Abstract
The concept of selective receptor modulators has been established for the nuclear steroid hormone receptors. Such selective modulators have been used therapeutically with great success in the treatment of cancer. However, this concept has not been examined with regard to the aryl hydrocarbon receptor (AHR) because of the latent toxicity commonly associated with AHR activation. AHR-mediated toxicity is primarily derived from AHR binding to its dioxin response element (DRE) and driving expression of CYP1 family members, which have the capacity to metabolize procarcinogens to genotoxic carcinogens. Recent evidence using a non-DRE binding AHR mutant has established the DRE-independent suppression of inflammatory markers by the AHR. We wished to determine whether such DRE-independent repression with wild-type AHR could be dissociated from canonical DRE-dependent transactivation in a ligand-dependent manner and, in doing so, prove the concept of a selective AHR modulator (SAhRM). Here, we identify the selective estrogen receptor (ER) modulator Way-169916 as a dually selective modulator, binding both ER and AHR. Inflammatory gene expression associated with the cytokine-inducible acute-phase response (e.g., SAA1 and CRP) are diminished by Way-169916 in an AHR-dependent manner. Furthermore, activation of AHR by Way-169916 fails to stimulate canonical DRE-driven AHR-mediated CYP1A1 expression, thus eliminating the potential for AHR-mediated genotoxic stress. Such anti-inflammatory activity in the absence of DRE-mediated expression fulfills the major criteria of an SAhRM, which suggests that selective modulation of AHR is possible and renders the AHR a therapeutically viable drug target for the amelioration of inflammatory disease.
For many decades, the ascribed function of the aryl hydrocarbon receptor (AHR) has been that of a xenobiotic sensor, modulating gene expression, principally P450-detoxifying enzymes (e.g., CYP1A1) in response to environmental ligands (e.g., dioxin). Such contaminants are products of the industrial age and fail to account for the evolutionary persistence of the AHR. A paradigm shift has occurred regarding AHR function. Although the detoxification role of AHR is not in doubt, the development of Ahr-null and transgenic rodent models indicates physiological roles for AHR beyond that of xenobiotic metabolism. Reports provide evidence for the involvement of AHR in immune function from both toxicological and physiological perspectives. AHR ligands alter embryonic immune development and programming (Hogaboam et al., 2008); induce thymic atrophy in rodents through enhanced FasL-mediated apoptosis (Kamath et al., 1997; Camacho et al., 2005); diminish B-lymphopoiesis (Schneider et al., 2008) and the B-cell IgM response (North et al., 2009); and promote the polarization of TH1/2 cells, generating a TH1 bias (Negishi et al., 2005). Focus has turned to the role of the AHR in facilitating the differentiation of CD4+ lymphocytes into TH17 and TReg cells. The TH17 population is suboptimal in the presence of an AHR antagonist (Veldhoen et al., 2009) with the implication that endogenous AHR ligands stimulate TH17 commitment. Furthermore, Ahr knockout models exhibit attenuated TH17 differentiation, substantiating AHR involvement (Kimura et al., 2008). Despite the diversity of immunological effects prompted by the AHR, little is known regarding a mode of action, but it is likely to involve cross-talk mechanisms. Notwithstanding, the immunosuppressive activity exhibited by the AHR raises the question of whether the AHR represents a novel drug target for the treatment of inflammatory or autoimmune conditions.
An established in vivo model of multiple sclerosis, experimental acute encephalitis, has been shown to be ameliorated by the prototypical AHR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and yet be enhanced by FICZ, also an AHR ligand (Quintana et al., 2008; Veldhoen et al., 2008). In addition, nonobese diabetic mice predisposed to autoimmune diabetes fail to develop diabetes while exposed to TCDD (Kerkvliet et al., 2009). Both are suspected to involve the reprogramming of T cells. Although illustrating the involvement of AHR, the use of polycyclic hydrocarbon agonists is not therapeutically viable because of the perceived inherent toxicity associated with AHR activation. Indeed, the AHR has a dubious reputation as a mediator of various modes of toxicity, including the conversion of procarcinogens into genotoxic intermediates through enhanced phase I bioactivity (Sagredo et al., 2006). Deleterious effects result from ligand-activated AHR in combination with its dimerization partner ARNT binding to DREs within AHR target genes, thus facilitating the expression of downstream effectors. We hypothesize that a selective AHR ligand with the capacity to promote dissociation between the beneficial cross-talk modes of AHR action away from its DRE-dependent toxic activity could render the AHR a viable therapeutic target.
This hypothesis of ligand-dependent but DRE-independent AHR activity has been tested using a DNA binding AHR mutant, identifying numerous genes that were suppressed in response to ligand (Patel et al., 2009). Predominant among these were components of the inflammatory acute phase response (APR) [e.g., serum amyloid-associated (Saa1) and C-reactive protein (Crp)]. These targets emphasized the involvement of AHR in the modulation of inflammatory signaling and established a subset of targets to screen and validate potential selective AHR modulators (SAhRMs) (i.e., AHR ligands with the capacity to promote DNA-independent AHR activity at the expense of DRE-mediated expression). The concept of selective receptor modulators is well established for nuclear hormone receptors, especially within the context of the estrogen receptor, and has been used clinically in the treatment of hormone-sensitive cancer. The selective modulator concept has expanded to include other nuclear receptors; however, the idea and therapeutic viability of SAhRMs has not been expounded.
In line with its function as a xenobiotic sensor, the AHR is remarkably promiscuous, binding diverse ligands, including some ER ligands (e.g., resveratrol), which antagonize canonical AHR signaling (Casper et al., 1999). Like TCDD, resveratrol ameliorates experimental acute encephalitis by affecting T-cell function (Singh et al., 2007). It is interesting that the selective ER modulator (SERM) Way-169916 demonstrates anti-inflammatory properties in models of rheumatoid arthritis, suppressing the expression of the same APR targets identified with our DNA binding AHR mutant (Chadwick et al., 2005; Harnish et al., 2006), thus presenting a potential for Way-169916 to influence the DNA-independent repressive action of AHR. We investigated this hypothesis and present evidence that Way-169916 is an AHR ligand and represents the first report of a SAhRM with anti-inflammatory properties.
Materials and Methods
Materials.
TCDD was a generous gift provided by Dr. Stephen Safe (Texas A&M University, College Station, TX). Way-169916 was initially a kind gift from Dr. Robert Steffan (Wyeth Research, Princeton, NJ) and subsequently was synthesized in-house. Human and murine recombinant cytokines, interleukin-1β (IL1β) and IL6, were obtained commercially (PeproTech, Rocky Hill, NJ).
Chemical Synthesis.
Way-169916 was synthesized as described previously with minor modifications (Steffan et al., 2004). Comprehensive methods and synthesis scheme are detailed in the supplemental information. The structure and purity of Way-169916 were verified by electrospray ionization-mass spectrometry and NMR.
Cell Culture.
The human hepatoma Huh7 cell line was maintained in α-minimal essential medium (Sigma-Aldrich, St. Louis, MO) supplemented with 8% fetal bovine serum (HyClone Labs, Logan, UT), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). Cells were cultured at 37°C in a humidified atmosphere composed of 95% air and 5% CO2.
Electromobility Shift Assay.
DRE-specific electrophoretic mobility shift assays were performed using in vitro translated human AHR and ARNT proteins. Expression vectors for each protein were translated separately using the T7 TNT kit (Promega, Madison, WI), and reactions were modified to include 1.25 mM sodium molybdate to enhance AHR stability. Aliquots (4 μl) of AHR and ARNT were combined at a 1:1 M ratio in buffer containing 25 mM HEPES, 1 mM EDTA, 10 mM sodium molybdate, and 10% glycerol, pH 7.5, followed by the addition of 0.25 μl of ligand or vehicle, for a total reaction volume equaling 10 μl. Transformation reactions were incubated for 90 min at room temperature, followed by the addition of 15 μl of oligonucleotide binding buffer [42 mM HEPES, 0.33 M KCl, 50% glycerol, 16.7 mM dithiothreitol, 8.3 mM EDTA, 0.125 mg/ml CHAPS, and 42 ng/μl poly(dI:dC)]. After 15 min of incubation in binding buffer, ∼2 × 105 cpm of 32P-labeled DRE was added to each reaction. The samples were mixed with an appropriate amount of 5× loading dye and one half of each sample resolved on a 6% nondenaturing polyacrylamide gel (Novex; Invitrogen, Carlsbad, CA). DRE oligonucleotides (5′-GATCTGGCTCTTCTCACGCAACTCCG-3′ and 3′-ACCGAGAAGAGTGCGTTGAGGCCTAG-5′) were a gift from Dr. M.S. Denison (University of California, Davis, CA.).
Competitive AHR Ligand Binding Assay.
Competitive ligand binding assays were performed as described previously (Flaveny et al., 2009). In brief, the AHR photoaffinity ligand 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin (PAL) was synthesized as described previously (Poland et al., 1986). Hepatic cytosol extracts were isolated from B6.Cg-Ahrtm3.1 Bra Tg (Alb-cre, Ttr-AHR)1GHP “humanized” AHR mice (Flaveny et al., 2009) by homogenization in MENG (25 mM MOPS, 2 mM EDTA, 0.02% NaN3, and 10% glycerol, pH 7.4) containing 20 mM sodium molybdate and protease inhibitor cocktail (Sigma), followed by centrifugation at 100,000g for 1 h. All binding experiments were conducted in the dark until photo-cross-linking of the PAL had occurred. A saturating amount of PAL (0.21 pmol; i.e., 8 × 105 cpm/tube) was added to 150 μg of cytosolic protein. Samples were then coincubated with Way-169916 for 20 min at room temperature. Samples were photolyzed (402 nm, 8 cm, 4 min), and 1% dextran-coated charcoal was added, followed by centrifugation at 3000g for 10 min to remove unbound PAL. Labeled samples were resolved on 8% Tricine-polyacrylamide gels, transferred to polyvinylidene difluoride membrane, and visualized by autoradiography. Radioactive AHR bands were excised and quantified by γ-counting.
Competitive ER Ligand Binding Polarization Assay.
Competitive ER ligand binding assays were performed using the PanVera Estrogen Receptor-α Competitor Assay (Invitrogen) according to the manufacturer's directions. In brief, vehicle (DMSO) or test compounds were diluted to twice their final assay concentrations in PanVera-supplied ES2 screening buffer and added to 60 × 150-mm borosilicate glass cell culture tubes. A master mix was made of screening buffer with human recombinant ERα added for a final concentration of 6 pmol/μl, and ES2 fluoromone was added for a final concentration of 400 nM. The master mix was added to diluted test compounds in a 1:1 volume, mixed gently, and incubated in the dark at room temperature for 2 h. Samples were then measured for fluorescence polarization using the PanVera Beacon 2000 polarization reader with 485 nm excitation and 530 nm emission filters at 25°C.
Short-Interfering RNA-Mediated Knockdown.
Huh7 cells were seeded into six-well plates and cultured overnight in serum- and antibiotic-free media. Cells were transfected using the Geneporter 3000 reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's directions. siRNA oligonucleotides (Dharmacon RNA technologies, Lafayette, CO) were transfected at final concentration of 50 nM. Cells were cultured for a further 24 h before treatment, as indicated. RNA and protein were harvested as detailed.
Protein Isolation and Expression Analysis.
Total protein was isolated from Huh7 cells by lysis with MENG/1% Nonidet P-40/1× protease inhibitor cocktail. Protein concentrations were assayed using the BCA kit (Thermo Fisher Scientific). Protein samples were resolved on 8% Tricine-SDS-polyacrylamide gel electrophoresis gels and subsequently transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were probed as indicated with the following antibodies: rabbit anti-AHR Rpt1 (Thermo Fisher Scientific), mouse anti-ARNT (Thermo Fisher Scientific), rabbit anti-ERα sc-543 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-ERβ sc-8974 (Santa Cruz), and mouse anti-β-actin (Santa Cruz).
RNA Isolation and Reverse Transcription.
Total RNA was isolated from Huh7 cells cultured in six-well plates using TRIzol (Invitrogen). RNA concentration was determined via spectrophotometry at λ 260 and 280 nm. Total RNA was reverse-transcribed to cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA).
Real-Time Quantitative PCR.
Quantitative PCR was performed on a DNA Engine Opticon system using DyNAmo SYBR Green reagent (New England Biolabs, Ipswich, MA) according to the manufacturer's instructions. Nucleotide sequences of primers (Integrated DNA Technologies, Coralville, IA) used in this study are as published previously (Patel et al., 2009). In all cases, melting point analysis revealed amplification of a single amplicon. Data acquisition and analyses were achieved using MyIQ software (Bio-Rad Laboratories, Hercules, CA).
Statistical Analysis.
Data were analyzed using the GraphPad Prism 5.0 software package (GraphPad Software Inc., San Diego, CA). One-way analysis of variance with Tukey-Kramer multiple comparison post-test and Student's t test were applied to determine statistical significance, and P < 0.05 was deemed significant and is indicated with an asterisk.
Results
Way-169916 Induces AHR/ARNT Dimerization and Weakly Stimulates DRE Binding through Direct Interaction with AHR.
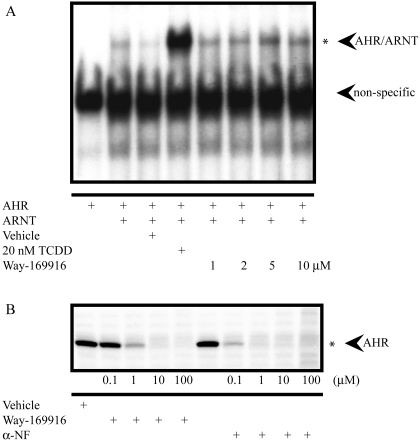
While investigating the mutual cross-talk between AHR and ER, we obtained data suggesting that the nonsteroidal SERM Way-169916 (Fig. 1B) may also bind to the human AHR. To confirm this hypothesis, we examined the potential of Way-169916 to mimic the characteristics of an AHR agonist (i.e., to promote the dimerization and subsequent binding of AHR/ARNT to the DRE containing CYP1A1 enhancer). Electromobility shift analyses using in vitro-translated AHR identified the formation of an AHR/ARNT/DRE complex after exposure to increasing (1, 2, 5, and 10 μM) concentrations of Way-169916. However, the level of complex formation elicited by Way-169916, even at the highest dose examined, was significantly less than that observed with 20 nM TCDD, the prototypical AHR agonist (Fig. 2A). To further substantiate the status of Way-169916 as a putative AHR ligand, we performed competitive in vitro ligand binding assays using a high-affinity photoreactive AHR ligand and human AHR expressing Huh7 hepatoma cytosol. Competition for photoaffinity ligand binding was not detected with vehicle alone but was observed with increasing concentrations of α-naphthoflavone (αNF), an established AHR ligand with a displacement of ∼80% achieved at 0.1 μM. Likewise, increasing concentrations of Way-169916 were able to effectively compete with the photoaffinity ligand for AHR binding with ∼15% displacement at 0.1 μM, ∼50% at 1 μM, and ∼65% at 100 μM (Fig. 2B). Such data confirm the status of Way-169916 as a ligand for the human AHR.
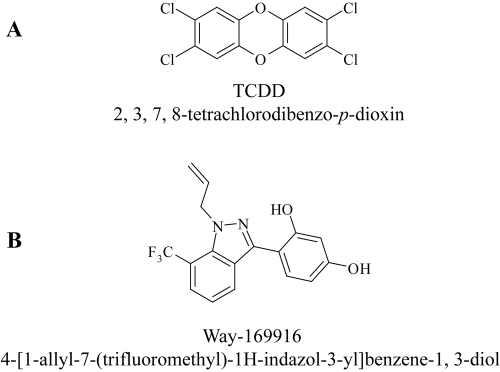
Fig. 1.
Chemical structures of AHR ligands. The chemical structures of the prototypical AHR agonist TCDD (A) is illustrated together with the putative selective AHR modulator (SAhRM) Way-169916 (B).
Fig. 2.
Way-169916 induces AHR/ARNT dimerization and weakly promotes DNA binding through direct interaction with AHR. A, gel-shift analysis of AHR/ARNT DNA binding in the presence of Way-169916. In vitro-translated AHR and ARNT were incubated with a 32P-labeled DNA probe encompassing the Cyp1a1 enhancer element. Binding reactions were coincubated with vehicle, TCDD, or increasing concentrations of Way-169916, as indicated. Specific binding of transformed AHR/ARNT to the probe is indicated with an asterisk. B, AHR ligand binding analysis. Competitive in vitro ligand binding assays were performed using human AHR containing Huh7 cytosol. Cytosolic extracts were incubated with vehicle, increasing concentrations (0.1, 1, 10, and 100 μM) of Way-169916 or αNF in the presence of the photoreactive AHR ligand 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin, as indicated.
The AHR Ligand Way-169916 Fails to Stimulate Xenobiotic Gene Expression.
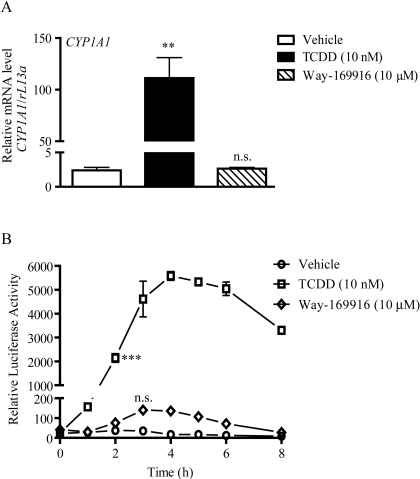
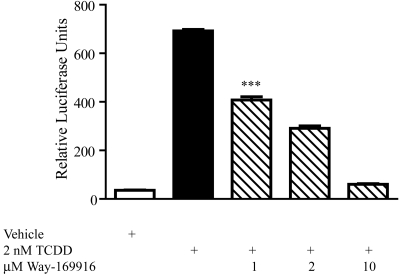
Having established Way-169916 as a ligand for AHR, we wished to examine the potential of Way-169916 to drive AHR-dependent gene expression. Exposure of Huh7 cells to 10 nM TCDD for 5 h resulted in an expected robust (>100-fold) increase in CYP1A1 mRNA level over vehicle-treated controls, as assessed by quantitative PCR (Fig. 3A). In contrast, Way-169916 failed to elicit a significant induction of CYP1A1 mRNA above that of vehicle-treated control over the same time frame. Likewise, the AHR-responsive target genes CYP1A1 and CYP1A2, although expressed in response to TCDD, were refractory to induction by Way-169916 in MCF-7 cells, revealing that failure to induce AHR-responsive genes is neither gene- nor cell-type-specific (Supplemental Fig. 2). Further examination using HepG2 cells stably integrated with a CYP1A1 enhancer-luciferase reporter construct revealed that the failure of Way-169916 to induce AHR-dependent gene expression was not associated with dose or temporal issues (Fig. 3B). Although a modest increase in reporter activity was observed with Way-169916 at 3 h, this proved not to be statistically significant, suggesting very weak agonist activity. We also examined the capacity of Way-169916 to antagonize TCDD-mediated expression of an AHR-dependent reporter construct. HepG2 (40/6) cells harboring the DRE-driven pGudluc 6.1 reporter were exposed to vehicle, TCDD, or in combination with increasing concentrations of Way-169916, and reporter expression was assayed. Exposure to TCDD prompted a robust induction of reporter activity, which was significantly repressed upon coexposure to Way-169916 (Fig. 4). These data in conjunction with our ligand-binding data indicate that Way-169916 is an extremely weak agonist with the capacity to be an effective competitive antagonist with regard to canonical DRE-dependent AHR transactivation.
Fig. 3.
Way-169916 is a weak partial agonist for AHR. A, Huh7 cells were treated with either vehicle (DMSO), 10 nM TCDD, or 10 μM Way-169916 as indicated for 4 h. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human CYP1A1 expression. Data represent the mean CYP1A1 mRNA level normalized against human rL13A mRNA. B, HepG2 40/6 cells were treated with either vehicle (DMSO), 10 nM TCDD, or 10 μM Way-169916. At the indicated time points, cells were lysed, and luciferase activity was determined. Data represent mean luciferase activity ± S.E.M. Significant induction is indicated by asterisks, n.s., not significant.
Fig. 4.
Way-169916 competes with AHR agonists to antagonize DRE-mediated expression. HepG2 (40/6) cells harboring the stably integrated AHR-dependent pGudluc 6.1 reporter construct were treated with either vehicle (DMSO), 2 nM TCDD, or TCDD, together with increasing concentrations of Way-169916, as indicated for 5 h. Cells were lysed, and luciferase activity was determined. Data represent mean luciferase activity ± S.E.M.
Way-169916 Stimulates DRE-Independent AHR Activity to Repress APR Gene Expression.
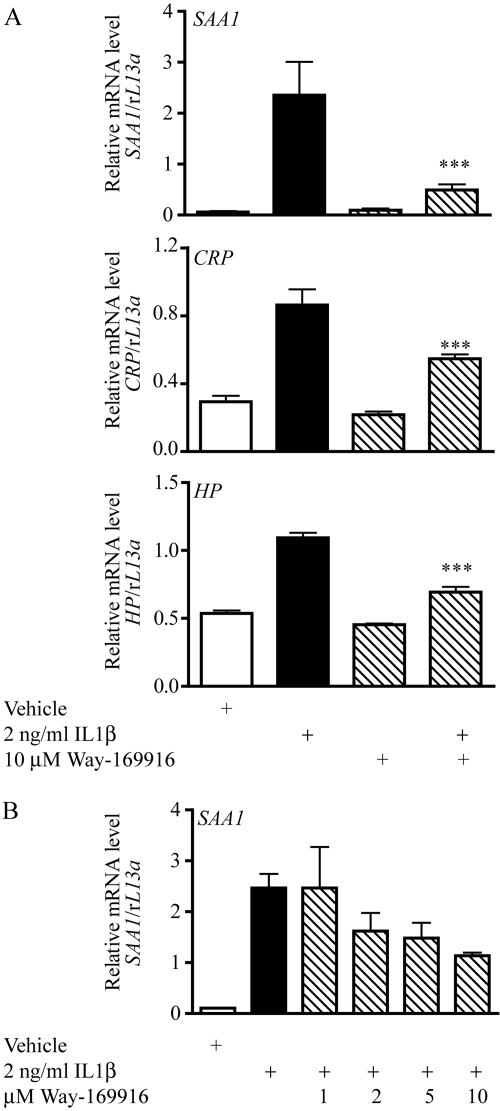
Evidence exists indicating the capacity of AHR to influence gene expression through the phenomenon of receptor cross-talk. Recent data obtained using a non-DRE binding AHR mutant has identified cytokine-regulated components of the inflammatory APR as being negatively regulated by AHR in the absence of cognate response element interaction (Patel et al., 2009). We thus assessed the potential of Way-169916 to modulate non-DRE-dependent gene expression using APR targets as a model. Exposure of Huh7 cells to IL1β elicited the expression of the APR genes serum amyloid-associated-1 (SAA1), C-reactive protein (CRP), and haptoglobin (HP), as determined by quantitative PCR (Figs. 5A). Pretreatment with AHR ligands αNF, βNF, B[a]P, M50354, or TCDD before IL1β exposure has been shown to result in a significant attenuation of acute phase gene expression (Patel et al., 2009). Treatment with 10 μM Way-169916 also resulted in significant repression of SAA1, CRP, and HP mRNA after IL1β induction (Fig. 5). Furthermore, exposure of Huh7 cells to increasing concentrations of Way-169916 identified a dose-dependent suppression of cytokine-mediated APR expression (Fig. 5B).
Fig. 5.
Way-169916 suppresses cytokine-mediated expression of acute phase reaction components. A, Huh7 cells were pretreated for 1 h with vehicle (DMSO) or 10 μM Way-169916 and then were incubated for a further 4 h while exposed to 2 ng/ml IL1β. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human SAA1, CRP, and HP expression. Data represent mean SAA1, CRP, and HP mRNA level normalized against human rL13A mRNA. B, dose-response analysis of Way-169916-mediated SAA1 repression. Huh7 cells were pretreated for 1 h with vehicle (DMSO) or increasing concentrations of Way-169916 and then were incubated for a further 4 h while exposed to 2 ng/ml IL1β. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human SAA1 expression. Data represent mean SAA1 mRNA level normalized against human rL13A mRNA.
Attenuation of APR Gene Expression by Way-169916 Is Independent of ER.
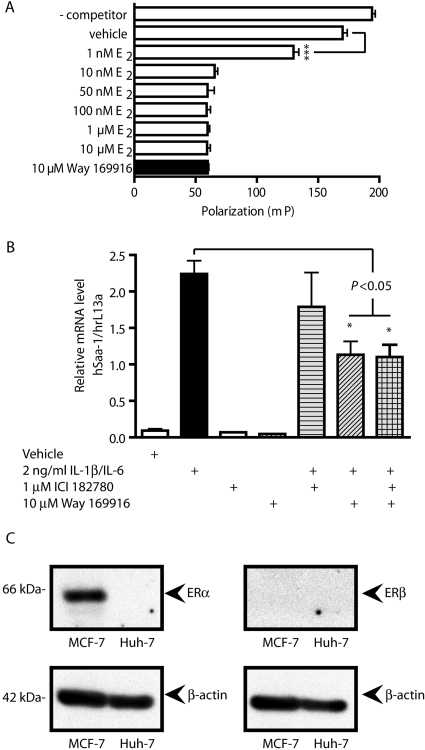
Way-169916 was originally developed as a SERM and has been shown to repress gene expression in rodent models of inflammation by a mechanism involving the estrogen receptor (Chadwick et al., 2005). Using a fluorescent polarization competition assay, we were able to confirm the status of Way-169916 as a ligand for ER (Fig. 6A). Thus, despite being a ligand for AHR, our data illustrating the attenuation of APR gene expression could be attributed to the SERM nature of Way-169916. We examined this scenario by cotreating Huh7 cells with Way-169916 and the ER antagonist ICI-182780 (ICI) at a dose shown previously to effectively inhibit ER-dependent signaling, before IL1β induction of APR gene expression. Analysis of human SAA1 mRNA revealed the same level of SAA1 repression with Way-169916 regardless of coexposure to ICI (Fig. 6B). Further confirmation of the ER-independent action of Way-169916 in the context of Huh7 cells was obtained from protein expression studies that demonstrated a lack of detectable ERα or ERβ expression by this cell line (Fig. 6C). Such data indicate that ER expression is not necessary to facilitate repression of SAA1 by Way-169916, at least in the context of the Huh7 cell line.
Fig. 6.
Way-169916 mediated the repression of inflammatory gene expression is independent of ERα/β. A, ER competition binding assays were performed using the PanVera Estrogen Receptor-α Competitor Assay. Fluoromone polarization was determined at 485 and 530 nm. Data represent mean polarization ± S.E.M. B, Huh7 cells were pretreated for 1 h with 1 μM ICI 182780 or 10 μM Way-169916 in isolation or in combination before 4-h exposure to 2 ng/ml IL1β. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human SAA1 expression. Data represent mean SAA1 mRNA level normalized against human rL13A mRNA. C, protein expression of ERα/β in Huh7 cells was analyzed by Western immunoblot. Protein from MCF-7 cells was used as a positive control for ER expression. β-Actin was used as a loading control.
Suppression of APR Expression by Way-169916 Is AHR-Dependent.
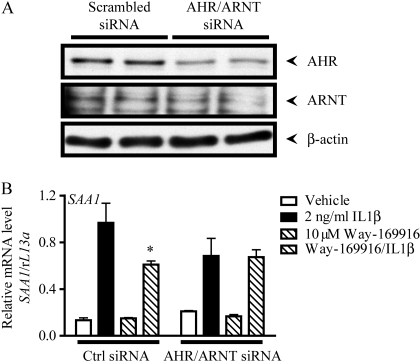
Having established that Way-169916 is an AHR ligand with the capacity to repress the cytokine-mediated expression of acute phase reaction components independently of ER, we wished to investigate whether the AHR/ARNT complex is a requirement for repression. Previous data indicated APR repression after exposure to AHR antagonists, thus rendering them unsuitable for AHR inhibition in this context (Patel et al., 2009). We therefore used siRNA to ablate AHR activity. Preliminary studies demonstrated that siRNA targeted against AHR alone was inefficient with regard to lowering AHR protein levels to a point that influenced the repression of APR expression. We therefore used a double knockdown approach, by using siRNA targeted against AHR and its obligatory dimerization partner ARNT. Huh7 cells transfected with a nontargeting scrambled siRNA oligonucleotide were sensitive to Way-169916-mediated repression of SAA1 (Fig. 7). By contrast, cells with diminished protein expression of AHR and ARNT arising from transfection with siRNA oligonucleotides targeted against AHR and ARNT were refractory to Way-169916 and demonstrated no significant repression of SAA1 (Fig. 7B). These data indicate that the AHR/ARNT complex is necessary and sufficient to mediate Way-169916-mediated APR repression.
Fig. 7.
Way-169916-mediated SAA1 repression is AHR-dependent. A, siRNA-mediated knockdown of AHR/ARNT expression in Huh7 cells transfected with scrambled control or AHR and ARNT-specific siRNA was assessed by Western immunoblotting. Forty-eight hours after transfection, cells were harvested, and protein isolated. Protein blots were probed for AHR, and ARNT, and β-actin was used as a loading control. B, 48 h after transfection with scrambled control or AHR and ARNT-specific siRNA Huh7 cells were pretreated for 1 h with vehicle (DMSO) or 10 μM Way-169916. Cells were then incubated with 2 ng/ml IL1β for a further 4 h. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human SAA1 expression. Data represent mean SAA1 mRNA level normalized against human rL13A mRNA.
Way-169916 Attenuates Cytokine-Mediated Gene Expression in a Context-Specific Manner.
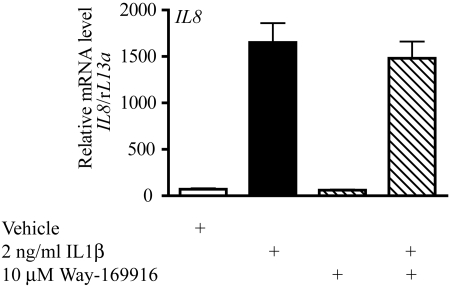
To determine whether the failure of DRE-dependent transcription together with the repression of APR gene expression is due to a globally repressive nature of Way-169916, we examined its effect on additional inflammatory targets. Exposure of Huh7 cells to IL1β can elicit the induction of the proinflammatory cytokine IL8 (Fig. 8). In contrast to the observed Way-169916-mediated repression of the APR targets SAA1, CRP, and HP, IL8 exhibited no significant repression after cotreatment with IL1β and Way-169916 (Fig. 8). Such data indicate that repression of inflammatory gene expression by the AHR ligand Way-169916 is context-specific, eliminating a role for Way-169916 as a global inflammatory transcription inhibitor.
Fig. 8.
Way-169916-mediated repression of cytokine-mediated gene expression is context-specific. Huh7 cells were pretreated for 1 h with 10 nM TCDD or 10 μM Way-169916 before 4-h exposure to 2 ng/ml IL1β. Total RNA was isolated, reverse-transcribed to cDNA, and used as a template for quantitative RT-PCR analysis of human IL8 expression. Data represent mean IL8 mRNA level normalized against human rL13A mRNA.
Discussion
The clinical application of SERMs has been a reality for many years, even predating the evolution of the SERM concept. It was the seminal observation that nuclear hormone receptors, particularly the steroid receptors (e.g., estrogen and glucocorticoid receptors), exhibit the phenomenon of DNA-independent transrepression and are not restricted solely to direct gene activation events through their cognate response elements and thus identified the physiological essence for selectivity (Reichardt et al., 1998). Whereas evolutionarily and structurally dissimilar to the steroid nuclear hormone receptor family, AHR, the only known ligand-activated member of the basic helix-loop-helix/PAS family of transcription factors behaves in an analogous fashion to elicit gene induction (Beischlag et al., 2008). Binding of an agonist (e.g., TCDD) increases the affinity of AHR for its cognate response element and facilitates the expression of AHR-responsive genes, principally members of the CYP1 P450 family, often leading to genotoxic stress or endocrine disruption (Safe, 1995; Sagredo et al., 2006). Despite the functional similarity between AHR and the nuclear receptor family, the notion of therapeutic modulation of AHR has, with the notable exception of its ability to antagonize ER function and mitigate hormone-sensitive tumor growth, largely been overlooked (Safe and McDougal, 2002). However, numerous recent reports have demonstrated the integration of the AHR into signaling pathways not directly connected with xenobiotic metabolism. Activation of AHR with putative endogenous ligands has been shown to exert profound effects upon the immune system, suggesting a physiological role for AHR (Stevens et al., 2009).
The association between AHR and the immune system, although known for a number of years from a toxicological immunosuppressive perspective (Kerkvliet, 2002), is only now being considered for its potential therapeutic applications in the treatment of autoimmune or inflammatory disease (Kerkvliet et al., 2009; Quintana et al., 2008; Veldhoen et al., 2008). The highly characterized role of AHR in mediating various aspects of toxicity has been a major obstacle in realizing the latent therapeutic modulation of immune signaling by the AHR. We have demonstrated that a number of genes associated with the inflammatory cytokine-mediated acute phase response (e.g., Saa1 and Crp) are negatively regulated by exposure to AHR agonists (Patel et al., 2009). The promoters of both genes contain putative but degenerate DRE sequences and could therefore be influenced through AHR binding to these elements. However, in vivo studies using an AHR mutant (AHRA78D) that confers non-DRE binding status upon the AHR while retaining ligand binding and heterodimerization functions, revealed that these APR genes as being repressed by AHR in a DRE-independent fashion, possibly involving a cross-talk mode of action and the inhibition of nuclear factor-κB function in a promoter-specific manner. It is interesting that the weak AHR partial agonist α-naphthoflavone (αNF) retained the capacity to repress APR gene induction with minimal CYP1A1 induction and thus supports the SAhRM hypothesis.
The concerted identification of AHR-mediated APR repression and establishment of the SAhRM hypothesis prompted us to screen for compounds with SAhRM activity. Preliminary evidence suggested that Way-169916, a nonsteroidal SERM, may be an AHR ligand. Competitive binding assays confirmed Way-169916 as an AHR ligand and corroborated its status as an ER ligand, although with an apparent affinity 3 orders of magnitude less relative to ER. Thus, like resveratrol, Way-169916 represents a dual-specificity ER/AHR ligand (Casper et al., 1999; Bhat and Pezzuto, 2001). It is interesting that resveratrol exhibits antagonistic activity for both ER and AHR. We therefore investigated the ability of Way-169916 to promote AHR transformation and DRE binding and stimulate AHR-dependent gene expression. Way-169916 proved to be a very weak partial AHR agonist in the context of both endogenous CYP1A1 mRNA expression and heterologous DRE-reporter activity. This weak activity can be attributed to greatly diminished association between AHR and its response element relative to typical AHR agonists. At this time, it is unclear whether Way-169916 is inefficient at inducing AHR transformation from the chaperone-bound complex into its heterodimeric 6S conformation and/or influences AHR structurally to directly diminish DRE affinity. In its capacity as a SERM, Way-169916 could influence DRE-dependent gene expression through the documented cross-talk of ER and AHR (Matthews and Gustafsson, 2006). This scenario was eliminated in the context of our Huh7-based expression analyses because of the absence of ERα and ERβ expression in this cell line, but it emphasizes a requirement to fully characterize putative SAhRMs because of the frequent overlap between AHR and ER with regard to ligands, modes of activation, repression, and mutual antagonism.
The revelation that Way-169916 binds AHR yet invokes minimal canonical DRE-driven AHR signaling and is thus unlikely to promote subsequent AHR toxicities fulfills one of the major criteria of a putative SAhRM. Indeed, previous in vivo gene profiling analyses in multiple tissues did not highlight the induction of any characteristic DRE-driven AHR targets in response to Way-169916 (Keith et al., 2005). The second defining facet of SAhRM activity is demonstrated with the repression of inflammatory APR gene targets by Way-169916. Anti-inflammatory action by Way-169916 is reported in various rodent models of inflammation, including rheumatoid arthritis, chronic intestinal inflammation, and ischemia-reperfusion injury, which highlights an ER-dependent suppression of NF-κB activity to account for diminished APR expression (Chadwick et al., 2005; Harnish et al., 2006; Booth et al., 2007). Although this mechanism of action is valid, it fails to explain the observed repression in Huh7 cells, which lack ER expression. Previous data using the DNA-binding AHR mutant, various structurally diverse AHR ligands, and the observation that Way-169916 is an AHR ligand all support our contention that repression of APR targets can occur through AHR independently of ER. Although different experimental approaches were used, it is interesting to note that despite having a much lower affinity for AHR, the Way-169916-mediated reduction in APR expression reported here is similar to that obtained through the ER-dependent mechanism (Keith et al., 2005).
As proof of the SAhRM concept, an examination of the mechanism behind the AHR-dependent APR repression is beyond the scope of this article. It is plausible that inhibition through AHR cross-talk is the underlying cause of repression. Such cross-talk between AHR and inflammatory mediators is not without precedent. Indeed, many of the toxic immunosuppressive outcomes attributed to sustained AHR activation by environmental contaminants are believed to involve cross-talk signaling (Tian et al., 2002). Studies showing APR repression with TCDD indicate a requirement for AHR translocation into the nucleus, because an AHR mutant deficient in translocation failed to mediate repression, thus eliminating a mechanism involving the AHR-mediated nuclear blockade of a necessary regulator (Patel et al., 2009). Furthermore, heterodimerization is also a prerequisite for AHR-mediated APR repression, as evidenced by AHR mutants lacking affinity for ARNT. We are currently investigating the molecular basis for SAhRM activity with the aim of identifying novel SAhRMs that exhibit enhanced efficacy and selectivity.
In conclusion, it is our contention that the anti-inflammatory nonsteroidal SERM Way-169916 also exhibits affinity for the AHR and in doing so retains anti-inflammatory characteristics with regard to APR expression. Furthermore, the AHR-mediated anti-inflammatory activity occurs in the absence of potentially toxic canonical DRE-dependent gene expression. As such, Way-169916 represents the first example of a selective AHR modulator. It is noteworthy that these data lend credence to the SAhRM concept and support the notion that the AHR represents a viable therapeutic target for the treatment of inflammatory disorders.
Supplementary Material
Acknowledgments
We thank Dr. Robert Steffan (Wyeth Research) for providing Way-169916 and Marcia Perdew for editorial assistance.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES04869].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061788.
- AHR
- aryl hydrocarbon receptor
- SERM
- selective estrogen receptor modulator
- SAhRM
- selective aryl hydrocarbon receptor modulator
- DRE
- dioxin response element
- APR
- acute phase response
- αNF
- α-napthoflavone
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- SAA1
- serum amyloid-associated 1
- ER
- estrogen receptor
- CRP
- C-reactive protein
- HP
- haptoglobin
- EAE
- experimental acute encephalitis
- Way-169916
- 4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]benzene-1, 3-diol
- ARNT
- aryl hydrocarbon receptor nuclear translocator
- IL
- interleukin
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate
- PAL
- 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin
- MOPS
- 3-(N-morpholino)propanesulfonic acid
- DMSO
- dimethyl sulfoxide
- siRNA
- short interfering
- PCR
- polymerase chain reaction
- M50354
- 2-[2-(2-phenylethyl) benzoimidazole-4-yl]-3-hydroxypropanoic acid
- ICI
- ICI-182780 (7a,17b-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol)
- RT-PCR
- reverse transcriptase polymerase chain reaction.
References
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. (2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 18: 207–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. (2001) Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 61: 6137–6144 [PubMed] [Google Scholar]
- Booth EA, Marchesi M, Knittel AK, Kilbourne EJ, Lucchesi BR. (2007) The pathway-selective estrogen receptor ligand WAY-169916 reduces infarct size after myocardial ischemia and reperfusion by an estrogen receptor dependent mechanism. J Cardiovasc Pharmacol 49: 401–407 [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. (2005) Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol 175: 90–103 [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. (1999) Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol 56: 784–790 [PubMed] [Google Scholar]
- Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC, Jr, Albert LM, Leathurby Y, Harris HA, Bhat RA, et al. (2005) Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci U S A 102: 2543–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Chiaro CR, Perdew GH. (2009) Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol 75: 1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish DC, Liu X, Kenney T, Winneker RC, Chadwick C, Friedrichs GS, Kilbourne EJ. (2006) The pathway-selective estrogen receptor ligand WAY-169916 displays differential activity in ischemia-reperfusion injury models. J Cardiovasc Pharmacol 47: 788–795 [DOI] [PubMed] [Google Scholar]
- Hogaboam JP, Moore AJ, Lawrence BP. (2008) The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system. Toxicol Sci 102: 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AB, Xu H, Nagarkatti PS, Nagarkatti M. (1997) Evidence for the induction of apoptosis in thymocytes by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vivo. Toxicol Appl Pharmacol 142: 367–377 [DOI] [PubMed] [Google Scholar]
- Keith JC, Jr, Albert LM, Leathurby Y, Follettie M, Wang L, Borges-Marcucci L, Chadwick CC, Steffan RJ, Harnish DC.Arthritis Res Ther 7: R427–R438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, Pham D, Mourich DV. (2009) Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunother 1: 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI. (2002) Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol 2: 277–291 [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. (2008) Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A 105: 9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. (2006) Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal 4: e016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T, Kato Y, Ooneda O, Mimura J, Takada T, Mochizuki H, Yamamoto M, Fujii-Kuriyama Y, Furusako S. (2005) Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J Immunol 175: 7348–7356 [DOI] [PubMed] [Google Scholar]
- North CM, Crawford RB, Lu H, Kaminski NE. (2009) Simultaneous in vivo time course and dose response evaluation for TCDD-induced impairment of the LPS-stimulated primary IgM response. Toxicol Sci 112: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. (2009) Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest 89: 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A, Glover E, Ebetino FH, Kende AS. (1986) Photoaffinity labeling of the Ah receptor. J Biol Chem 261: 6352–6365 [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71 [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Wessely O, Gass P, Schmid W, Schütz G. (1998) Analysis of glucocorticoid signalling by gene targeting. J Steroid Biochem Mol Biol 65: 111–115 [DOI] [PubMed] [Google Scholar]
- Safe S, McDougal A. (2002) Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int J Oncol 20: 1123–1128 [PubMed] [Google Scholar]
- Safe SH. (1995) Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther 67: 247–281 [DOI] [PubMed] [Google Scholar]
- Sagredo C, Øvrebø S, Haugen A, Fujii-Kuriyama Y, Baera R, Botnen IV, Mollerup S. (2006) Quantitative analysis of benzo[a]pyrene biotransformation and adduct formation in Ahr knockout mice. Toxicol Lett 167: 173–182 [DOI] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Crawford RB, Chen W, Kaminski NE. (2008) 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated impairment of B cell differentiation involves dysregulation of paired box 5 (Pax5) isoform, Pax5a. J Pharmacol Exp Ther 326: 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. (2007) Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72: 1508–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan RJ, Matelan E, Ashwell MA, Moore WJ, Solvibile WR, Trybulski E, Chadwick CC, Chippari S, Kenney T, Eckert A, et al. (2004) Synthesis and activity of substituted 4-(indazol-3-yl)phenols as pathway-selective estrogen receptor ligands useful in the treatment of rheumatoid arthritis. J Med Chem 47: 6435–6438 [DOI] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, Bradfield CA. (2009) The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Rabson AB, Gallo MA. (2002) Ah receptor and NF-kappaB interactions: mechanisms and physiological implications. Chem Biol Interact 141: 97–115 [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. (2009) Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med 206: 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. (2008) Nature 453: 106–109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.