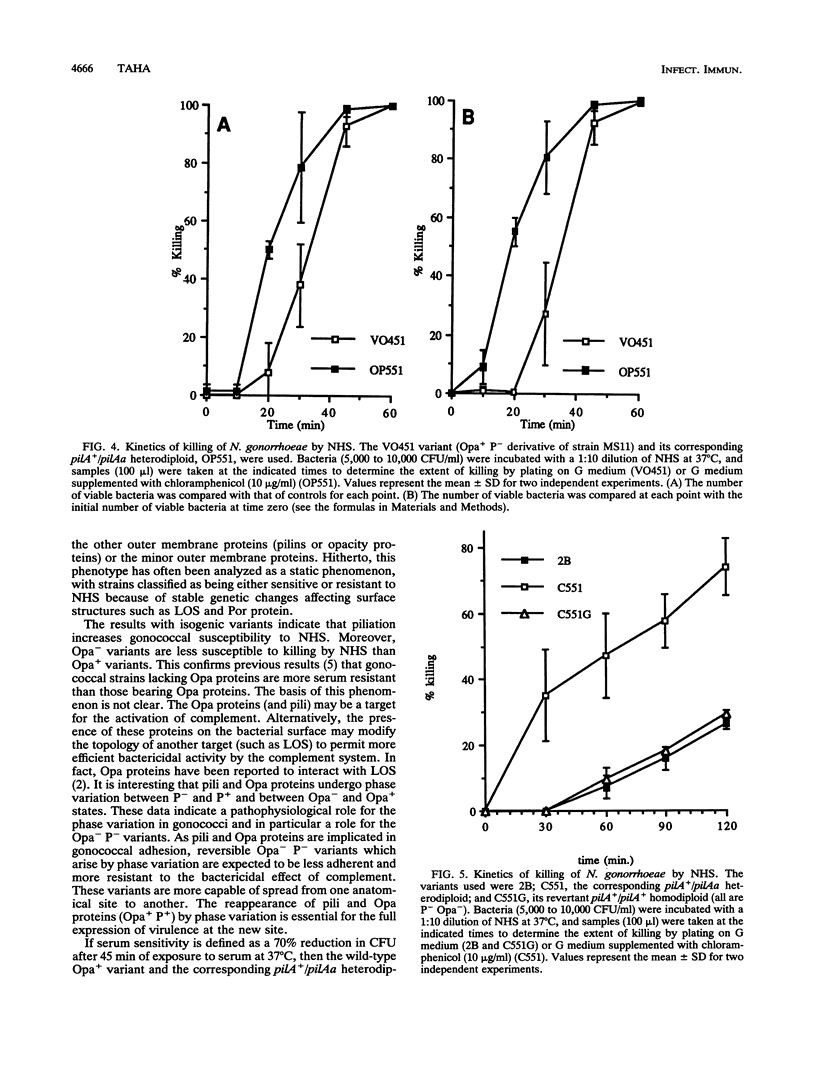
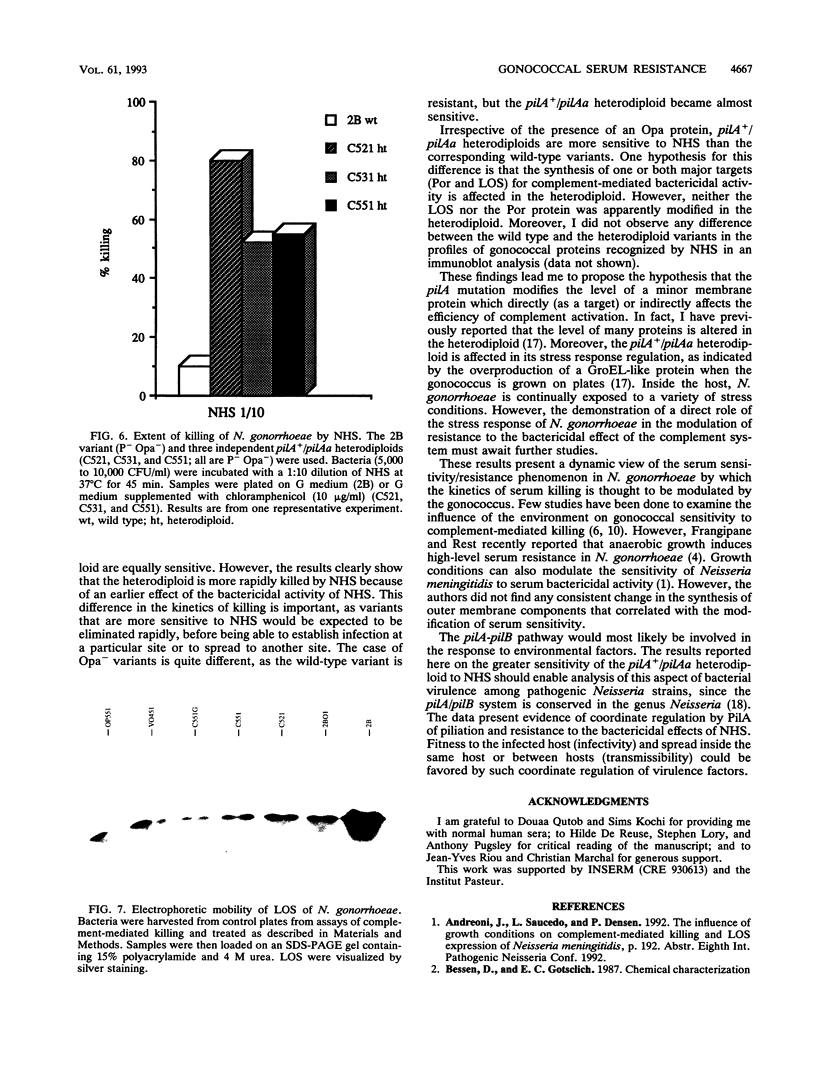
Abstract
PilA is a pleiotropic transcriptional regulator in Neisseria gonorrhoeae, encoded by an essential gene, pilA. It regulates pilin gene expression and stress response and it is implicated in gonococcal adaptation to external signals. All these phenomena may participate in gonococcal virulence. In this report, I tested the role of PilA in another aspect of gonococcal virulence, resistance to the bactericidal effect of normal human serum. Gonococcal mutants with impaired PilA function were more susceptible to the bactericidal effect of normal human serum than the isogenic wild-type strain. However, the major outer membrane protein and the lipooligosaccharide, targets for complement-mediated killing by the serum, were unchanged in the mutants. I discuss the role of PilA in modulating gonococcal sensitivity and resistance to normal human serum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen D., Gotschlich E. C. Chemical characterization of binding properties of opacity-associated protein II from Neisseria gonorrhoeae. Infect Immun. 1987 Jan;55(1):141–147. doi: 10.1128/iai.55.1.141-147.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco de Hormaeche R., van Crevel R., Hormaeche C. E. Neisseria gonorrhoeae LPS variation, serum resistance and its induction by cytidine 5'-monophospho-N-acetylneuraminic acid. Microb Pathog. 1991 Apr;10(4):323–332. doi: 10.1016/0882-4010(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Frangipane J. V., Rest R. F. Anaerobic growth and cytidine 5'-monophospho-N-acetylneuraminic acid act synergistically to induce high-level serum resistance in Neisseria gonorrhoeae. Infect Immun. 1993 May;61(5):1657–1666. doi: 10.1128/iai.61.5.1657-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., Davies D. B., Spillane B. J., Mahenthiralingam E. Influence of iron-limited and replete continuous culture on the physiology and virulence of Neisseria gonorrhoeae. J Gen Microbiol. 1989 Apr;135(4):851–863. doi: 10.1099/00221287-135-4-851. [DOI] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. A., Cole J. A., Patel P. V., Parsons N. J., Fox J. E., Smith H. Cytidine 5'-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988 Dec;134(12):3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S112–S117. doi: 10.1128/cmr.2.suppl.s112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Billyard E., So M., Storzbach S., Meyer T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985 Feb;40(2):293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Taha M. K., Dupuy B., Saurin W., So M., Marchal C. Control of pilus expression in Neisseria gonorrhoeae as an original system in the family of two-component regulators. Mol Microbiol. 1991 Jan;5(1):137–148. doi: 10.1111/j.1365-2958.1991.tb01834.x. [DOI] [PubMed] [Google Scholar]
- Taha M. K., Larribe M., Dupuy B., Giorgini D., Marchal C. Role of pilA, an essential regulatory gene of Neisseria gonorrhoeae, in the stress response. J Bacteriol. 1992 Sep;174(18):5978–5981. doi: 10.1128/jb.174.18.5978-5981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. K., Marchal C. Conservation of Neisseria gonorrhoeae pilus expression regulatory genes pilA and pilB in the genus Neisseria. Infect Immun. 1990 Dec;58(12):4145–4148. doi: 10.1128/iai.58.12.4145-4148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. K., So M., Seifert H. S., Billyard E., Marchal C. Pilin expression in Neisseria gonorrhoeae is under both positive and negative transcriptional control. EMBO J. 1988 Dec 20;7(13):4367–4378. doi: 10.1002/j.1460-2075.1988.tb03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Glynn A. A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature. 1970 Jul 25;227(5256):382–384. doi: 10.1038/227382a0. [DOI] [PubMed] [Google Scholar]