Abstract
The recrudescence of infection with Campylobacter jejuni after appropriate antibiotic treatment has not been previously reported in an immunocompetent adult. We present the complete clinical, microbiologic, and immunologic evaluation of a closely monitored healthy male with recrudescent C. jejuni infection occurring in the absence of immunodeficiency following experimental infection with a well-characterized strain. After antibiotic treatment, the initial infection was clinically cleared and microbiologically undetectable. Subsequently, two episodes of recrudescence occurred, with no change in in vitro antibiotic sensitivity being detected. The immune responses of the individual were compared to those of other participants in the experimental infection study: innate immune responses, including fecal cytokines and C-reactive protein, were intact; however, measures of Campylobacter-specific adaptive immune responses were absent, including serum antibodies, antibody-secreting cells, and in vitro gamma interferon responses. No primary or secondary immunodeficiency was identified. Recrudescent Campylobacter infections after treatment may be more common than has previously been appreciated. This work adds to our understanding of the human immune response to natural Campylobacter infection and reiterates the importance of pathogen-specific adaptive immune responses to this globally important pathogen.
Campylobacter jejuni is a leading cause of food-borne bacterial enteritis throughout the world (5, 39). The clinical manifestations of campylobacteriosis include inflammatory diarrhea associated with fever, malaise, and abdominal cramping (11). In healthy individuals, extraintestinal disease from C. jejuni is rarely reported and gastrointestinal manifestations often resolve completely without the use of antibiotics. When antibiotic use is necessary for severe disease and is used early, symptoms abate rapidly in healthy hosts (36).
Unlike individuals with immunodeficiencies, recrudescent infection with C. jejuni in healthy hosts who have received antibiotic therapy has not been previously reported (13, 21, 27). The recrudescence of infection with or without illness and in the absence of repeat exposure suggests that the original pathogen has not been completely eliminated from the host due to an insufficient immunologic response, containment of the microbe beyond the reach of antibiotics or host immunity, or the development of antibiotic resistance.
We report the first description of a healthy adult who experienced two episodes of C. jejuni recrudescence after appropriate antibiotic therapy and the findings of immunologic and microbiologic evaluations of this individual.
CASE REPORT
The subject (subject 006) was a healthy 23-year-old male with no significant medical history except mild, well-controlled depression. In particular, the subject had no known immunodeficiency, atopy or allergies, recurrent sinopulmonary or gastrointestinal disease, or risk factors for HIV infection. The screening laboratory results performed for study eligibility are summarized in Table 1 .
TABLE 1.
Clinical immunology at screening (preinfection), during the period of the second recrudescence, and at resolutiona
| Laboratory assay | Clinical findings at: |
Normal range | ||
|---|---|---|---|---|
| Screening | Recrudescence | Resolution | ||
| Complete blood count | ||||
| Leukocytes | 6,900 | 7,600 | 4,000-10,400/mm3 | |
| Hematocrit | 44.3 | 42.8 | 39.5-50.2% | |
| Platelets | 175,000 | 242,000 | 141,000-320,000/mm3 | |
| Lymphocytes | 14.8 | 16.0 | 15.0-46.8% | |
| Neutrophils | 75.1 | 73.0 | 45.5-79.9% | |
| Monocytes | 7.6 | 7.0 | 1.8-12.0% | |
| Eosinophils | 2.2 | 2.0 | 0.03-0.61% | |
| Basophils | 0.02 | 0 | 0.01-0.11% | |
| Serum chemistry | ||||
| Total protein | 7.4 | 7.7 | 6.5-8.3 g/dl | |
| Albumin | 4.9 | 4.9 | 3.4-4.9 g/dl | |
| Serum or salivary levels | ||||
| Serum IgA | 104 | 81 | 100 | 82-453 mg/dl |
| IgA1 | 73b | —c | ||
| IgA2 | 25b | — | ||
| Serum IgG | 800 | 950 | 650-1,500 mg/gl | |
| IgG1 | 438 | 490-1,140 mg/dl | ||
| IgG2 | 326 | 150-640 mg/dl | ||
| IgG3 | 26.6 | 20-110 mg/dl | ||
| IgG4 | 27.9 | 8-140 mg/dl | ||
| Serum IgM | 149 | 171 | 60-300 mg/dl | |
| Serum IgE | 29 | 0-41 mg/dl | ||
| Total complement | 60 | 30-75 U/ml | ||
| Mannose binding protein | 1,282d | 1,000-3,000 ng/ml | ||
| Salivary IgA | 5.4 | 6.2-14.5 mg/dl | ||
The clinical assays performed before recruitment to the study included assays for antibodies to HIV and hepatitis C virus, hepatitis B virus surface antigens, and HLA-B27. The results of these tests were negative. At the time of recrudescence, antibodies to diphtheria and tetanus were present and the absolute CD4 count was 600 U/liter (range, 602 to 1,791 U/liter). A repeat test for HIV was negative.
Units are mg/dl.
—, no clinically validated reference range.
Testing for total mannose binding lectin was repeated with samples collected on days 7, 9, and 28 postinfection; and there was no significant change in the results.
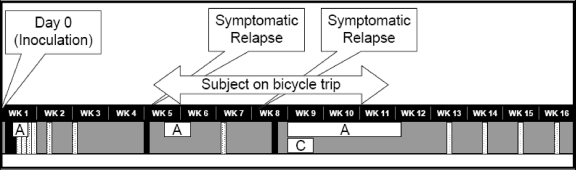
As illustrated in Fig. 1, a stool sample collected 23 h after dosing with C. jejuni CG8421 grew Campylobacter. At 48.5 h, the subject became symptomatic with diarrhea, mild malaise, back pain, fatigue, and myalgias. Fever, vomiting, dysentery, and severe abdominal cramping were not present. Although the subject was never hypotensive or dizzy and drank oral fluids without difficulty, he met the study criteria for severe diarrhea (more than six loose/liquid stools in 24 h or >800 g loose/liquid stools in 24 h) at 58 h and, according to the protocol, received early antibiotics and 1 liter of intravenous normal saline. Azithromycin was given at 500 mg orally for three daily doses in the hospital under supervision. The total loss through diarrhea for the episode was 2,978 g in 14 diarrheal stool samples. Similar to the other dosed subjects, he had a rapid clinical improvement during the azithromycin treatment, and the stool cultures became and remained negative by 75 h postdosing. He was asymptomatic at discharge on day 6 postdosing. Stool cultures remained negative at follow-up visits 9 and 14 days after dosing.
FIG. 1.
Clinical time course of initial inoculation and recrudescence episodes. A and C, the subject's courses of azithromycin and ciprofloxacin, respectively; black boxes, positive culture for C. jejuni CG8421; speckled boxes, negative culture for C. jejuni.
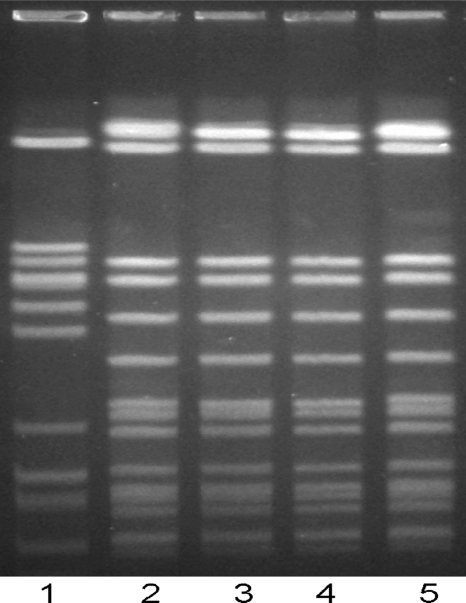
On day 28, the final day of outpatient observation, the subject remained asymptomatic; however, a stool sample collected on this date demonstrated the growth of C. jejuni on Campy CVA agar. On day 31, the subject noted three episodes of loose stools with visible blood and mild abdominal cramping. He was treated with a second course of azithromycin (500 mg orally for 5 days) and became asymptomatic within 24 h. The bacterial isolate was found to be identical to the original C. jejuni strain by pulsed-field gel electrophoresis (Fig. 2). A complete antibiotic sensitivity panel confirmed at two clinical laboratories revealed no change in antibiotic sensitivities, including sensitivity to ciprofloxacin (MICs, 0.064 and 0.032 μg/ml) and azithromycin (MICs, 0.125 and 0.094 μg/ml) (Table 2). After the second course of azithromycin, a follow-up stool culture was performed 7 days after the end of antibiotic treatment and was negative for C. jejuni.
FIG. 2.
Pulsed-field gel electrophoresis performed with genomic DNA from C. jejuni reference strain 81-176 (lane 1), the original C. jejuni CG8421 inoculum strain (lane 2 and 5), and strains cultured during the first and second recrudescence episodes (lanes 3 and 4, respectively).
TABLE 2.
Antibody sensitivity panels performed with C. jejuni CG8421 used for the initial study inoculum and at the first and second episodes of recrudescence
| Antibiotic | Etest (μg/ml) MIC for C. jejuni CG8421a |
||
|---|---|---|---|
| Original | Recrudescence |
||
| First isolate | Second isolate | ||
| Ciprofloxacin | 0.064 | 0.047 | 0.032 |
| Levofloxacin | 0.094 | 0.064 | 0.047 |
| Tetracycline | 32 | 32 | 12 |
| Trimethoprim-sulfamethoxazole | >32 | >32 | >32 |
| Azithromycin | 0.125 | 0.125 | 0.094 |
| Erythromycin | 0.75 | 1 | 0.5 |
| Ampicillin | 0.5 | 0.5 | 1 |
| Imipenem | 0.016 | 0.016 | 0.032 |
C. jejuni CG8421 is a known tetracycline-resistant strain. All susceptibility testing was performed in accordance with CLSI guidelines.
On day 53 after dosing (18 days after the last antibiotic administration), the subject again experienced mild diarrhea and fatigue. A stool culture grew a C. jejuni strain and was confirmed to be the same strain (CG8421), and the antibiotic sensitivities remained unchanged (Fig. 2; Table 2). A more complete clinical immunologic workup was initiated (Table 1). While awaiting the antibiotic sensitivity results, the subject was restarted on azithromycin (500 mg orally daily), and ciprofloxacin at 500 mg orally twice daily for 5 days was added. Since the subject was out of contact with reliable medical care on a cross-country bicycling trip (2,800 miles, begun on day 29), azithromycin was continued until his return, for a total of 22 days.
The subject was reevaluated upon his return, 10 days after the cessation of antibiotic treatment. He appeared healthy and robust and had no physical findings of postinfectious sequelae or malnutrition. To increase the sensitivity of detection, six CVA agar plates were used for each culture performed. Weekly cultures were done for an additional 4 weeks, and all remained negative. The subject remained healthy and asymptomatic thereafter.
MATERIALS AND METHODS
Screening and clinical trial.
The subject participated in an inpatient clinical trial designed to develop a challenge model of human Campylobacter jejuni infection for future use in Campylobacter vaccine testing (44). The trial was approved by the Institutional Review Boards of the University of Vermont and the U.S. Naval Medical Research Center and was performed under Good Clinical Practices and a Food and Drug Administration Investigational New Drug (IND) application. The genome of the C. jejuni challenge strain (CG8421) has been fully sequenced, and structural studies indicated that the lipooligosaccharide of the strain has no molecular mimicry to human gangliosides that are associated with Guillain-Barré syndrome (38). The subjects were healthy adults who were excluded if they had evidence of prior infection with C. jejuni, serum IgA to C. jejuni glycine extract (titer, >1:2,000), or C. jejuni-specific in vitro gamma interferon (IFN-γ) levels of >400 pg/ml of culture supernatant (see below). The enrolled subjects were monitored as inpatients for up to 10 days. The challenge inoculum was taken orally in a bicarbonate buffer as a dose of 106 CFU. Seven other individuals were dosed at the same time as the case subject (immunological data for three other subjects are presented).
The subjects were treated with azithromycin (500 mg orally daily for 3 days) starting at day 6 postdosing or if they met the study criteria for early antibiotic treatment (20). The subjects were discharged after two serial stool cultures were negative for Campylobacter and the symptoms had resolved. While they were inpatients, all subjects had a minimum of two stool specimens cultured daily for Campylobacter on Campy CVA agar at 42°C under microaerophilic conditions. Stool cultures were performed by a research microbiology laboratory qualified to perform validated procedures for the detection of Campylobacter jejuni. The subjects were reevaluated; and postdischarge stool cultures were performed on days 14, 21, and 28. The subjects were instructed in strict hand-washing techniques throughout the study.
Immune responses.
C. jejuni CG8421 glycine extract antigens were used for the detection of antibodies in serum or fecal extracts by enzyme-linked immunosorbent assay (ELISA) and for the detection of antibody-secreting cells (ASCs) by enzyme-linked immunospot (ELISPOT) assay, and a formalin-fixed whole-cell preparation was used to detect cellular immune responses by the in vitro induction of IFN-γ. All immunological laboratory assays employed have been described elsewhere (6, 7). The total IgA titer in each stool homogenate (1:2, wt/vol) was determined by ELISA, and the antigen-specific titers were adjusted to 1,000 μg/ml of total IgA. For cellular (IFN-γ) responses, peripheral blood mononuclear cells (PBMCs; 105) were stimulated in vitro with 105 formalin-fixed C. jejuni CG8421 whole-cell particles. Following 72 h of incubation at 37°C in 5% CO2, the culture supernatant was collected and the IFN-γ levels were determined by a Luminex bead assay (Bio-Rad, Hercules, CA). The fecal interleukin-1 (IL-1) and IL-8 levels were determined with human-specific cytokine Luminex bead kits (Bio-Rad). Plasma levels of C-reactive protein (CRP) were determined with a commercially available kit (RapiTex; Dade Behring, Marburg, Germany). Clinical immunology assays (for immunoglobulins and subsets, salivary IgA, and mannose-binding lectin [MBL]) are described elsewhere.
PFGE.
The C. jejuni strains were grown for 18 h on Mueller-Hinton agar plates at 37°C under microaerobic conditions. Cells were harvested and adjusted to an optical density at 600 nm of 1.7 to 1.9. The bacterial cells were aliquoted to 100-μl plugs (Bio-Rad) with low-melting-point agarose (Invitrogen) and were allowed to solidify for 15 min at 5°C. The bacterial cells within the plugs were lysed by standard procedures (28). The plugs were resuspended in 1 ml of 1× restriction buffer for 1 h at room temperature. The plugs were removed and put into 500 μl of fresh restriction buffer and BssHII enzyme (8 U; New England Biolabs, Beverly, MA), and the mixture was incubated overnight at 37°C.
Pulsed-field gel electrophoresis (PFGE) was done with a contour-clamped homogeneous electric field (CHEF) apparatus (Bio-Rad) (12). Samples were electrophoresed in 1% pulsed-field gel agarose (Bio-Rad) and electrophoresed with a switch time of 2.2 to 17.6 s and a field strength of 6 V/cm for 23 h. The gels were stained in ethidium bromide, visualized under UV light, and photographed in a Kodak Image Station 2000 (29).
PCR analyses.
Strains isolated from the subject were also compared to the original inoculum strain by PCR analyses of four genes that were identified by complete genome sequencing of strain CG8421, as shown in Table S1 in the supplemental material (38). The genes amplified by these primers are found in a phage-like element found in CG8421 that was originally described in a Campylobacter coli strain, strain RM2228 (2, 16). An additional PCR amplification was done with primers pg06.14 and pg06.15 to amplify the C. jejuni-specific fspA gene, as shown in Table S1 in the supplemental material (37). PCR amplification was done for 30 cycles, as follows: denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and an extension at 72°C for 1 min. Controls included DNA from C. jejuni strains CG8421 and 81-176 and negative controls lacking DNA templates. The PCR products were analyzed on 0.8% agarose gels.
RESULTS
Immunology and microbiology results.
Table 1 demonstrates that the clinical immunology assay results for the subject conducted at the screening, during the second recrudescence, and upon resolution were all within normal reference ranges for all measures except the serum IgA and IgG1 titers (at recrudescence) and the salivary IgA titer at resolution, which were slightly lower than the reference range but which were not likely to be clinically significant.
The PFGE band pattern demonstrates that the two recrudescent strains were indistinguishable from each other and from strain CG8421, and their pattern was distinct from that of a reference strain, strain 81-176 (Fig. 2). PCR primer sets were designed for four genes that have not been described together in the same strain of C. jejuni except in the genome of strain CG8421 (13). Both recrudescent strains were positive for these four genes (see Table S1 in the supplemental material). The antibiotic sensitivities of the C. jejuni strain used for initial dosing and the two isolates obtained during the recurrence were similar, as determined by Etest. Hence, no evidence of a change in antibiotic susceptibility was seen.
The Campylobacter-specific immune responses and the nonspecific innate inflammatory responses for subject 006 and three other subjects dosed simultaneously are shown in Table 3. In contrast to the other three subjects who did not experience clinical or microbiologic recrudescence, subject 006 showed low and transient seroconversion detected only by the presence of Campylobacter-specific serum IgM, whereas no change in serum IgA or IgG was seen. Similarly, no evidence of mucosal immune stimulation postinfection was detected in subject 006, whereas the other three subjects showed vigorous increases in the Campylobacter antigen-specific fecal IgA titers as well as IgA ASCs in the circulation postinfection. The pre- and postinfection IFN-γ levels produced by the PBMCs of subject 006 remained the same, whereas the other three subjects showed three- to sixfold increases in IFN-γ levels after infection. The levels of IFN-γ detected from phytohemagglutinin (PHA)-stimulated PBMCs were vigorous and were similar in all subjects (Table 3).
TABLE 3.
Comparison of Campylobacter-specific immune responses of the case subject (subject 006) with those of other subjects dosed concurrentlya
| Response | Case subject 006 |
Subject 002 |
Subject 004 |
Subject 008 |
||||
|---|---|---|---|---|---|---|---|---|
| Preinfection findings | Postinfection response (fold)b | Preinfection findings | Postinfection response (fold) | Preinfection findings | Postinfection response (fold) | Preinfection findings | Postinfection response (fold) | |
| Systemic humoral response | ||||||||
| Plasma IgA titer (mg/dl) | 640 | 2 | 640 | 8 | 1,280 | 4 | 1,280 | 16 |
| Plasma IgG titer (mg/dl) | 6,400 | 2 | 1,600 | 2 | 5,120 | 1 | 1,600 | 16 |
| Plasma IgM titer (mg/dl) | 1,600 | 4 | 12,800 | 8 | 6,400 | 8 | 6,400 | 16 |
| Mucosal response | ||||||||
| Antigen-specific fecal IgA titer (mg/dl) | 4 | 1 | 4 | 108 | 3 | 48 | 3 | 1,296 |
| Total fecal IgA (μg/ml) | 1,975 | 10,090 | 1,550 | 870 | 1,745 | 4,510 | 1,995 | 2,885 |
| No. of IgA ASCs/106 PBMCs | <1 | 1 | 2 | 31 | <1 | 78 | <1 | 448 |
| No. of IgG ASCs/106 PBMCs | <1 | 1 | <1 | <1 | <1 | 2 | <1 | 38 |
| Cellular immune response, antigen-specific IFN-γ (pg/ml) | 99 | 91 | 211 | 1,265 | 279 | 736 | 80 | 319 |
| PHA (pg/ml) | 97,884 | 95,650 | 86,113 | 84,144 | 107,810 | 113,270 | 87,710 | 84,742 |
| Inflammatory response | ||||||||
| Plasma CRP (μg/ml) | 3 | 48 | 3 | 48 | 3 | 3 | 3 | 12 |
| Stool IL-1 (pg/ml) | 4 | 95,457 | 82 | 3,518 | 7 | 560 | 3 | 1,924 |
| Stool IL-8 (pg/ml) | 9 | 665 | 62 | 161 | 0.5 | 30 | 0.5 | 86 |
Assay detection limits are as follows: for ASCs, 1 ASC/106 PBMCs, and values below the detection limit are assigned a value of <1; for all cytokines, 1 pg/ml, and a sample showing a value below the detection limit was assigned a value of 0.5; and for CRP, 6 μg/ml, and samples showing levels below the detection limit were assigned a value of 3.
Postinfection responses are the maximum fold rise after infection from the value at the baseline.
In contrast to the acquired immune responses, the innate immune response to C. jejuni infection in subject 006 was vigorous and was distinct from that in the other subjects. In particular, a dynamic increase in the levels of the inflammatory cytokines IL-1β and IL-8 was seen. The magnitude of the change in IL-1β levels from pre- to postinfection in subject 006 was >2 log units higher than that in the other three subjects, who did not experience clinical or microbiologic recrudescence.
DISCUSSION
We report two episodes of recrudescence of C. jejuni infection in a young healthy adult who had been appropriately treated with strain-sensitive antibiotics, who had no evidence of a known underlying primary or secondary immunodeficiency, and who had no family history of infectious disease susceptibility. Infection with Campylobacter is the second most common cause of food-borne bacterial disease in the United States and a common cause of enteritis throughout the world (5, 39). Despite the frequency of Campylobacter infections, the recrudescence of campylobacteriosis after appropriate antibiotic use has never been described in an immunocompetent adult. To assess this individual, we were fortunate to have comparable clinical data from other subjects who received the C. jejuni inoculum simultaneously, as well as specimens and isolates for immunologic and microbiologic testing. Clinical assessments as well as the microbiologic evaluation of the C. jejuni isolate demonstrated that recrudescence was not due to antibiotic resistance and was unlikely to have been related to increased strain virulence. In the case subject, the evidence of a robust nonspecific (innate) inflammatory response was evident postinfection, as measured by increases in C-reactive protein and fecal IL-8, IL-1β, and total IgA levels. However, Campylobacter-specific systemic (plasma IgG and IgA), mucosal (circulating IgG and IgA antibody-secreting cells and fecal IgA), and cellular (INF-γ) immune responses were strikingly absent. A brief and nonsustained increase in the plasma IgM level was seen. In contrast, robust humoral and cellular responses were mounted by the other subjects. Our subject appeared to have failed to make protective Campylobacter-specific immune responses, despite protracted exposure to the pathogen.
Campylobacteriosis in healthy hosts is generally associated with a spontaneous recovery and the full resolution of symptoms, although postinfectious sequelae (including reactive arthritis, Guillain-Barré syndrome, and irritable bowel syndrome) are well described (3, 22, 43). The use of antibiotics for the treatment of Campylobacter infections is generally reserved for patients with moderate or severe disease (dehydrating diarrhea, dysentery), signs of systemic infection (fever, prostration) or underlying immunodeficiency (42). The antimicrobial therapy recommended for C. jejuni infection includes a macrolide antibiotic (i.e., azithromycin or erythromycin) for 3 days, as was initially done for our case subject (20). In general, fluoroquinolone use is limited secondary to the mounting of Campylobacter resistance, but it is highly effective against sensitive strains (14).
In contrast to healthy hosts, prolonged clinical symptoms and the recrudescence of disease have been specifically reported in subjects with HIV infection and those with hypogammaglobulinemia (1, 13, 21, 25, 27, 31, 35). In these populations, campylobacteriosis may be associated with protracted shedding and gastrointestinal symptoms, as well as systemic disease. Evaluation of the case subject for underlying primary or secondary immunodeficiency was performed and included testing for HIV and measurement of immunoglobulin classes, IgG/IgA subclasses, secreted IgA (via salivary IgA), total complement, and mannose binding lectin. A single IgG1 measurement for the case subject was slightly below the reference range of normal, but it is not likely to have been clinically significant. The subject's ability to mount an adaptive antibody response was confirmed by measuring antibodies to recently given protein-based vaccines (diphtheria, tetanus). Functional T-cell testing was measured by stimulation of peripheral lymphocytes with the mitogen phytohemagglutinin. We considered the possibility of transient immunodeficiency due to intense exercise; however, the first microbiologic relapse occurred prior to the onset of the subject's prolonged bicycling trip (17-19, 30). Despite our attempts, no previously characterized immunodeficiency was detected.
In otherwise healthy adults and in the absence of antibiotics, a relapse of infection with C. jejuni has been reported. In untreated patients of all ages infected with C. jejuni or C. coli, Kapperud et al. found that 17.8% of patients shed C. jejuni or C. coli a mean of 31 days after they became asymptomatic. The proportion of infections due to C. jejuni and the patient ages were not reported in that case series (26). In infants and children, Pai et al. reported shedding of Campylobacter spp. for a mean of 16.8 days in untreated patients, whereas antibiotic-treated patients shed for 2.1 days after antibiotic treatment (34). As noted above, apart from the report of one child who had a relapsed Campylobacter jejuni infection after a course of erythromycin, there have been no reports of recrudescent C. jejuni infections in healthy adults following a course of strain-sensitive antibiotics (34). An asymptomatic recrudescent infection after antibiotic therapy that is not explained by acquired resistance or biliary disease is well described for acute nontyphoidal salmonellosis (Salmonella java) (33).
Knowledge about what constitutes a disease-limiting or protective immune response in humans infected with Campylobacter is incomplete, although epidemiologic studies and human challenge models have helped define innate and adaptive humoral and cellular markers of infection which likely contribute to the host's overall defense (7, 8, 45). Work with human intestinal cell lines and primary human intestinal tissues has evaluated the role of innate immune responses and demonstrated that these responses at the epithelial surfaces include the rapid production of antimicrobial peptides and proinflammatory chemokines and cytokines, with both Th1 and Th2 cytokines being represented (4, 23, 47). These data are consistent with clinical observations of inflammatory diarrhea. For humoral immunity, a large number of trials have demonstrated the production of Campylobacter-specific antibodies in response to infection or exposure; however, it is unknown which (if any) antibodies represent correlates of immunity (24, 40, 41, 46). In our subjects, IgG (plasma and antibody-secreting cell) responses were not uniformly positive in all subjects; IgG may be less critical for the control of this nonsystemic infection. ASC data and the presence of antibodies in fecal specimens demonstrate the likely importance of IgA at mucosal surfaces for Campylobacter control.
In contrast to several studies evaluating antibody responses, work on adaptive cellular immune responses to human campylobacteriosis is limited (7, 9, 10, 32). For adaptive cellular immunity, previous challenge models and a recent comprehensive evaluation of an incidentally infected patient (with specimens being available pre- and postinfection) suggest that IFN-γ may be a marker of protection against infection; the functional role of T-cell subsets and the importance of other cytokines have not been clarified (7, 8; D. Tribble, S. Baqar, D. Scott, M. Oplinger, F. Trespalacios, D. M. Rollins, R. I. Walker, J. D. Clements, S. E. Walz, P. Gibbs, E. F. Burg, A. P. Moran, and A. L. Bourgeois, submitted for publication).
Other reasons for recrudescence in this individual were considered, including the possibility of accidental self-reinfection; the improper administration or early use of antibiotics; and carriage or shielding of Campylobacter in a gut location, such as a diverticulum, crypt abscess, or biofilm (15). Gallbladder carriage of Campylobacter has never been described. We hypothesize that either the organism was shielded from both antibiotics and the immune system in a gut focus or a Campylobacter-specific immunodeficiency was present. The latter is unlikely in an otherwise healthy adult; however, antigen-specific responses were not mounted after three episodes of symptomatic disease.
As noted, the final resolution of infection occurred after the use of prolonged antibiotics and the addition of a 5-day course of ciprofloxacin to azithromycin. Since the subject was traveling out of reach of predictable medical services at that time, antibiotics were continued for a protracted period (22 days). In this setting, it remains unclear whether the infection would have cleared spontaneously or whether either the addition of a fluoroquinolone or the extended duration of antibiotic treatment was responsible for the final clearance of infection.
In summary, we have identified a young healthy male with normal innate inflammatory immune responses but with no Campylobacter-specific immune responses. This work reiterates the importance of adaptive immunity for the complete eradication of infection. We suspect that in the absence of extensive follow-up for positive stool cultures, as performed in this clinical study, “healthy” individuals with protracted or recrudescent Campylobacter infection may be underrecognized. We hypothesize that, similar to our case, these individuals may not mount adaptive immune responses. Further work on the systemic and gut immune responses to Campylobacter may shed light on the precise immunologic deficit of our subject and/or a hidden locus of infection in the gut. As we work toward vaccines and better therapeutics for Campylobacter infections, the immunologic evaluation of this subject adds important information to our understanding of the human immune response to Campylobacter infection.
Supplementary Material
Acknowledgments
The University of Vermont General Clinical Research Center received NIH award MO1 RR000109. Support was also provided by the Naval Medical Research Center and ACE Biosciences. The laboratory immunologic and molecular biology studies were supported by U.S. Navy Research and Development Command Work Unit 6000.RAD1.DA3.A0308.
We acknowledge the assistance of the many individuals who assisted in the cross-country care and evaluation of this individual, including Martin Blaser (NY), John Brooks (MI), Gaither Brynum (MN), and Abdulhamid Alkhalaf (ND). We also thank Howard Lederman, Charlotte Cunningham-Rundle, and Jiri Mestecky, who willingly discussed evaluations to rule out primary immunodeficiencies.
We acknowledge the hard work and dedication of the Campylobacter Study Team (at UVM, Cassandra Ventrone, Elizabeth Dill, Patrick Daunais, Garth Cummings, Caroline Lyon, and Ann Fingar; at NMRC, Theron Gilliland, Jr., Erika Jones, Cheryl P. Ewing, Sandra Dadouk, Stephanie Sincock, and Lanfong Lee; at ACE Biosciences, Charlotte Buchwaldt, Pascale Puel, Robert Miller, and Dennis Mattiesen), the study subjects, and the UVM/FAHC General Clinical Research Center staff.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position or the U.S. Department of the Navy, the U.S. Department of Defense, or the U.S. government.
We have no conflicting financial interests. S.B., D.R.T., and P.G. are employees of the U.S. government. This work was prepared as part of their official duties. Title 17 U.S.C. 101 defines U.S. government work as work prepared by a military service member or employee of the U.S. government as part of that person's official duties.
Footnotes
Published ahead of print on 18 November 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Ahnen, D. J., and W. R. Brown. 1982. Campylobacter enteritis in immune-deficient patients. Ann. Intern. Med. 96:187-188. [DOI] [PubMed] [Google Scholar]
- 2.Ajjampur, S. S., B. P. Gladstone, D. Selvapandian, J. P. Muliyil, H. Ward, and G. Kang. 2007. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J. Clin. Microbiol. 45:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allos, B. M. 1997. Association between Campylobacter infection and Guillain-Barre syndrome. J. Infect. Dis. 176(Suppl. 2):S125-S128. [DOI] [PubMed] [Google Scholar]
- 4.Al-Salloom, F. S., A. Al Mahmeed, A. Ismaeel, G. A. Botta, and M. Bakhiet. 2003. Campylobacter-stimulated INT407 cells produce dissociated cytokine profiles. J. Infect. 47:217-224. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. 2005. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 sites, United States, 2004. MMWR Morb. Mortal. Wkly. Rep. 54:352-356. [PubMed] [Google Scholar]
- 6.Baqar, S., A. L. Bourgeois, P. J. Schultheiss, R. I. Walker, D. M. Rollins, R. L. Haberberger, and O. R. Pavlovskis. 1995. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine 13:22-28. [DOI] [PubMed] [Google Scholar]
- 7.Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. [DOI] [PubMed] [Google Scholar]
- 8.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 9.Blaser, M. J., R. E. Black, D. J. Duncan, and J. Amer. 1985. Campylobacter jejuni-specific serum antibodies are elevated in healthy Bangladeshi children. J. Clin. Microbiol. 21:164-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser, M. J., and D. J. Duncan. 1984. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect. Immun. 44:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser, M. J., W. J. Feldman, R. A. Pollard, and J. R. Allen. 1983. Campylobacter enteritis in the United States. A multicenter study. Ann. Intern. Med. 98:360-365. [DOI] [PubMed] [Google Scholar]
- 12.Bourke, B., P. Sherman, H. Louie, E. Hani, P. Islur, and V. L. Chan. 1995. Physical and genetic map of the genome of Campylobacter upsaliensis. Microbiology 141(Pt 10):2417-2424. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin, B., G. P. Wormser, R. A. Abdoo, F. Cabello, M. E. Aguero, and S. L. Sivak. 1986. Persistence of multiply antibiotic-resistant Campylobacter jejuni in a patient with the acquired immune deficiency syndrome. Am. J. Med. 80:965-970. [DOI] [PubMed] [Google Scholar]
- 14.Engberg, J., J. Neimann, E. M. Nielsen, F. M. Aerestrup, and V. Fussing. 2004. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerg. Infect. Dis. 10:1056-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields, J. A., and S. A. Thompson. 2008. Campylobacter jejuni CsrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J. Bacteriol. 190:3411-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouts, T. R., A. L. DeVico, D. Y. Onyabe, M. T. Shata, K. C. Bagley, G. K. Lewis, and D. M. Hone. 2003. Progress toward the development of a bacterial vaccine vector that induces high-titer long-lived broadly neutralizing antibodies against HIV-1. FEMS Immunol. Med. Microbiol. 37:129-134. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson, M. 2000. Special feature for the Olympics: effects of exercise on the immune system. Overview: exercise immunology. Immunol. Cell Biol. 78:483-484. [DOI] [PubMed] [Google Scholar]
- 18.Gleeson, M., and N. C. Bishop. 2000. Elite athlete immunology: importance of nutrition. Int. J. Sports Med. 21(Suppl. 1):S44-S50. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson, M., and D. B. Pyne. 2000. Special feature for the Olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol. Cell Biol. 78:536-544. [DOI] [PubMed] [Google Scholar]
- 20.Guerrant, R. L., T. Van Gilder, T. S. Steiner, N. M. Thielman, L. Slutsker, R. V. Tauxe, T. Hennessy, P. M. Griffin, H. DuPont, R. B. Sack, P. Tarr, M. Neill, I. Nachamkin, L. B. Reller, M. T. Osterholm, M. L. Bennish, and L. K. Pickering. 2001. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32:331-351. [DOI] [PubMed] [Google Scholar]
- 21.Hammarstrom, V., C. I. Smith, and L. Hammarstrom. 1993. Oral immunoglobulin treatment in Campylobacter jejuni enteritis. Lancet 341:1036. [PubMed] [Google Scholar]
- 22.Hannu, T., L. Mattila, H. Rautelin, P. Pelkonen, P. Lahdenne, A. Siitonen, and M. Leirisalo-Repo. 2002. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 41:312-318. [DOI] [PubMed] [Google Scholar]
- 23.Hu, L., and T. E. Hickey. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect. Immun. 73:4437-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janvier, B., S. Ayraud, A. Beby-Defaux, and J. Louis Fauchere. 2000. Immunogens of interest for the diagnosis of Campylobacter jejuni infections. FEMS Immunol. Med. Microbiol. 27:263-268. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, R. J., C. Nolan, S. P. Wang, W. R. Shelton, and M. J. Blaser. 1984. Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann. Intern. Med. 100:832-834. [DOI] [PubMed] [Google Scholar]
- 26.Kapperud, G., J. Lassen, S. M. Ostroff, and S. Aasen. 1992. Clinical features of sporadic Campylobacter infections in Norway. Scand. J. Infect. Dis. 24:741-749. [DOI] [PubMed] [Google Scholar]
- 27.Kerstens, P. J., H. P. Endtz, J. F. Meis, W. J. Oyen, R. J. Koopman, P. J. van den Broek, and J. W. van der Meer. 1992. Erysipelas-like skin lesions associated with Campylobacter jejuni septicemia in patients with hypogammaglobulinemia. Eur. J. Clin. Microbiol. Infect. Dis. 11:842-847. [DOI] [PubMed] [Google Scholar]
- 28.Maslow, J. N., M. E. Mulligan, and R. D. Arbeit. 1993. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin. Infect. Dis. 17:153-162. [DOI] [PubMed] [Google Scholar]
- 29.Maslow, J. N., A. M. Slutsky, and R. D. Arbeit. 1993. The application of pulsed-field gel electrophoresis to molecular epidemiology, p. 563-572. In D. H. Persing, F. C. Tenover, T. F. Smith, and T. J. White (ed.), Diagnostic molecular microbiology. American Society for Microbiology, Washington, DC.
- 30.McKune, A. J., L. L. Smith, S. J. Semple, and A. A. Wadee. 2005. Influence of ultra-endurance exercise on immunoglobulin isotypes and subclasses. Br. J. Sports Med. 39:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melamed, I., Y. Bujanover, Y. S. Igra, D. Schwartz, V. Zakuth, and Z. Spirer. 1983. Campylobacter enteritis in normal and immunodeficient children. Am. J. Dis. Child. 137:752-753. [DOI] [PubMed] [Google Scholar]
- 32.Nachamkin, I., and A. M. Hart. 1985. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J. Clin. Microbiol. 21:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill, M. A., S. M. Opal, J. Heelan, R. Giusti, J. E. Cassidy, R. White, and K. H. Mayer. 1991. Failure of ciprofloxacin to eradicate convalescent fecal excretion after acute salmonellosis: experience during an outbreak in health care workers. Ann. Intern. Med. 114:195-199. [DOI] [PubMed] [Google Scholar]
- 34.Pai, C. H., F. Gillis, E. Tuomanen, and M. I. Marks. 1983. Erythromycin in treatment of Campylobacter enteritis in children. Am. J. Dis. Child. 137:286-288. [DOI] [PubMed] [Google Scholar]
- 35.Perlman, D. M., N. M. Ampel, R. B. Schifman, D. L. Cohn, C. M. Patton, M. L. Aguirre, W. L. Wang, and M. J. Blaser. 1988. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV). Ann. Intern. Med. 108:540-546. [DOI] [PubMed] [Google Scholar]
- 36.Pichler, H., G. Diridl, and D. Wolf. 1986. Ciprofloxacin in the treatment of acute bacterial diarrhea: a double blind study. Eur. J. Clin. Microbiol. 5:241-243. [DOI] [PubMed] [Google Scholar]
- 37.Poly, F., C. Ewing, S. Goon, T. E. Hickey, D. Rockabrand, G. Majam, L. Lee, J. Phan, N. J. Savarino, and P. Guerry. 2007. Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect. Immun. 75:3859-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poly, F., T. D. Read, Y. H. Chen, M. A. Monteiro, O. Serichantalergs, P. Pootong, L. Bodhidatta, C. J. Mason, D. Rockabrand, S. Baqar, C. K. Porter, D. Tribble, M. Darsley, and P. Guerry. 2008. Characterization of two Campylobacter jejuni strains for use in volunteer experimental infection studies. Infect. Immun. 76:5655-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Palacios, G. M. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin. Infect. Dis. 44:701-703. [DOI] [PubMed] [Google Scholar]
- 40.Strid, M. A., J. Engberg, L. B. Larsen, K. Begtrup, K. Molbak, and K. A. Krogfelt. 2001. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin. Diagn. Lab. Immunol. 8:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, B. V., J. Williamson, D. Jones, D. Coleman, J. Luck, and A. McGregor. 2007. Utility of serum Campylobacter specific antibodies in determining prior Campylobacter infection in neurological disease. J. Clin. Neurosci. 14:116-121. [DOI] [PubMed] [Google Scholar]
- 42.Ternhag, A., T. Asikainen, J. Giesecke, and K. Ekdahl. 2007. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin. Infect. Dis. 44:696-700. [DOI] [PubMed] [Google Scholar]
- 43.Thornley, J. P., D. Jenkins, K. Neal, T. Wright, J. Brough, and R. C. Spiller. 2001. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J. Infect. Dis. 184:606-609. [DOI] [PubMed] [Google Scholar]
- 44.Tribble, D., S. Baqar, M. P. Carmolli, C. K. Porter, K. K. Pierce, S. K. Sadigh, P. Guerry, C. Larsson, D. Rockabrand, C. Ventrone, F. Poly, C. E. Lyon, S. Dakdouk, A. Fingar, T. C. Gilliland, Jr., P. Daunais, E. Jones, S. L. Rymarchyk, C. D. Huston, M. Darsley, and B. D. Kirkpatrick. 2009. Campylobacter jejuni strain CG8421: a refined model for the study of campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin. Infect. Dis. 49:1512-1519. [DOI] [PubMed] [Google Scholar]
- 45.Tribble, D. R., J. W. Sanders, L. W. Pang, C. Mason, C. Pitarangsi, S. Baqar, A. Armstrong, P. Hshieh, A. Fox, E. A. Maley, C. Lebron, D. J. Faix, J. V. Lawler, G. Nayak, M. Lewis, L. Bodhidatta, and D. A. Scott. 2007. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin. Infect. Dis. 44:338-346. [DOI] [PubMed] [Google Scholar]
- 46.Walz, S., S. Baqar, H. Beecham, et al. 2001. Pre-exposure anti-Campylobacter jejuni immunoglobulin a levels associated with reduced risk of Campylobacter diarrhea in adults traveling to Thailand. Am. J. Trop. Med. Hyg. 65:652-656. [DOI] [PubMed] [Google Scholar]
- 47.Zilbauer, M., N. Dorrell, P. K. Boughan, A. Harris, B. W. Wren, N. J. Klein, and M. Bajaj-Elliott. 2005. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect. Immun. 73:7281-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.