Abstract
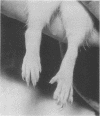
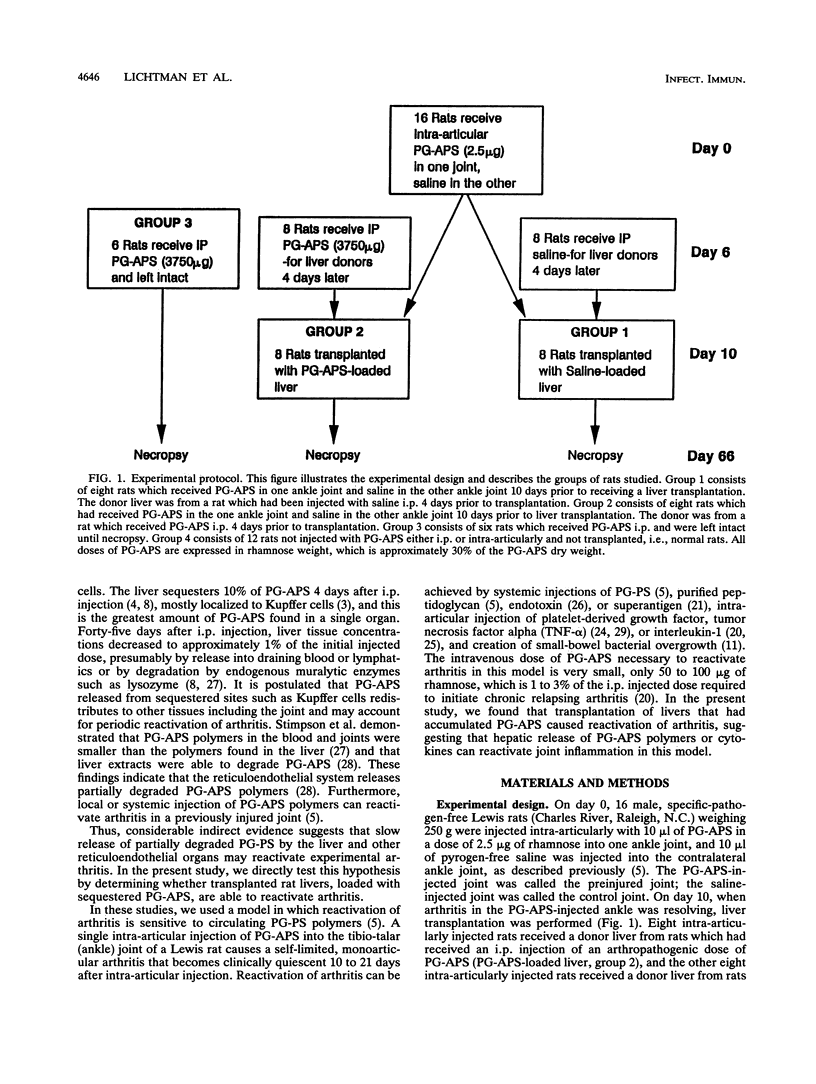
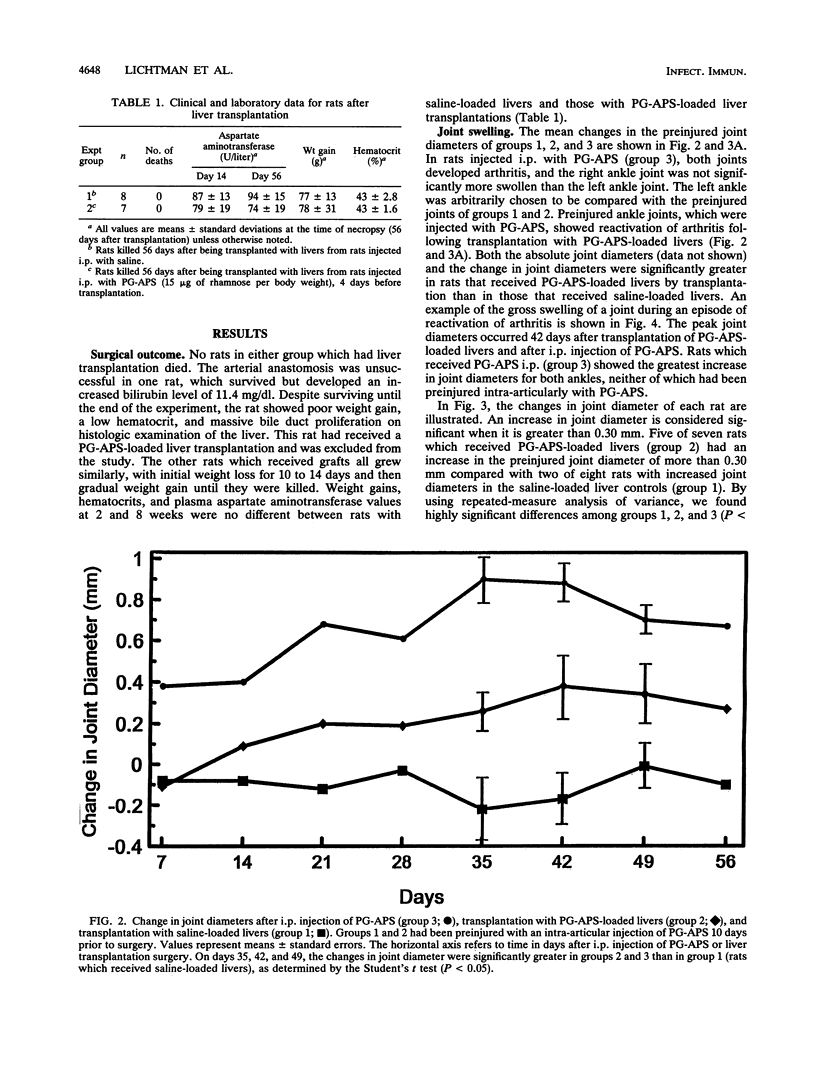
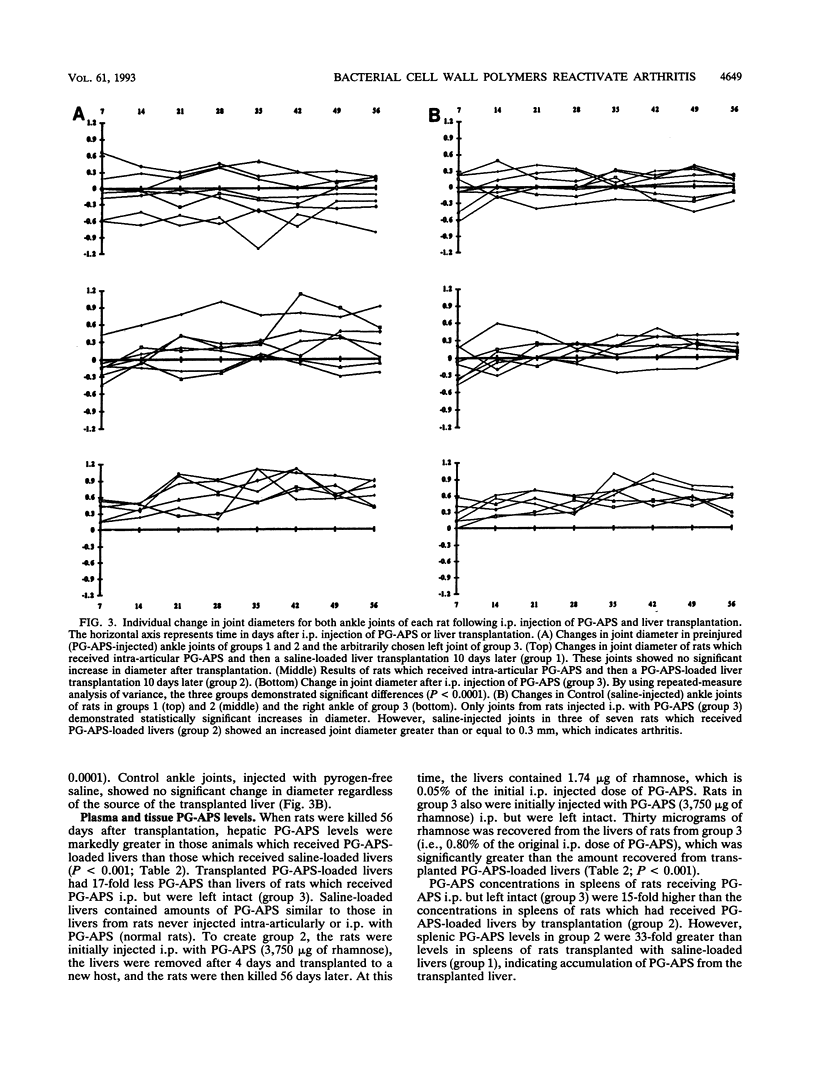
Intraperitoneal (i.p.) injection of peptidoglycan-polysaccharide derived from group A streptococci (PG-APS) causes chronic arthritis with spontaneous remissions and exacerbations. We hypothesized that, following i.p. injection, PG-APS released from hepatic stores mediated spontaneous recurrences of arthritis. We tested whether transplanted livers with large amounts of PG-APS were able to reactivate quiescent arthritis. Saline-loaded (group 1) or PG-APS-loaded (group 2) livers were transplanted into rats which had been injected intra-articularly 10 days earlier with PG-APS in one joint and saline in the other. A comparison was made with the arthritis that occurred in rats injected i.p. with PG-APS which did not receive transplants (group 3). Arthritis was monitored by serial measurement of joint diameters. Transplantation of saline-loaded livers (group 1) caused no reactivation of arthritis. However, transplantation of PG-APS-loaded livers (group 2) reactivated arthritis (P < 0.0001). Injection of PG-APS i.p. (group 3) induced the most-severe arthritis. PG-APS levels in plasma decreased with time, and PG-APS accumulated in the spleen in groups 2 and 3. Plasma and hepatic levels of PG-APS in rats injected i.p. with PG-APS were greater than levels in rats transplanted with PG-APS-loaded livers, which in turn were greater than levels in rats with saline-loaded livers. Plasma tumor necrosis factor did not correlate with recurrence of arthritis. Transplantation with PG-APS-loaded livers induced reactivation of arthritis in preinjured joints. The extent of arthritis was proportional to hepatic PG-APS content. Reactivation of arthritis may be mediated by slow release of liver-sequestered PG-APS or cytokines (not tumor necrosis factor) released by the liver.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Stimpson S. A., Cromartie W. J., Schwab J. H. Reactivation of streptococcal cell wall-induced arthritis by homologous and heterologous cell wall polymers. Arthritis Rheum. 1985 Dec;28(12):1402–1411. doi: 10.1002/art.1780281213. [DOI] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. S., Thomas P., Broitman S. A. Hepatic mechanisms for clearance and detoxification of bacterial endotoxins. J Nutr Biochem. 1990 Dec;1(12):620–628. doi: 10.1016/0955-2863(90)90020-l. [DOI] [PubMed] [Google Scholar]
- Janusz M. J., Chetty C., Eisenberg R. A., Cromartie W. J., Schwab J. H. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin. J Exp Med. 1984 Nov 1;160(5):1360–1374. doi: 10.1084/jem.160.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz M. J., Esser R. E., Schwab J. H. In vivo degradation of bacterial cell wall by the muralytic enzyme mutanolysin. Infect Immun. 1986 May;52(2):459–467. doi: 10.1128/iai.52.2.459-467.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Calne R. Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979 Jul;28(1):47–50. [PubMed] [Google Scholar]
- Lichtman S. N., Keku J., Schwab J. H., Sartor R. B. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect Immun. 1991 Feb;59(2):555–562. doi: 10.1128/iai.59.2.555-562.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman S. N., Okoruwa E. E., Keku J., Schwab J. H., Sartor R. B. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. J Clin Invest. 1992 Oct;90(4):1313–1322. doi: 10.1172/JCI115996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey C. L., Kossmann T., Allen J. B., Corcoran M. L., Brandes M. E., Wahl S. M. Role of Kupffer cells in developing streptococcal cell wall granulomas. Streptococcal cell wall induction of inflammatory cytokines and mediators. Am J Pathol. 1992 May;140(5):1205–1214. [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B., Anderle S. K., Rifai N., Goo D. A., Cromartie W. J., Schwab J. H. Protracted anemia associated with chronic, relapsing systemic inflammation induced by arthropathic peptidoglycan-polysaccharide polymers in rats. Infect Immun. 1989 Apr;57(4):1177–1185. doi: 10.1128/iai.57.4.1177-1185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B., Bond T. M., Schwab J. H. Systemic uptake and intestinal inflammatory effects of luminal bacterial cell wall polymers in rats with acute colonic injury. Infect Immun. 1988 Aug;56(8):2101–2108. doi: 10.1128/iai.56.8.2101-2108.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Anderle S. K., Brown R. R., Dalldorf F. G., Thompson R. C. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect Immun. 1991 Dec;59(12):4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Brown R. R., Anderle S. K., Schlievert P. M. Superantigen can reactivate bacterial cell wall-induced arthritis. J Immunol. 1993 May 1;150(9):4151–4159. [PubMed] [Google Scholar]
- Steffen R., Ferguson D. M., Krom R. A. A new method for orthotopic rat liver transplantation with arterial cuff anastomosis to the recipient common hepatic artery. Transplantation. 1989 Jul;48(1):166–168. doi: 10.1097/00007890-198907000-00044. [DOI] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Carter P. B., Sartor R. B., Cromartie W. J., Schwab J. H. Lipopolysaccharide induces recurrence of arthritis in rat joints previously injured by peptidoglycan-polysaccharide. J Exp Med. 1987 Jun 1;165(6):1688–1702. doi: 10.1084/jem.165.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Cromartie W. J., Schwab J. H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986 May;52(2):390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Lerch R. A., Cleland D. R., Yarnall D. P., Clark R. L., Cromartie W. J., Schwab J. H. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun. 1987 Jan;55(1):16–23. doi: 10.1128/iai.55.1.16-23.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Dougherty S., Evequoz V., Pluznik D. H., Wilder R. L., Hand A. R., Wahl L. M. T lymphocyte-dependent evolution of bacterial cell wall-induced hepatic granulomas. J Immunol. 1986 Oct 1;137(7):2199–2209. [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Yoshino S., Cleland L. G., Mayrhofer G., Brown R. R., Schwab J. H. Prevention of chronic erosive streptococcal cell wall-induced arthritis in rats by treatment with a monoclonal antibody against the T cell antigen receptor alpha beta. J Immunol. 1991 Jun 15;146(12):4187–4189. [PubMed] [Google Scholar]