Abstract
Over the last decade, the development of in vitro, human, three-dimensional (3D) tissue models, known as human skin equivalents (HSEs), has furthered understanding of epidermal cell biology and provided novel experimental systems. Signaling pathways that mediate the linkage between growth and differentiation function optimally when cells are spatially organized to display the architectural features seen in vivo, but are uncoupled and lost in two-dimensional culture systems. HSEs consist of a stratified squamous epithelium grown at an air-liquid interface on a collagen matrix populated with dermal fibroblasts. These 3D tissues demonstrate in vivo–like epithelial differentiation and morphology, and rates of cell division, similar to those found in human skin. This unit describes fabrication of HSEs, allowing the generation of human tissues that mimic the morphology, differentiation, and growth of human skin, as well as disease processes of cancer and wound re-epithelialization, providing powerful new tools for the study of diseases in humans.
Keywords: organotypic culture, three-dimensional model, human skin, wound repair, intraepithelial neoplasia
INTRODUCTION
The development of human, three-dimensional (3D) models for advancing insights into the biology of skin keratinocytes requires the ability to construct tissues that mimic their in vivo counterparts. The linkage that exists in tissues between growth and differentiation is optimal only when cells are spatially organized to display the architectural features seen in vivo; it is lost in two-dimensional culture systems (Bissell and Radisky, 2001). Over the last decade, the development of tissue-engineered models that mimic human skin, known as human skin equivalents (HSEs) or organotypic cultures, have provided novel experimental systems for studying epidermal biology (Kolodka et al., 1998; Andriani et al., 2003). This unit describes methods for constructing 3D models of human epidermis that mimic the architectural features and behavior of normal human skin and the changes that occur during early skin cancer progression and wound re-epithelialization. This unit describes the construction of a multilayered epithelium that is composed of skin keratinocytes and grown upon a collagen substrate populated with dermal fibroblasts. When these protocols are used, keratinocytes differentiate upon exposure to an air-liquid interface to enable the tissue to recapitulate the morphologic and biochemical processes of human epidermis.
This unit describes the fabrication of normal skin and includes modifications to these protocols that will create novel environments that mimic disease states in cutaneous tissues. Basic Protocol 1 describes the fabrication of normal HSEs using primary keratinocytes, which allows the generation of tissues that mimic the morphology, differentiation, and growth of human epidermis. An Alternate Protocol provides a tissue model that mimics the early stages of skin cancer progression; it incorporates keratinocytes from both a normal and a squamous cell carcinoma cell line. Basic Protocol 2 describes a skin-like tissue model for cutaneous re-epithelialization, in which 3D tissues are wounded and keratinocytes close the wound defect to re-establish epithelial integrity. A Support Protocol addresses alternative substrates for fabrication of HSEs.
While the methods described below are for the fabrication of HSEs using skin keratinocytes, they can be adapted to grow tissues from other stratified epithelia, e.g., the oral and cervical mucosa, esophageal lining, laryngeal epithelium, and conjunctiva. It is suggested that efforts be made to optimize the growth of these cells in monolayer culture before incorporating them into HSEs.
NOTE: All solutions and equipment coming into contact with cells must be sterile, and proper aseptic technique should be used accordingly.
NOTE: All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
CONSTRUCTION OF A THREE-DIMENSIONAL MODEL OF NORMAL HUMAN SKIN
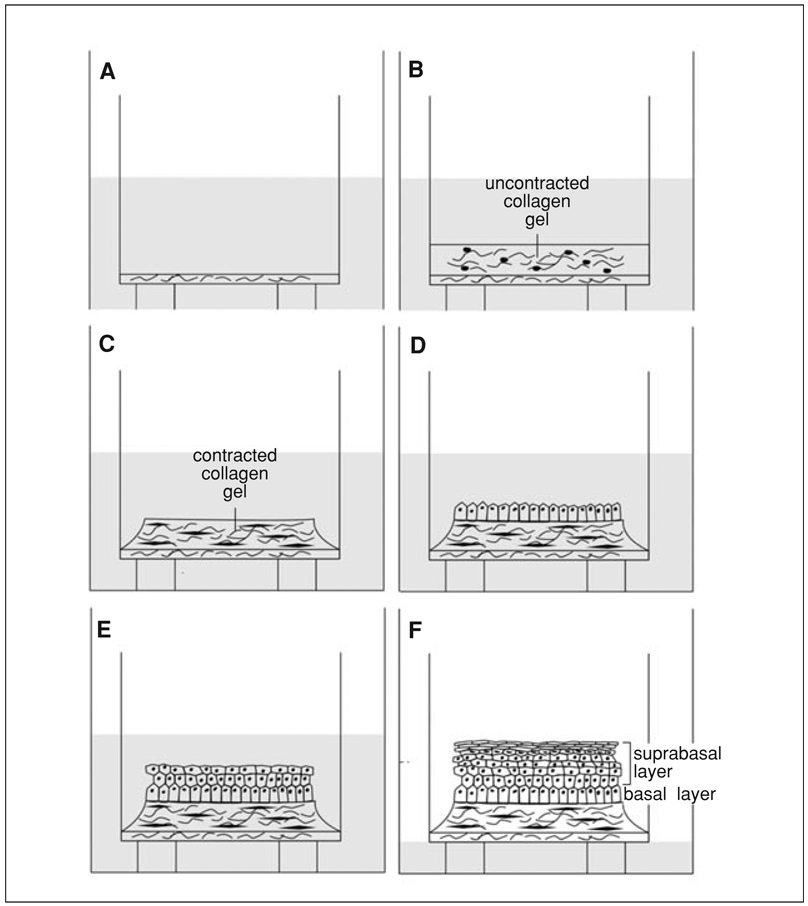
The 3D tissue model of normal skin consists of a stratified epithelium harboring differentiated keratinocytes that are grown on a contracted collagen matrix populated with dermal fibroblasts. As seen in the schematic figure describing HSE assembly (Fig. 19.9.1), normal tissues are constructed through the following sequential steps: (1) A thin, acellular layer of collagen (acellular collagen layer) is constructed first and acts as an attachment substrate for the cellular collagen that is fabricated above it. This thin layer prevents the cellular collagen from contracting from the insert membrane and detaching from it. (2) A collagen matrix with human dermal fibroblasts (cellular collagen layer) is constructed and allowed to contract for 7 days while submerged in medium. Fibroblasts mixed within the collagen gel remodel the matrix upon production of extracellular matrix (ECM) proteins and cause contraction of the gel. (3) After contraction is complete and the matrix is stabilized, keratinocytes are added to the surface of the matrix and allowed to attach to this substrate to generate a confluent cellular monolayer that will initiate tissue stratification (epithelialization). (4) Tissues are raised to an air-liquid interface to enable complete stratification, as well as full morphological and biochemical differentiation (tissue stratification, organization, and differentiation).
Figure 19.9.1.
Schematic of three-dimensional tissue construction. (A) A thin, acellular layer of collagen is first constructed; it provides an attachment substrate for the cellular collagen. (B) A collagen gel embedded with human dermal fibroblasts is layered onto the acellular layer. (C) While submerged in medium for 7 days, dermal fibroblasts remodel the collagen matrix, causing it to contract away from the walls of the insert. The contracted collagen forms a plateau. (D) Keratinocytes are then added to the center of the plateau of contracted collagen and allowed to attach to the collagen (or intervening substrate such as AlloDerm) to create a monolayer that will form the basal layer of the tissue. (E) Tissues are raised to an air-liquid interface to initiate stratification. Keratinocytes stratify and differentiate and form a suprabasal layer that mimics in vivo skin both morphologically and biochemically. (F) Further exposure to the air-liquid interface and additional feedings with cornification medium results in an increase in the thickness of the spinous and cornified layers of the tissue.
Materials
-
Human foreskin fibroblasts
Fibroblast culture medium (see recipe)
Acellular and cellular collagen matrices (see recipe)
Feeder layer of mitotically inactivated mouse 3T3 fibroblasts (e.g., see Rheinwald and Green, 1975)
Human neonatal foreskin keratinocytes
Keratinocyte culture medium (see recipe)
Trypsin/EDTA (see recipe)
EDTA/PBS (see recipe)
PBS (phosphate-buffered saline; Invitrogen, cat. no. 14190; also see APPENDIX 2A)
Human skin equivalent (HSE) culture media (see Table 19.9.1) including:-
Epidermalization medium IEpidermalization medium IICornification medium
10% (v/v) formalin
2 M sucrose
Embedding medium: Tissue-Tek optimal cutting temperature (OCT) medium (Ted Pella)
Liquid nitrogen
-
-
Pipets, chilled 15 min at −20°C before use
10-cm2 cell culture plates
6-well tissue culture plate with 3-µm porous polycarbonate membrane inserts (Organogenesis, cat. no. MS-10-3-5)
15-ml centrifuge tubes
Centrifuge capable of 500–1000 × g
Scalpel
Tissue processing cassette
Aluminum foil
~2-cm bottle or cap (to use as a mold form)
Narrow-tip forceps
Metal rack (for embedded tissue)
Styrofoam box
Additional reagents and equipment for trypsinizing (UNIT 1.1) and counting (UNIT 1.1) cells
Table 19.9.1.
Supplements for HSE Organotypic Mediaa
| Ingredient | Stock concentration |
Final concentration |
Epidermalization I (ml) |
Epidermalization II (ml) |
Cornification (ml) |
|---|---|---|---|---|---|
| O1O mediumb | 363 | 363 | 237 | ||
| Ham’s F12 supplements | 120 | 120 | 237 | ||
| l-glutamine | 200 mM | 4 mM | 10 | 10 | 10 |
| Adenineb | 18 mM | 40 µM | 1 | 1 | 1 |
| Hydrocortisoneb | 500× | 1 µM | 1 | 1 | 1 |
| Triiodothyronine (T3)b | 500× | 20 nM | 1 | 1 | 1 |
| Transferrinc | 5 mg/ml | 10 µg/ml | 1 | 1 | 1 |
| Insulinb | 5 mg/ml | 10 µg/ml | 1 | 1 | 1 |
| Progesteroneb | 2 µM | 2 nM | 0.5 | 0.5 | 0 |
| PESc | 500× | 1× | 1 | 1 | 1 |
| Calcium chloride | 0.5 M | 1.8 mM | 0 | 1.8 | 1.8 |
| FBSd | 0.5 | 0.5 | 10 |
Media construction for epidermalization I, epidermalization II, and cornification media, including stock concentrations, final concentration, and volume of each supplement for a 500 ml total volume.
See recipe.
Biosource.
Fabricate cellularized collagen
-
1.
Seed ~1 × 106 human foreskin fibroblasts (HFFs) in 10 ml fibroblast culture medium to a standard 10-cm2 cell culture dish so that they are almost confluent 1 day before incorporation into the collagen. Change the medium every 2 days.
After the fibroblasts have been passaged once from the frozen state, they will reach confluency in ~3 days.
-
2.
The day before incorporation, passage fibroblasts at a 9:10 split ratio, i.e., resuspend the trypsinized cells (see UNIT 1.1) in 10 ml fibroblast culture medium, and add 9 ml of the cell suspension to a new plate.
Passaging cells from a confluent plate ensures a higher fraction of actively dividing cells upon incorporation into collagen gels.
-
3.
On the day of incorporation, prepare the acellular collagen mixture on ice. Use chilled pipets (15 min at −20°C) to prevent warming of collagen when it is mixed. Avoid creating air bubbles when mixing.
Preparing the mixture on ice prevents premature gelation.
Collagen should be a straw-yellow to light pink color to ensure optimal gelation. If the color is bright yellow, add a single drop of sodium bicarbonate and triturate until a straw-yellow color is seen.
-
4.
Add 1 ml acellular collagen matrix to each 6-well tissue culture plate insert. Ensure that the gel coats the entire bottom surface of the insert and allow it to gel 20 min at room temperature. Do not move the plate while the mixture is undergoing gelation.
The color will turn pink when the collagen has fully gelled.
-
5.
Trypsinize (UNIT 1.1) with trypsin/EDTA, count viable cells in an aliquot (UNIT 1.1), and resuspend the fibroblasts to a final concentration of 3 × 105 cells/ml.
A total of 5 × 105 fibroblasts will be used per 6-well plate.
-
6.
Prepare the cellular collagen mixture on ice, adding the reagents in the order listed so that the cells (added last) will not be damaged by the acidic pH of the unneutralized collagen.
It is important to neutralize the collagen so that the cells will not be damaged by the acid pH that exists before neutralization.
-
7.
Add the fibroblasts to the mixture last, gently triturate the cellular matrix to evenly incorporate fibroblasts into the collagen matrix, and add 3 ml into each insert on top of the gelled acellular collagen matrix. Gently transfer the mixture to the incubator for 30 min.
-
8.
When the cellular matrix has turned pink and is completely gelled (usually less than 30 min), feed the gels with 12 ml of fibroblast culture medium by adding 10 ml of medium to the well around the insert and 2 ml of medium directly onto the insert.
-
9.
Incubate the matrix 5 to 7 days to allow complete gel contraction.
During the first few days, the sides of the gel contract and will form a plateau in the center. Gels are stable from 5 to 10 days after initial construction.
Add keratinocytes
-
10.
Aspirate off the medium from a feeder layer of mitotically inactivated mouse 3T3 fibroblasts and seed the feeder layer with 1 × 106 keratinocytes in 10 ml keratinocyte culture medium. Grow keratinocytes to no more than 50% confluence to minimize the number of differentiated cells seeded onto the collagen matrix.
We typically expand 3T3 feeder cells (J2 line preferred in our lab) in ~20 T-125 flasks, which yield ~1 × 108 cells. It is important when expanding 3T3 cells to not let them lose their “stellate” morphology before irradiating; passage at ~70% confluency during expansion is optimal.
Trypsinized suspensions of the cells should be lethally irradiated. The irradiation level is determined by the user to provide cells that will still attach and survive for ~1 week, but will not continue to divide.
Frozen, irradiated cells last ~3 months at −80°C, or indefinitely in liquid nitrogen. Each batch should be tested to verify support of normal human keratinocyte growth before large 3D experiments are started.
After the keratinocytes have been passaged once from the frozen state, they will reach confluency in ~3 days. One change of medium may be necessary.
Alternatively, keratinocytes can be grown in monolayer culture in a low-calcium, serum-free medium (see Critical Parameters).
-
11.
Remove the 3T3 feeder cells from the culture by incubating the dish in 5 ml EDTA/PBS for 5 min at 37°C. Displace the 3T3 cells by gentle pipetting so that keratinocytes will remain attached.
It is important not to allow the cultures to incubate for an excessive time in EDTA/PBS, as the keratinocytes may detach from the dish as well.
-
12.
As soon as the 3T3 cells have begun to detach, replace the EDTA/PBS with 5 ml PBS, gently rinse the plate three times with 5 ml PBS until all 3T3 cells have been completely removed, then remove the PBS, leaving only keratinocyte colonies attached to the dish.
-
13.
Trypsinize (see UNIT 1.1) the keratinocytes with 2 ml trypsin/EDTA for 5 min at 37°C to obtain a single-cell suspension. Transfer the detached cells into a 15-ml tube containing 9 ml keratinocyte culture medium (to neutralize the trypsin) and count viable cells in an aliquot (UNIT 1.1).
-
14.
Dispense the desired number of cells into a 15-ml tube. Centrifuge the cells 5 min at 1000 × g, room temperature, and resuspend in a volume of epidermalization I medium so that a total of 5 × 105 keratinocytes can be used per insert in 50 µl.
-
15.
Remove all fibroblast culture medium from the plates with the contracted collagen 20 min before seeding keratinocytes so that keratinocytes can be seeded onto a moist collagen gel.
Keratinocytes should be seeded directly onto the contracted collagen gels in an aliquot of 50 µl containing 5 × 105 cells. To modify the nature of the substrate on which keratinocytes are seeded, de-epidermalized dermis or coated polycarbonate inserts can be applied directly on top of the contracted collagen gels at this point (see the Support Protocol for specialized substrates).
-
16.
Carefully add 50 µl of the cell suspension to the center of the contracted collagen gel (or onto the center of the intervening substrate placed on the collagen gel). Do not move the plate for 15 min, to allow the keratinocytes to attach. Then incubate 30 to 60 min at 37°C (without any additional medium) to allow the keratinocytes to fully adhere.
-
17.
Add 12 ml of epidermalization I medium to each insert, adding 10 ml to the bottom of the well and 2 ml gently into the insert on top of the keratinocytes. Incubate at 37°C.
-
18.Feed each culture with medium every 2 days with appropriate HSE culture media as follows:
- Day 4: Add 12 ml epidermalization II medium.
-
Day 6 until termination of the experiment: Add 7 ml cornification medium to the bottom of the well so that the insert just contacts the medium every two days. Aspirate medium from the inside of the insert so that tissues can be grown at the air-liquid interface.The morphological appearance of tissues at various stages of their development is shown in Figure 19.9.2.
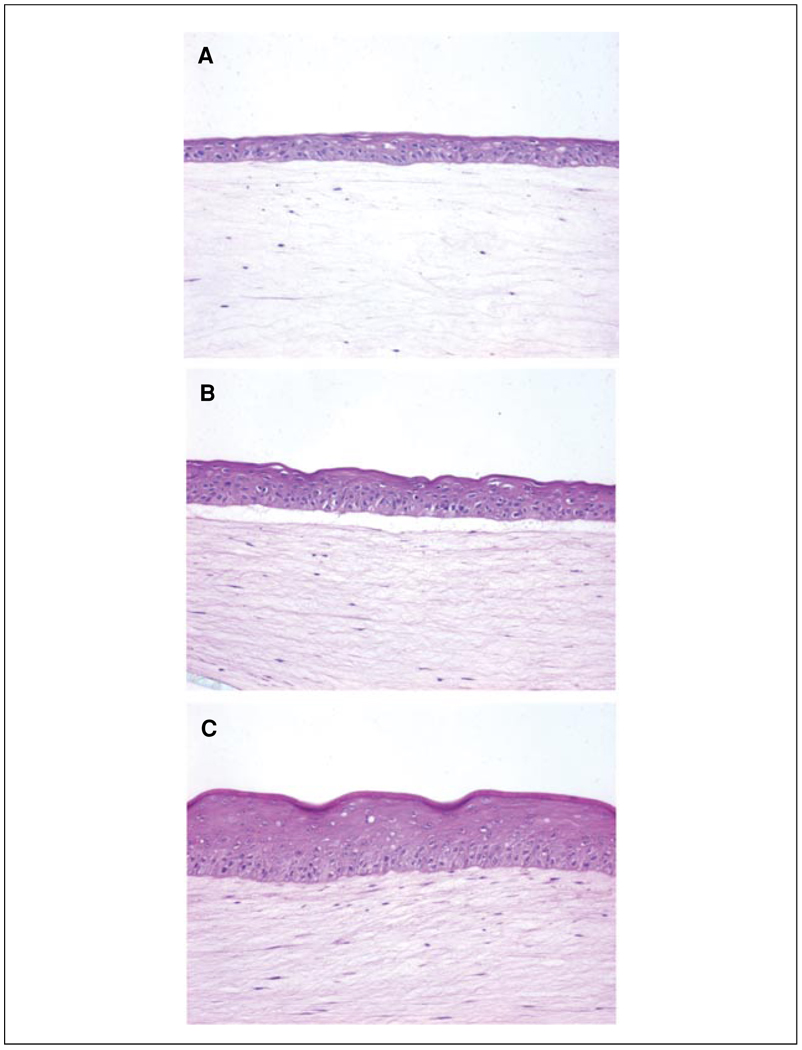
Figure 19.9.2.
Morphologic development of human skin equivalents. Keratinocytes were grown directly on collagen gels for 4 days (A), 6 days (B), and 10 days (C). (A) Early epithelial development, evidenced by a thin epithelium, is seen while tissues are still submerged in epidermalization II medium. (B) The epithelium demonstrates a greater degree of tissue architecture and organization, demonstrated by the presence of cuboidal basal cells, after the tissue is exposed to the air-liquid interface for 2 days. (C) Full morphologic differentiation and stratification are seen after cells are exposed to the air-liquid interface for 6 days. Note that the clear space between the epidermis and dermis in panels A and B likely represents separation along the epithelium-stromal interface due to incomplete organization of the basement membrane at early time points of tissue development. A more mature tissue (C) has a more well developed basement membrane and is more resistant to separation during tissue processing.
Harvest tissues for analysis
-
19.
Remove the medium from the inserts and gently rinse tissues twice in 5 ml PBS.
-
20.
Cut away the insert membrane from the plastic insert at its base using a scalpel, and bisect the culture.
To fix the tissue
-
21a.
Place one half of the culture in a tissue processing cassette and immediately immerse in 10% formalin for 1 hr.
Tissues are very thin and thus only require a short fixation (1 hr).
-
22a.
Proceed with tissue processing and paraffin embedding by sequential dehydration in graded ethanols and xylene, using standard techniques.
To preserve the tissue by freezing
-
21b.
Place the other half of the tissue in a 2 M sucrose solution. Soak the tissue in the sucrose solution at least 1 hr, but not more than 24 hr, at 4°C.
Sucrose replaces water in the hydrated collagen gel and protects against freezing damage during embedding and processing.
-
22b.
Make a small mold with aluminum foil, using the cap or bottom of a small bottle (roughly 2 cm in diameter) as a form. Fill three-quarters of the mold with embedding medium.
-
23b.
Gently remove the tissue from the sucrose using narrow-tip forceps, making sure to keep the tissue on the nylon insert membrane. Gently touch the membrane side of the tissue to a Kimwipe to remove excess sucrose solution.
-
24b.
Place the tissue in the embedding medium and allow the tissue to soak 20 to 30 min at room temperature.
-
25b.
Place a metal rack inside a Styrofoam box and fill it with liquid nitrogen to a height just under the top of the metal rack. Place the aluminum foil mold on top of the rack and stand the tissue inside to an upright position.
The liquid nitrogen vapors will freeze the embedding medium and tissue in about 5 min. The tissue can then be stored for decades at −80°C.
INCULDING SPECIALIZED SUBSTRATES FOR GROWTH OF HUMAN SKIN EQUIVALENTS ON BASEMENT MEMBRANE OR EXTRACELLULAR MATRIX PROTEINS
Specific applications may necessitate growing 3D tissues on basement membrane or defined extracellular matrix proteins using either de-epidermalized dermis or a coated polycarbonate membrane, respectively.
Materials
-
AlloDerm (de-epidermalized basement membrane; LifeCell, cat. no. 102-009)
PBS (phosphate-buffered saline; Invitrogen, cat. no. 14190; also see APPENDIX 2A)
6-well cell culture PET membrane inserts coated with the appropriate material:-
Collagen I (Becton-Dickinson, cat. no. 354442)Laminin I (Becton-Dickinson, cat. no. 354446)Fibrillar Collagen I (Becton-Dickinson, cat. no 354472)Fibronectin/collagen I (Becton-Dickinson, cat. no 354633)Fibronectin (Becton-Dickinson, cat. no 354440)Collagen IV (Becton-Dickinson, cat. no 354544)
Serum-free, low-glucose Dulbecco’s modified Eagle medium (DMEM; Invitrogen)
-
-
1.4-cm stainless steel dermatological punch (Delasco, cat. no. KP-14)
Scalpel
To make de-epidermalized dermis substrate
-
1a.
Cut the de-epidermalized dermis (AlloDerm) with a dermatological punch to fit the diameter of the contracted collagen gel.
-
2a.
Rehydrate the substrate in PBS 1 hr at 37°C before use.
To make coated insert substrates
-
1b.
Cut the coated membrane from the inserts with a dematological punch.
-
2b.
Rehydrate the coated membranes in serum-free DMEM for 1 hr at 37°C.
Add membrane or coated insert to collagen
-
3.
Layer the de-epidermalized membrane or the coated membrane from the insert onto the contracted collagen gel 20 min before adding keratinocytes in step 15 of Basic Protocol 1.
FABRICATION OF THREE-DIMENSIONAL MODEL OF HUMAN SKIN CANCER
The tissue microenvironment is defined by the complex network of intercellular interactions that are mediated by physical attachment (as in direct cell-cell or cell-extracellular matrix interactions) and by biochemical signals mediated by soluble molecules (Hagios et al., 1998). Cell adhesion is essential for the assembly of cells into 3D tissues and is required to maintain normalization of tissue architecture and homeostasis. In this way, cells receive adhesive cues that control their polarity, proliferation, differentiation, and survival. In recent years, evidence has accumulated that cancer is a disease of altered tissue architecture and that neoplastic progression is a consequence of abnormal interactions between tumor cells and their tissue microenvironment. As a result, it is essential to study the impact of the tissue microenvironment on cancer progression in human tissues that incorporate 3D tissue context so that they faithfully mimic their in vivo counterparts. Monolayer, 2D culture systems do not generate the spatially organized, 3D structures that are seen in vivo and have been of limited use in studying complex cellular responses. These inherent limitations have driven the construction of 3D tissue models that now provide novel experimental paradigms for studying cancer progression in biologically relevant tissues.
In one example of such tissue fabrication, it is now possible to construct 3D models of cutaneous, human squamous cell carcinoma that replicate multiple stages of its progression. To recapitulate the precancerous stages of progression of skin cancer, 3D tissue models must be constructed to reflect alterations in cell-cell interactions that could propel the tissue towards a neoplastic fate (Margulis et al., 2005). This has been accomplished by developing tissue models in which the fate of potentially malignant cells can be mapped by mixing normal keratinocytes with genetically-marked (with β-galactosidase) tumor cells at varying ratios to mimic precancerous conditions. We have previously found that interactions between genetically marked tumor cells and adjacent normal cells lead to tissue normalization and elimination of tumor cells when the two cell types are mixed in a ratio greater than 1:1 (normal cells/tumor cells) (Javaherian et al., 1998). Thus potentially malignant cells enter a quiescent state known as intraepithelial dormancy, in which the neoplastic potential of the tissue is conditionally suppressed and not realized. These studies have used 3D tissue models to directly implicate tissue architecture mediated by cell-cell interactions as a dominant regulator of the neoplastic phenotype.
Carcinoma cell lines can be grown as pure cultures at an air-liquid interface to simulate a carcinoma in situ, or they can be grown as mixtures with normal keratinocytes to mimic an earlier stage of epithelial dysplasia. Previous studies with different SCC cell lines indicate that tumor cells may either be maintained as individual cells within the tissue (Javaherian et al., 1998), or they may undergo intraepithelial expansion (Vaccariello et al., 1999). These tissues can be constructed by following the steps in Basic Protocol 1 for construction of normal skin equivalents and including the modifications presented below.
Additional Materials (also see Basic Protocol 1)
Cancer cell line of interest
Prepare cellularized collagen as described in Basic Protocol 1, steps 1 to 9.
Prepare a single cell suspension of normal keratinocytes as described in Basic Protocol 1, steps 10 to 13.
Culture the desired cancer cell line in a 10-cm2 tissue culture dish in medium appropriate to the cell line.
Prepare a single cell suspension by incubating the cells with trypsin/EDTA for 5 min. at 37°C. Transfer the detached cells to a 15-ml tube and count viable cells in an aliquot (UNIT 1.1).
Dispense the desired number of normal keratinocytes and cancer cells into separate 15-ml tubes. Centrifuge the cells 5 min at 2000 × g, room temperature.
Resuspend each cell pellet in an appropriate volume of epidermalization I medium at 5 × 105 cells/50 µl.
Mix cancer cells with normal keratinocytes in the desired ratio, and seed a total of 5 × 105 combined cells/50 µl for each insert.
Proceed with Basic Protocol 1, starting at step 15, to culture tissues at the air-liquid interface and harvest for analysis.
FABRICATION OF THREE-DIMENSIONAL WOUND HEALING MODEL OF HUMAN SKIN
In vitro studies of wound re-epithelialization have often been limited by their inability to simulate wound repair as it occurs in humans. For example, wound models using skin explants or monolayer, submerged keratinocyte cultures demonstrate limited stratification, partial differentiation, and hyperproliferative growth culture systems have been helpful in studying keratinocyte migration in response to wounding. However, they have been of limited use in studying the complex nature of keratinocyte response during wound repair, as these cultures do not provide the proper tissue architecture to study the in vivo wound response.
We have previously found that HSEs adapted to study wound repair in human epithelium simulate the chronology of events that occur during re-epithelialization in human skin, and we have advanced the understanding of the healing of wounds in human skin and other stratified epithelia (Garlick and Taichman, 1994b; Garlick et al., 1996). This tissue model has allowed direct determination of the key response parameters of wounded epithelium including cell proliferation, migration, differentiation, growth-factor response, and protease expression. The application of tissue engineering technology demonstrates the utility of HSEs in studying phenotypic responses that are characteristic of the switch from a normal to a regenerative epithelial tissue during wound re-epithelialization.
This protocol describes a method for determining the response of HSEs that mimics re-epithelialization of wounded human skin, from the initiation of keratinocyte activation until restoration of epithelial integrity. Using these protocols, HSEs are fabricated as described in Basic Protocol 1. They are wounded 7 days after keratinocytes are seeded onto the contracted collagen gel. One week before wounding these tissues, an additional collagen gel, using both acellular and cellular collagen, is fabricated; this gel serves as a substrate to which the wounded tissue will be transferred. This method requires sequencing the experiment so that preparation of the additional collagen gel will be initiated one week in advance of wounding (see Fig. 19.9.3), i.e., at approximately the same time that the keratinocytes are seeded onto the first collagen matrix.
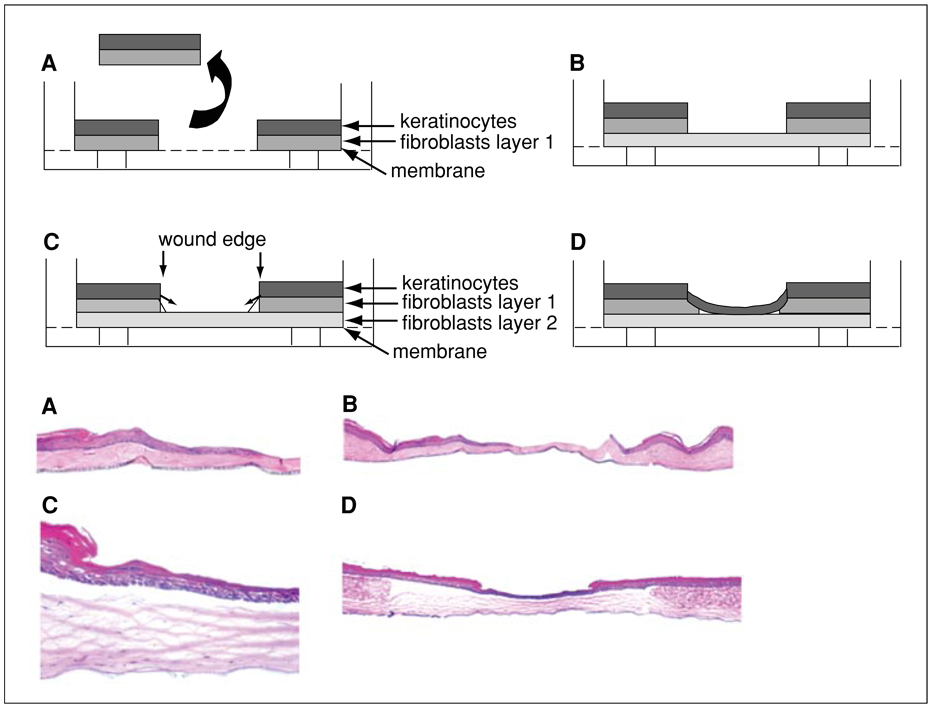
Figure 19.9.3.
Three-dimensional tissue model of wound re-epithelialization: schematic (upper panels) and photomicrographs (lower panels; magnification: 5×). (A) A wound is generated through the full thickness of a human skin equivalent (HSE) and the excised tissue is removed. (B) The wounded tissue is placed on a second, contracted collagen gel. (C) Keratinocytes undergo migration to close the wound gap. (D) Keratinocytes have restored epithelial integrity, have closed the wound gap, and undergo stratification.
Materials
-
Human skin equivalent (HSE; see Basic Protocol 1, steps 1–18) with keratinocyte cultures at day 7
Contracted collagen gel (prepared 1 week in advance of the wounding; Basic Protocol 1 steps 1–9)
Cornification medium (see Table 19.9.1)
10-cm2 sterile tissue culture dish
1.4-cm stainless steel dermatological punch (Delasco, cat. no. KP-14), optional
Forceps
Dental mirror
Scalpel with #22 blade, sterile
Wound the tissue
-
1.
Aspirate all medium from the HSE culture after 7 to 10 days of culture.
-
2.
Remove the insert from the well and place it upside down in a sterile dish.
-
3.
Using a scalpel, cut away the insert membrane and place the culture in a 10-cm2 sterile dish right-side up.
-
4.
Trim the culture with the scalpel by cutting around the raised, mesa-like region to remove the part of the collagen gel not covered by keratinocytes.
This will facilitate the removal and transfer of the wounded tissue from the membrane.
-
5.
Wound the cultures with either an incisional or excisional wound.
An incisional wound can be generated by incising tissues with a scalpel in a way that will allow the wound edges to be separated to generate an elliptical wound.
An excisional wound can be generated using an elliptical dermatological punch that completely penetrates the center of the tissue through the epidermis, collagen, and membrane. The excised tissue can be fixed and preserved for H&E staining in 10% formalin.
Transfer tissue to new gel
-
6.
Use forceps to gently lift the edge of the wounded tissue by separating the collagen gel from the membrane. Drag the tissue onto a dental mirror while leaving the membrane behind.
The transfer may be easier if the mirror is moistened with medium.
-
7.
Unfold any wrinkles in the culture by gently moving the tissue back and forth on the mirror using the forceps. Once the culture is smooth, pull one side of the culture slightly over the edge of the mirror.
-
8.
Move the mirror directly over the second contracted collagen matrix so that the edge of the mirror and wounded tissue are in contact with the matrix. Slide the tissue onto the second collagen gel by teasing it gently with a closed forceps as the mirror is slowly pulled away, leaving the culture on the contracted collagen gel.
-
9.
Using the forceps, tease apart the tissue wounded by incision to create an elliptical space that should be 2 to 3 mm at its greatest width. Smooth the tissue with the forceps to ensure that it is completely free of any folds or wrinkles.
-
10.
Maintain the tissue at an air/liquid interface by adding 8 ml of cornification medium beneath the insert, and change the medium every 2 days until the end of the experiment.
Histologically analyze wound repair
-
11.
For histological analysis, process the tissue as described in Basic Protocol 1, steps 19 to 25, making sure to bisect the tissue perpendicular to the long axis of the wound (i.e., along the greatest width of the wound). Mount the tissue for sectioning en face so that the greatest width of the wound is sectioned first.
It is essential to capture the first two sections as these will be the most informative sections of the wound.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Acellular and cellular collagen matrices
-
10× MEM (minimum essential medium with Earle’s salts; Cambrex, cat. no. 12-684F)
200 mM l-glutamine (Invitrogen, cat. 25030-081)
Fetal bovine serum (FBS; Hyclone, cat. SH30071)
187 µl 71.2 mg/ml NaHCO3 (Cambrex, cat. no. 17-605E)
Bovine type I collagen (Organogenesis, cat. no. 200-055)
3 × 105 fibroblasts/ml (see Basic Protocol 1)
NOTE: Keep all components on ice and mix in the order listed to avoid alteration in pH that will result in inability of collagen to gel.
See Table 19.9.2 for volumes to use for acellular and cellular matrix construction. Prepare fresh and use immediately. Do not store the final matrix.
Table 19.9.2.
Construction of Collagen Matrix
| Componenta | Acellular collagen matrixb | Cellular collagen matrixb |
|---|---|---|
| 10× MEM | 0.6 ml | 1.8 ml |
| l-glutamine | 54 µl | 162.5 µl |
| FBS | 0.68 ml | 2.02 ml |
| Sodium bicarbonate | 187 µl | 0.56 ml |
| Collagen | 5 ml | 15 ml |
| Fibroblasts | – | 1.65 ml |
See recipe for acellular and cellular collagen matrix components for more information about component solutions and the fibroblast suspension.
Component volumes are listed for the construction of acellular collagen and cellular collagen matrices. Volumes are for six inserts. Use 1 ml per insert for acellular collagen matrix and 3 ml per insert for cellular collagen matrix.
Adenine, 18 mM
Dissolve 0.972 g adenine (MP Biomedicals, cat. no. 100190) in 2.4 ml 4 N NaOH. Bring to 400 ml with water. Store up to 1 year at −20°C.
Cholera toxin, 10−7 M
Dissolve cholera toxin (Sigma, cat. no. C-8052) at 9 ng/ml in water and store up to 1 year at −20°C.
EDTA/PBS
50 ml of 5 mM EDTA: dilute 5 ml of 0.5 M EDTA, pH 8.0 (Invitrogen, cat. no. 15575) to 500 ml with PBS (Invitrogen, cat. no. 14190; also see APPENDIX 2A); store up to 1 year at room temperature
EGF, 10 µg/ml
Dissolve human recombinant epidermal growth factor (EGF; Austral Biological) at 10 µg/ml in 0.1% (v/v) bovine serum albumin (BSA; tissue culture tested); store up to 1 year at −20°C.
Fibroblast culture medium
-
500 ml serum-free, low-glucose Dulbecco’s modified Eagle medium (DMEM; Invitrogen)
55.6 ml fetal bovine serum (FBS; Hyclone, cat. no. SH30071; 10% final)
5.6 ml 800 mM HEPES (see recipe; 8 mM final)
3.4 ml 100× penicillin/streptomycin (Invitrogen)
Store up to 2 weeks at 4°C
HEPES, 800 mM
Dissolve 47.24 g HEPES (Sigma) in 250 ml H2O. Store up to 1 year at −20°C.
Hydrocortisone, 0.25 mg/ml (500×)
-
Dissolve 0.0538 g hydrocortisone (Sigma) in 200 ml H2O. Store up to 1 year at −20°C.
The concentration in this solution is 0.55 mM.
Insulin, 5 mg/ml
Dissolve 50 mg insulin (Sigma) in 10 ml of 0.005N HCl. Store up to 1 year at −20°C.
Keratinocyte culture medium
-
338 ml serum-free, low-glucose Dulbecco’s modified Eagle medium (DMEM; Invitrogen)
112 ml F12 nutrient mixture (Ham), containing l-glutamine (Invitrogen)
25 ml fetal bovine serum (FBS; Hyclone, cat. no. SH30071; 5% final)
5 ml 18 mM adenine (see recipe; 0.18 mM final)
3.4 ml 100× penicillin/streptomycin (Invitrogen)
5 ml 800 mM HEPES (see recipe; 8 mM final)
1 ml 0.25 mg/ml hydrocortisone (see recipe; 0.5 µg/ml final)
0.5 ml 10−7 M cholera toxin (see recipe; 10−10 M final)
0.5 ml 10 µg/ml EGF (see recipe; 10 ng/ml final)
0.5 ml 5 mg/ml insulin (see recipe; 5 µg/ml final)
Store up to 2 weeks at 4°C
CAUTION:Cholera toxin is very toxic. Use appropriate precautions when handling stock solutions.
O1O Medium
-
43 g DME powder, containing no glucose and no CaCl2 (JRH Biosciences, special order)
5 liters H2O
0.5 g MgSO4
18.5 g NaHCO3
Store up to 6 months at 4°C
Progesterone, 2 µM
Dissolve 1 mg progesterone (Sigma, cat. no. P-8783) in 1 ml absolute ethanol and then add 14.7 ml H2O. Dilute 1 ml of this solution in 100 ml serum-free, low glucose Dulbecco’s modified Eagle medium (DMEM; Invitrogen). Store up to 1 year at −20°C
Triiodothyronine (T3), 500×
-
1 ml 3,3′,5-triiodo-l-thyronine sodium salt (Sigma, T-5516)
99 ml H2O
Store up to 6 months at 4°C
Trypsin/EDTA
-
50 ml of 0.1% (v/v) trypsin: prepared by diluting 50 ml 0.25% trypsin (Invitrogen, cat. no. 15050) with 75 ml PBS (Invitrogen, cat. no. 14190; also see APPENDIX 2A); store up to 1 year at −20°C
50 ml of 5 mM EDTA: prepared by diluting ultrapure 0.5 M EDTA, pH 8.0 (Invitrogen, cat. no. 15575) with PBS; store up to 6 months at room temperature
Store up to 1 year at −20°C
COMMENTARY
Background Information
A biologically relevant 3D human tissue model of epidermis must faithfully recreate the morphological and biochemical features of the in vivo tissue. Upon fabrication, HSEs become spatially organized as they undergo growth and differentiation, due to stromal-epidermal communication (see Fig. 19.9.2). This provides numerous advantages in the study of epidermal biology when compared to 2D monolayer culture systems (Carlson et al., 2007), as components of the model can be altered and tissue outcomes monitored in order to investigate morphological and molecular responses. The ability to manipulate either the cells or the tissue microenvironment provides opportunities to investigate cancer progression and injury response in human skin. For example, soluble factors (e.g., growth factors) can be added to the medium to directly observe HSE response. A pulse of bromodeoyuridine (BrdU) can be added to the medium to enable analysis of the proliferation rates of the cells in 3D tissues.
The protocols described above enable tissue growth on a variety of ECM substrates. Substrates can be selected to answer specific questions about the impact of different environments and how they affect tissue development. De-epidermalized dermis (AlloDerm, LifeCell) provides an interface on which the assembly of basement membrane is optimized, while HSEs grown on a contracted collagen gel do not assemble structured basement membrane (Andriani et al., 2004). Tissues grown on polycarbonate membranes coated with specific ECM proteins can be used to study how specific ECM or basement membrane (BM) proteins affect tissue growth and architecture (Segal et al., 2008).
Observations from early studies performed in monolayer 2D culture systems revealed that cell-cell contact between normal cells and adjacent tumorigenic cells could suppress the transformed phenotype. However, the mechanistic basis for these observations remained unclear due to a lack of human tissue models that could more fully mimic the biologically meaningful pathways that couple the cell-cell adhesion and in vivo phenotype, and more accurately represent these early events in tumorigenesis as they occur. Three-dimensional tissue models have now been designed to reflect the incipient stages of spontaneous tumors in human epithelial tissues, where potentially neoplastic cells are found in the context of normal, cellular neighbors during the premalignant stage of epithelial cancer development.
Three-dimensional tissue models can be adapted to understand the intraepithelial dynamic that occurs in precancerous legions. The fate of potentially malignant cells can be monitored in 3D tissues by mixing normal keratinocytes with genetically marked (with β-glactosidase) tumor cells at varying ratios. These models can reflect the alterations in cell-cell interactions that could propel the tissue towards a neoplastic fate. Using this approach, we had previously found that interactions between genetically marked tumor cells and adjacent normal cells led to normalization and elimination of tumor cells. Thus, potentially malignant cells entered a quiescent state known as intraepithelial dormancy, in which the neoplastic potential of the tissue was conditionally suppressed and not realized. This dormant state can be overcome by altering tissue dynamics in response to the tumor promoter TPA (Karen et al., 1999), irradiating with UV light (Mudgil et al., 2003), decreasing adhesive interactions between tumor cells and adjacent epithelia, or enabling tumor cells to interact with basement membrane proteins (Andriani et al., 2004). Both TPA and UV have different effects on normal versus premalignant cells. For example, premalignant cells are resistant to UV-induced apoptosis and undergo selective expansion in the presence of TPA, leading to intraepithelial expansion of premalignant cells. Thus, the opportunities created through the use of 3D tissue models have directly implicated tissue architecture, mediated by cell-cell interactions, as a dominant regulator of the neoplastic phenotype since maintenance of normal tissue architecture can constrain cancer progression at a premalignant stage.
Critical Parameters
The proliferative potential of keratinocytes is a critical factor in the successful fabrication of HSEs with normalized tissue architecture. Most keratinocytes seeded onto HSE cultures will adhere to the connective tissue substrate, but only replicating cells will grow after seeding. Keratinocytes that have undergone terminal differentiation while in submerged culture will also attach to the substrate, but will not undergo further proliferation to become well stratified HSEs. In order to generate tissues with normalized tissue architecture, it is critical that keratinocytes seeded to generate HSEs have an elevated growth potential. Nearly all keratinocytes seeded in HSEs will adhere to their connective tissue substrate, but only replicating cells will grow.
It is, therefore, important to grow keratinocytes so that a high growth fraction is present in the monolayer cultures at the time of passage to HSEs. This can be accomplished by growing keratinocytes as small colonies at high clonal density in submerged cultures on 3T3 feeder layers so that terminal differentiation will be minimized and the fraction of replicating cells will be maximized. Alternatively, keratinocytes can be grown in low calcium medium before passage to HSEs, using defined medium conditions without 3T3 fibroblasts.
Keratinocyte strains can be tested in HSEs to determine those that will provide the best growth and morphologic differentiation. This may also be accomplished by testing the clonogenic growth of keratinocyte strains in monolayer cultures on feeder layers in order to determine the growth potential of these cells. We have observed variations in growth and morphology of HSEs when different keratinocyte and fibroblast strains are incorporated. Therefore, keratinocyte strains should be screened by constructing HSEs to identify strains that can achieve optimal morphologic differentiation and tissue architecture.
An advantage of studying keratinocyte phenotype in HSEs lies in the ability to control and modify the cellular milieu in which these tissues are grown. For example, it is possible to add soluble factors directly to these cultures to determine the phenotypic response to such environmental conditions. In addition, growth conditions can be modified by culturing tissues in the absence of fibroblasts by eliminating them after contraction of the collagen gel (Andriani et al., 2003). Furthermore, control of the milieu facilitates analysis of these tissues, as it is possible to directly determine proliferation indices by adding a pulse of BrdU directly to the medium.
The protocols described in this unit provide techniques that allow growth of epithelial tissues on a variety of connective tissue substrates, each of which can be tailored to answer experimental questions. The interface of tissues grown on the de-epidermalized dermis promotes the rapid assembly of structured basement membrane and optimization of tissue morphology (Andriani et al., 2003). One source of such a dermal substrate is AlloDerm, a commercially available cadaveric dermis that is used in a variety of clinical applications to treat burns, periodontal disease, and surgical defects (LifeCell). Tissues grown directly on contracted collagen gels do not assemble intact basement membrane, but provide excellent support for keratinocyte growth and differentiation. Cultures grown on polycarbonate membranes coated with specific extracellular matrix proteins can be used to directly study the effect of these proteins on tissue architecture and phenotype of these tissues (Segal et al., 2008).
Troubleshooting
We have found some variability in the degree to which fibroblast strains support keratinocyte growth after their incorporation into collagen gels. It appears that fibroblast support of HSE organization and growth is directly related to the degree to which fibroblasts are able to contract the collagen gel. Fibroblast strains demonstrating more shrinkage of the collagen gel before adding keratinocytes are better able to support keratinocyte growth. This parameter can be used to screen fibroblast strains for optimal growth-support when planning initial experiments.
It is critical to preserve tissue architecture during processing of tissues after culture is complete. Since collagen gels are greatly hydrated, they can undergo significant artifactual damage during tissue processing. For this reason, tissues are soaked in 2 M sucrose for 2 hr at room temperature or overnight at 4°C in order to replace water in the tissue and prevent freezing damage. Furthermore, tissues need to be gradually frozen in liquid nitrogen vapor to prevent destruction of tissue architecture. Tissues should never be snap-frozen by immersing them in liquid nitrogen.
Fabrication of the collagen matrix requires that all components be kept on ice until the gel mixture is placed onto the insert. This will ensure that collagen will not prematurely precipitate from these solutions. Plastic pipets used for collagen should be chilled before use.
Anticipated Results
Several points regarding some subtleties of keratinocyte behavior in HSEs should be mentioned. The first concerns the length of time which cultures can be maintained at the air-liquid interface. In our experience, cultures can be kept at this interface for 7 to 14 days. After this time, the surface layers of the epithelium become very thick due to a failure to desquamate. As a result, lower layers of the epithelium become compressed and the longevity of cultures is limited. A second and related issue concerns the growth potential of keratinocytes in HSEs. While HSEs demonstrate a basal level of proliferation that is greater than human skin, it has been shown that these cultures have a tremendous potential for cell growth and are very responsive to external growth stimuli. For example, we have shown that disruption of HSEs upon wounding results in a 10-fold increase in basal cell proliferation (Garlick and Taichman, 1994a).
It should be kept in mind that although keratinocytes grown in HSEs share many morphologic and biochemical features with their in vivo skin and mucosal keratinocytes, there are differences in tissue phenotype. For example, integrin receptors (the αv integrins) not normally expressed in skin are constituitively expressed in keratinocytes grown in HSEs. In addition, HSEs are somewhat deficient in barrier function. Keratinocytes grown in HSEs can be thought of as tissues undergoing de novo development (e.g., newly re-epithelialized, healed wounds in vivo) wherein morphologic differentiation is complete, but cells are still in a somewhat activated state.
Time Considerations
Construction of HSEs requires ~3 to 4 weeks from the time HFF are seeded in monolayer culture until 3D tissues are fully mature. HFF cells should be confluent two days before construction of the collagen gels. At that time, cells should be passaged at a 9:10 ratio, to provide cells with a growth stimulus before incorporation into collagen gels. Complete contraction of the collagen gels requires 7 to 10 days, during which time the normal human keratinocytes cell cultures are initiated and expanded. Growth and differentiation of keratinocytes on the contracted collagen gels requires, sequentially, 2 days in epidermalization I medium, 2 days in epidermalization II medium, and 7 to 10 days in cornification medium to allow full tissue organization and stratification. Histological analysis takes 2 to 3 days.
Literature Cited
- Andriani F, Margulis A, Lin N, Griffey S, Garlick JA. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J. Invest. Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- Andriani F, Garfield J, Fusenig NE, Garlick JA. Basement membrane proteins promote progression of intraepithelial neoplasia in 3-dimensional models of human stratified epithelium. Int. J. Cancer. 2004;108:348–357. doi: 10.1002/ijc.11525. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer JID. 2001;1011241681:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MW, Iyer VR, Marcotte EM. Quantitative gene expression assessment identifies appropriate cell line models for individual cervical cancer pathways. BMC Genomics. 2007;8:117. doi: 10.1186/1471-2164-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB. Effect of TGF-beta 1 on re-epithelialization of human keratinocytes in vitro: An organotypic model. J. Invest. Dermatol. 1994a;103:554–559. doi: 10.1111/1523-1747.ep12396847. [DOI] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB. Fate of human keratinocytes during re-epithelialization in an organotypic culture model. Lab. Invest. 1994b;70:916–924. [PubMed] [Google Scholar]
- Garlick JA, Parks WC, Welgus HG, Taichman LB. Re-epithelialization of human oral keratinocytes in vitro. J. Dent. Res. 1996;75:912–918. doi: 10.1177/00220345960750030801. [DOI] [PubMed] [Google Scholar]
- Hagios C, Lochter A, Bissell MJ. Tissue architecture: The ultimate regulator of epithelial function? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998;353:857–870. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Vaccariello M, Fusenig NE, Garlick JA. Normal keratinocytes suppress early stages of neoplastic progression in stratified epithelium. Cancer Res. 1998;58:2200–2208. [PubMed] [Google Scholar]
- Karen J, Wang Y, Javaherian A, Vaccariello M, Fusenig NE, Garlick JA. 12-O-tetradecanoylphorbol-13-acetate induces clonal expansion of potentially malignant keratinocytes in a tissue model of early neoplastic progression. Cancer Res. 1999;59:474–481. [PubMed] [Google Scholar]
- Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: Long-term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A, Zhang W, Alt-Holland A, Crawford HC, Fusenig NE, Garlick JA. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res. 2005;65:1783–1791. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- Mudgil AV, Segal N, Andriani F, Wang Y, Fusenig NE, Garlick JA. Ultraviolet B irradiation induces expansion of intraepithelial tumor cells in a tissue model of early cancer progression. J. Invest. Dermatol. 2003;121:191–197. doi: 10.1046/j.1523-1747.2003.12320.x. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Segal N, Andriani F, Pfeiffer L, Kamath P, Lin N, Satyamurthy K, Egles C, Garlick J. The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 2008;27:163–170. doi: 10.1016/j.matbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccariello M, Javaherian A, Wang Y, Fusenig NE, Garlick JA. Cell interactions control the fate of malignant keratinocytes in an organotypic model of early neoplasia. J. Invest. Dermatol. 1999;113:384–391. doi: 10.1046/j.1523-1747.1999.00701.x. [DOI] [PubMed] [Google Scholar]