Abstract
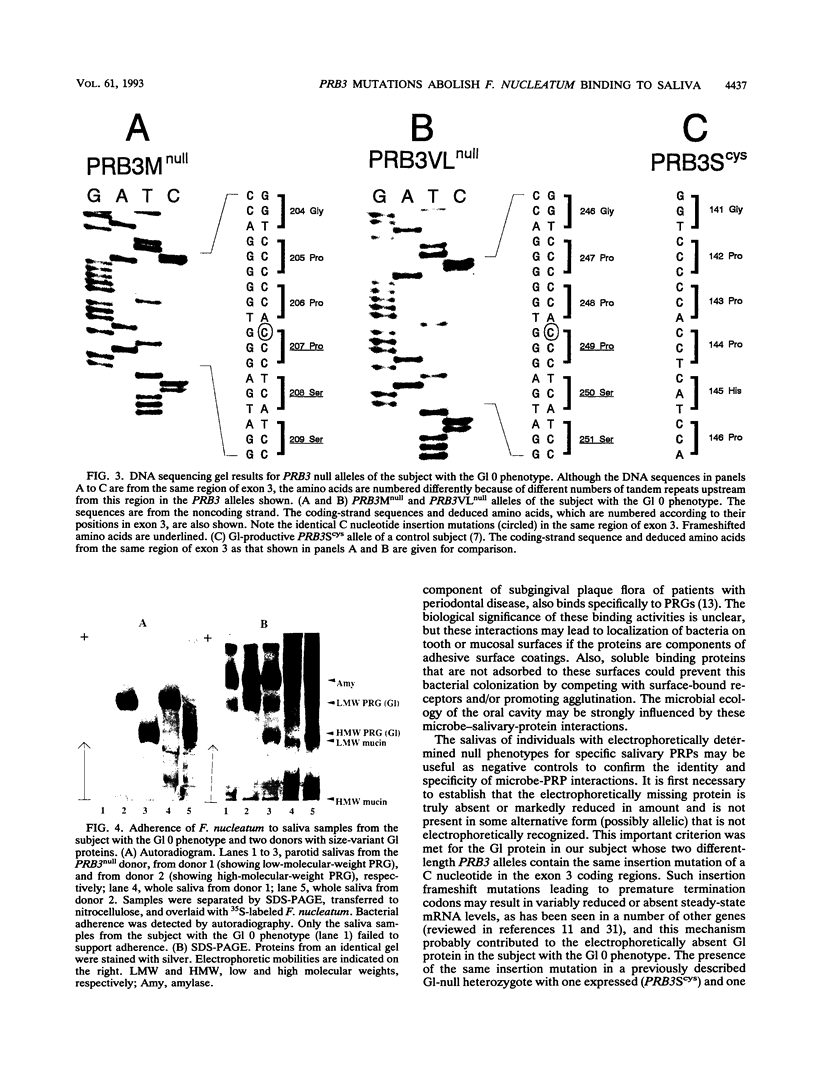
The glycosylated proline-rich glycoprotein (Gl or PRG), a product of the PRB3 gene, is a major constituent of human parotid saliva. Important functions proposed for Gl include acting as a bacterial receptor. The Gl proteins of several subjects were typed by two polyacrylamide gel electrophoresis (PAGE) systems: acid-lactate PAGE followed by staining with the periodic acid-Schiff reagent and sodium dodecyl sulfate-PAGE followed by electrophoretic transfer and staining with amido black or concanavalin A. The results showed one subject who apparently lacked Gl. The four exons, including splice junctions, for both PRB3 alleles of this subject were completely sequenced. Unexpressed (null) mutations were detected with an identical C nucleotide insertion in the same coding region of exon 3 of both alleles. This C nucleotide insertion leads to a frameshift with a premature termination codon that probably results in markedly reduced or absent PRB3 gene expression. We then used a nitrocellulose blot overlay assay to assay the bacterial receptor activity of parotid saliva from the PRB3null subject. No interactions with Fusobacterium nucleatum, shown previously to interact selectively with Gl, were detected. Together, these results suggest that this subject does not express the PRB3 gene and that one of the consequences is an altered ability to interact with a bacterium known to colonize the oral cavity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azen E. A., Denniston C. Genetic polymorphism of PIF (parotid isoelectric focusing variant) proteins with linkage to the PPP (parotid proline-rich protein) gene complex. Biochem Genet. 1981 Jun;19(5-6):475–485. doi: 10.1007/BF00484620. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Denniston C. Polymorphism of Ps (parotid size variant) and detection of a protein (PmS) related to the Pm (parotid middle band) system with genetic linkage of Ps and Pm to Gl, Db, and Pr genetic determinants. Biochem Genet. 1980 Jun;18(5-6):483–501. doi: 10.1007/BF00484396. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Hurley C. K., Denniston C. Genetic polymorphism of the major parotid salivary glycoprotein (Gl) with linkage to the genes for Pr, Db, and Pa. Biochem Genet. 1979 Apr;17(3-4):257–279. doi: 10.1007/BF00498967. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Latreille P., Niece R. L. PRBI gene variants coding for length and null polymorphisms among human salivary Ps, PmF, PmS, and Pe proline-rich proteins (PRPs). Am J Hum Genet. 1993 Jul;53(1):264–278. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., Maeda N. Molecular genetics of human salivary proteins and their polymorphisms. Adv Hum Genet. 1988;17:141–199. doi: 10.1007/978-1-4613-0987-1_5. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Minaguchi K., Latreille P., Kim H. S. Alleles at the PRB3 locus coding for a disulfide-bonded human salivary proline-rich glycoprotein (Gl 8) and a null in an Ashkenazi Jew. Am J Hum Genet. 1990 Oct;47(4):686–697. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., O'Connell P., Kim H. S. PRB2/1 fusion gene: a product of unequal and homologous crossing-over between proline-rich protein (PRP) genes PRB1 and PRB2. Am J Hum Genet. 1992 Apr;50(4):842–851. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., Yu P. L. Genetic polymorphism of CON 1 and CON 2 salivary proteins detected by immunologic and concanavalin A reactions on nitrocellulose with linkage of CON 1 and CON 2 genes to the SPC (salivary protein gene complex). Biochem Genet. 1984 Feb;22(1-2):1–19. doi: 10.1007/BF00499283. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Yu P. L. Genetic polymorphisms of Pe and Po salivary proteins with probable linkage of their genes to the salivary protein gene complex (SPC). Biochem Genet. 1984 Dec;22(11-12):1065–1080. doi: 10.1007/BF00499632. [DOI] [PubMed] [Google Scholar]
- Azen E., Lyons K. M., McGonigal T., Barrett N. L., Clements L. S., Maeda N., Vanin E. F., Carlson D. M., Smithies O. Clones from the human gene complex coding for salivary proline-rich proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5561–5565. doi: 10.1073/pnas.81.17.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece-Castro B. L., Prakobphol A., Burlingame A. L., Leffler H., Fisher S. J. Structure and bacterial receptor activity of a human salivary proline-rich glycoprotein. J Biol Chem. 1991 Sep 15;266(26):17358–17368. [PubMed] [Google Scholar]
- Ikemoto S., Minaguchi K., Tomita K., Suzuki K. A variant protein in human parotid saliva detected by SDS polyacrylamide gel electrophoresis and its inheritance. Ann Hum Genet. 1979 Jul;43(1):11–14. doi: 10.1111/j.1469-1809.1979.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Lyons K. M., Saitoh E., Azen E. A., Smithies O., Maeda N. The structure and evolution of the human salivary proline-rich protein gene family. Mamm Genome. 1993;4(1):3–14. doi: 10.1007/BF00364656. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O., Maeda N. A physical map of the human salivary proline-rich protein gene cluster covers over 700 kbp of DNA. Genomics. 1990 Feb;6(2):260–267. doi: 10.1016/0888-7543(90)90565-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Weill J. C., Ellison S. A. The isolation and analysis of a glycoprotein from parotid saliva. Biochim Biophys Acta. 1969 Aug 12;188(1):165–167. doi: 10.1016/0005-2795(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Azen E. A., Goodman P. A., Smithies O. Many protein products from a few loci: assignment of human salivary proline-rich proteins to specific loci. Genetics. 1988 Sep;120(1):255–265. doi: 10.1093/genetics/120.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K. M., Stein J. H., Smithies O. Length polymorphisms in human proline-rich protein genes generated by intragenic unequal crossing over. Genetics. 1988 Sep;120(1):267–278. doi: 10.1093/genetics/120.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Inheritance of the human salivary proline-rich proteins: a reinterpretation in terms of six loci forming two subfamilies. Biochem Genet. 1985 Jun;23(5-6):455–464. doi: 10.1007/BF00499086. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Minaguchi K., Bennick A. Genetics of human salivary proteins. J Dent Res. 1989 Jan;68(1):2–15. doi: 10.1177/00220345890680010201. [DOI] [PubMed] [Google Scholar]
- Minaguchi K., Takaesu Y., Tsutsumi T., Suzuki K. Studies of genetic markers in human saliva. (VII). Frequencies of the major parotid salivary glycoprotein (GI) system in a Japanese population. Bull Tokyo Dent Coll. 1981 Feb;22(1):1–6. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Prakobphol A., Lee T., Hoover C. I., Fisher S. J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992 Jan;60(1):31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobphol A., Murray P. A., Fisher S. J. Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Jul;164(1):5–11. doi: 10.1016/0003-2697(87)90359-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M., Minaguchi K., Lim K. A., Hashimoto M., Suzuki K. Salivary proline-rich protein polymorphisms in Chinese, Malays and Indians in Singapore. Hum Hered. 1990;40(2):89–98. doi: 10.1159/000153912. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle B. E. Phage lambda and plasmid expression vectors with multiple cloning sites and lacZ alpha-complementation. Gene. 1986;45(1):95–99. doi: 10.1016/0378-1119(86)90136-8. [DOI] [PubMed] [Google Scholar]