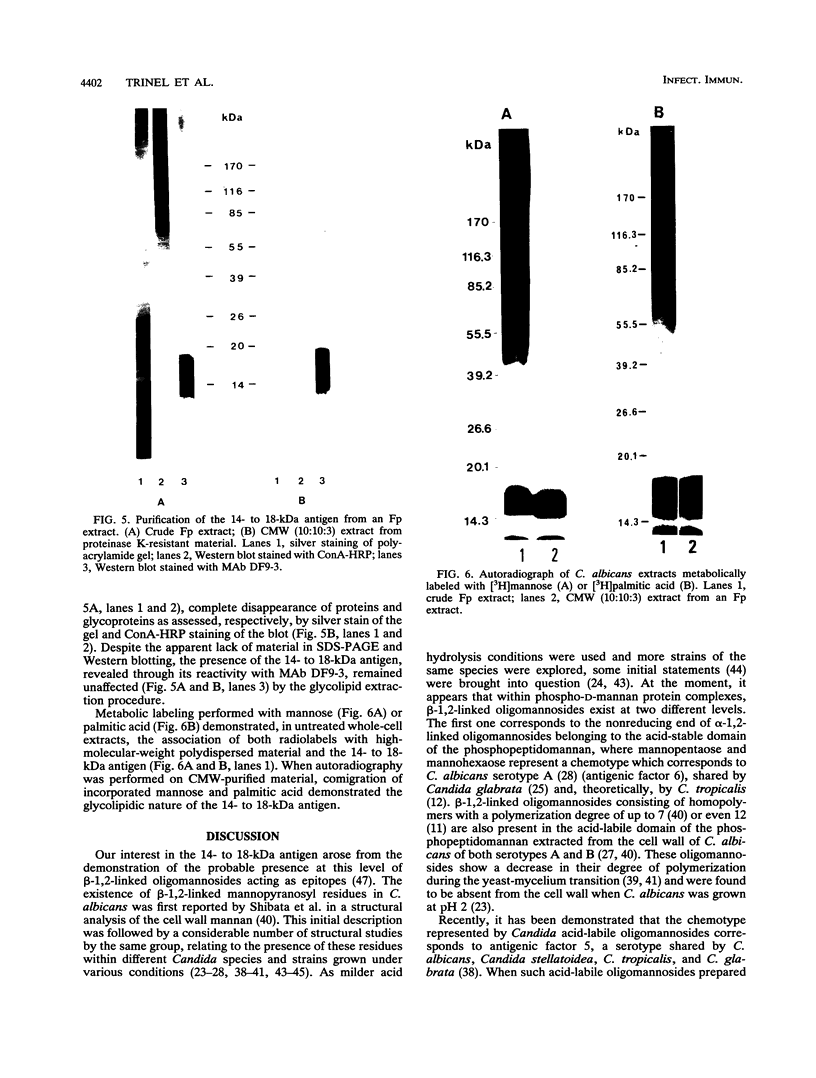
Western blot (immunoblot) analysis of Candida albicans germ tube extracts has demonstrated the probable presence of beta-1,2-linked oligomannosides acting as epitopes distributed over a 14- to 18-kDa antigen unreactive to concanavalin A. These conclusions about the existence of these non-mannan-associated oligomannoside species were reinforced in the present study by the demonstration of reactivity of factor serum 5 (Iatron Laboratories) with the same antigen. A monoclonal antibody which reacted in an enzyme immunoassay with beta-1,2-linked oligomannosides converted into neoglycolipids and in Western blotting with the 14- to 18-kDa antigen from yeast and germ tubes, through metaperiodate-sensitive epitopes, was used for further characterization of the molecule. Reducing agents and strong protease digestion, which have deleterious effects on C. albicans proteins and mannoproteins, affected neither the antigenicity nor the relative molecular weight of the molecule. Western blots performed after migration of protease-treated extracts in polyacrylamide gels without sodium dodecyl sulfate (SDS) showed that the 14- to 18-kDa antigen could be negatively charged, whereas metabolic radiolabeling demonstrated that these charges could originate, at least in part, from the presence of phosphorus within the molecule. Chloroform-methanol-water extraction of protease-resistant material led to purification of the 14- to 18-kDa antigen, as determined by SDS-polyacrylamide gel electrophoresis and Western blotting. Metabolic radiolabeling with mannose confirmed the presence of these sugar residues within the purified 14- to 18-kDa antigen (despite its nonreactivity to concanavalin A), whereas radiolabeling with palmitic acid demonstrated its lipopolysaccharidic nature. Together, these results led to the conclusion that the 14- to 18-kDa antigen is a phospholipomannan.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Borg-von Zepelin M., Grüness V. Characterization of two monoclonal antibodies against secretory proteinase of Candida tropicalis DSM 4238. J Med Vet Mycol. 1993;31(1):1–15. [PubMed] [Google Scholar]
- Casanova M., Chaffin W. L. Phosphate-containing proteins and glycoproteins of the cell wall of Candida albicans. Infect Immun. 1991 Mar;59(3):808–813. doi: 10.1128/iai.59.3.808-813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Torosantucci A., Boccanera M., Pellegrini G., Palma C., Malavasi F. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J Med Microbiol. 1988 Dec;27(4):233–238. doi: 10.1099/00222615-27-4-233. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Hunter S. W., McNeil M., Brennan P. J. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J Biol Chem. 1992 Mar 25;267(9):6228–6233. [PubMed] [Google Scholar]
- Conzelmann A., Puoti A., Lester R. L., Desponds C. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):457–466. doi: 10.1002/j.1460-2075.1992.tb05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faille C., Mackenzie D. W., Michalski J. C., Poulain D. Evaluation of an enzyme immunoassay using neoglycolipids constructed from Candida albicans oligomannosides to define the specificity of anti-mannan antibodies. Eur J Clin Microbiol Infect Dis. 1992 May;11(5):438–446. doi: 10.1007/BF01961859. [DOI] [PubMed] [Google Scholar]
- Faille C., Michalski J. C., Strecker G., Mackenzie D. W., Camus D., Poulain D. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect Immun. 1990 Nov;58(11):3537–3544. doi: 10.1128/iai.58.11.3537-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faille C., Wieruszeski J. M., Lepage G., Michalski J. C., Poulain D., Strecker G. 1H-NMR spectroscopy of manno-oligosaccharides of the beta-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW-32 (serotype A). Biochem Biophys Res Commun. 1991 Dec 31;181(3):1251–1258. doi: 10.1016/0006-291x(91)92073-s. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y. Antigenic structure of Candida albicans. Immunochemical basis of the serologic specificity of the mannans in yeasts. Immunol Ser. 1989;47:37–62. [PubMed] [Google Scholar]
- Ghannoum M. A., Janini G., Khamis L., Radwan S. S. Dimorphism-associated variations in the lipid composition of Candida albicans. J Gen Microbiol. 1986 Aug;132(8):2367–2375. doi: 10.1099/00221287-132-8-2367. [DOI] [PubMed] [Google Scholar]
- Gil M. L., Casanova M., Martínez J. P., Sentandreu R. Antigenic cell wall mannoproteins in Candida albicans isolates and in other Candida species. J Gen Microbiol. 1991 May;137(5):1053–1061. doi: 10.1099/00221287-137-5-1053. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayette M. P., Strecker G., Faille C., Dive D., Camus D., Mackenzie D. W., Poulain D. Presence of human antibodies reacting with Candida albicans O-linked oligomannosides revealed by using an enzyme-linked immunosorbent assay and neoglycolipids. J Clin Microbiol. 1992 Feb;30(2):411–417. doi: 10.1128/jcm.30.2.411-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando F. L., Estevez J. J., Cebrian M., Poulain D., Ponton J. Identification of Candida albicans cell wall antigens lost during subculture in synthetic media. J Med Vet Mycol. 1993;31(3):227–237. [PubMed] [Google Scholar]
- Hoberg K. A., Cihlar R. L., Calderone R. A. Characterization of cerulenin-resistant mutants of Candida albicans. Infect Immun. 1986 Jan;51(1):102–109. doi: 10.1128/iai.51.1.102-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Ishiguro A., Homma M., Sukai T., Higashide K., Torii S., Tanaka K. Immunoblotting analysis of sera from patients with candidal vaginitis and healthy females. J Med Vet Mycol. 1992;30(4):281–292. doi: 10.1080/02681219280000371. [DOI] [PubMed] [Google Scholar]
- Kaku H., Goldstein I. J., Oscarson S. Interactions of five D-mannose-specific lectins with a series of synthetic branched trisaccharides. Carbohydr Res. 1991 Jun 25;213:109–116. doi: 10.1016/s0008-6215(00)90602-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Giummelly P., Takahashi S., Ishida M., Sato J., Takaku M., Nishidate Y., Shibata N., Okawa Y., Suzuki S. Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without beta-1,2 linkage and phosphate group. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1003–1009. doi: 10.1016/0006-291x(91)91664-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Kojimahara T., Takahashi K., Takikawa M., Takahashi S., Shibata N., Okawa Y., Suzuki S. Structural determination of D-mannans of pathogenic yeasts Candida stellatoidea type I strains: TIMM 0310 and ATCC 11006 compared to IFO 1397. Carbohydr Res. 1991 Jul 18;214(1):131–145. doi: 10.1016/s0008-6215(00)90536-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Mitobe H., Takahashi K., Yamamoto T., Shibata N., Suzuki S. Structural study of a cell wall mannan-protein complex of the pathogenic yeast Candida glabrata IFO 0622 strain. Arch Biochem Biophys. 1992 May 1;294(2):662–669. doi: 10.1016/0003-9861(92)90739-j. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Mitobe H., Ohkubo Y., Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989 Aug 1;272(2):364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Nakada M., Chaki S., Mizugami K., Ohkubo Y., Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990 Apr;278(1):195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Suzuki S. Evidence for oligomannosyl residues containing both beta-1,2 and alpha-1,2 linkages as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun. 1992 May;60(5):2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell J. P. The resolution of bacteroides lipopolysaccharides by polyacrylamide gel electrophoresis. J Med Microbiol. 1991 May;34(5):253–257. doi: 10.1099/00222615-34-5-253. [DOI] [PubMed] [Google Scholar]
- Menon A. K., Mayor S., Schwarz R. T. Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J. 1990 Dec;9(13):4249–4258. doi: 10.1002/j.1460-2075.1990.tb07873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Paul T. R., Smith S. N., Brown M. R. Effect of iron depletion on cell-wall antigens of Candida albicans. J Med Microbiol. 1989 Feb;28(2):93–100. doi: 10.1099/00222615-28-2-93. [DOI] [PubMed] [Google Scholar]
- Poulain D., Faille C., Delaunoy C., Jacquinot P. M., Trinel P. A., Camus D. Probable presence of beta(1-2)-linked oligomannosides that act as human immunoglobulin G3 epitopes and are distributed over a Candida albicans 14- to 18-kilodalton antigen. Infect Immun. 1993 Mar;61(3):1164–1166. doi: 10.1128/iai.61.3.1164-1166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzo G. The carbohydrate- and lipid-containing cell wall of mycobacteria, phenolic glycolipids: structure and immunological properties. Crit Rev Microbiol. 1990;17(4):305–327. doi: 10.3109/10408419009105730. [DOI] [PubMed] [Google Scholar]
- Qadri A., Gupta S. K., Talwar G. P. Monoclonal antibodies delineate multiple epitopes on the O antigens of Salmonella typhi lipopolysaccharide. J Clin Microbiol. 1988 Nov;26(11):2292–2296. doi: 10.1128/jcm.26.11.2292-2296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüchel R., Böning B., Borg M. Characterization of a secretory proteinase of Candida parapsilosis and evidence for the absence of the enzyme during infection in vitro. Infect Immun. 1986 Aug;53(2):411–419. doi: 10.1128/iai.53.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Arai M., Haga E., Kikuchi T., Najima M., Satoh T., Kobayashi H., Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as beta-1,2-linked oligomannosyl residues. Infect Immun. 1992 Oct;60(10):4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Fukasawa S., Kobayashi H., Tojo M., Yonezu T., Ambo A., Ohkubo Y., Suzuki S. Structural analysis of phospho-D-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res. 1989 Apr 15;187(2):239–253. doi: 10.1016/0008-6215(89)80006-0. [DOI] [PubMed] [Google Scholar]
- Shibata N., Ichikawa T., Tojo M., Takahashi M., Ito N., Okubo Y., Suzuki S. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch Biochem Biophys. 1985 Dec;243(2):338–348. doi: 10.1016/0003-9861(85)90511-9. [DOI] [PubMed] [Google Scholar]
- Shibata N., Kobayashi H., Tojo M., Suzuki S. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100T. Arch Biochem Biophys. 1986 Dec;251(2):697–708. doi: 10.1016/0003-9861(86)90379-6. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Enzymatic release of germ tube-specific antigens from cell walls of Candida albicans. Infect Immun. 1985 Sep;49(3):609–614. doi: 10.1128/iai.49.3.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Ban Y., Suzuki S. Structure of the D-mannan of Candida stellatoidea IFO 1397 strain. Comparison with that of the phospho-D-mannan of Candida albicans NIH B-792 strain. Carbohydr Res. 1990 Jun 1;199(2):215–226. doi: 10.1016/0008-6215(90)84263-t. [DOI] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Kobayashi M., Mikami T., Suzuki M., Suzuki S. Preparation of monoclonal antibodies reactive with beta-1,2-linked oligomannosyl residues in the phosphomannan-protein complex of Candida albicans NIH B-792 strain. Clin Chem. 1988 Mar;34(3):539–543. [PubMed] [Google Scholar]
- Tomavo S., Dubremetz J. F., Schwarz R. T. A family of glycolipids from Toxoplasma gondii. Identification of candidate glycolipid precursor(s) for Toxoplasma gondii glycosylphosphatidylinositol membrane anchors. J Biol Chem. 1992 Jun 15;267(17):11721–11728. [PubMed] [Google Scholar]
- Trinel P. A., Faille C., Jacquinot P. M., Cailliez J. C., Poulain D. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect Immun. 1992 Sep;60(9):3845–3851. doi: 10.1128/iai.60.9.3845-3851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S. J. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990 Feb;70(2):241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- Varki A. Radioactive tracer techniques in the sequencing of glycoprotein oligosaccharides. FASEB J. 1991 Feb;5(2):226–235. doi: 10.1096/fasebj.5.2.2004668. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]