Abstract
Immunity to the asexual blood stage of Plasmodium falciparum is complex and likely involves several effector mechanisms. Antibodies are thought to play a critical role in malaria immunity, and a corresponding in vitro correlate of antibody-mediated immunity has long been sought to facilitate malaria vaccine development. The growth inhibition assay (GIA) measures the capacity of antibodies to limit red blood cell (RBC) invasion and/or growth of P. falciparum in vitro. In humans, naturally acquired and vaccine-induced P. falciparum-specific antibodies have growth-inhibitory activity, but it is unclear if growth-inhibitory activity correlates with protection from clinical disease. In a longitudinal study in Mali, purified IgGs, obtained from plasmas collected before the malaria season from 220 individuals aged 2 to 10 and 18 to 25 years, were assayed for growth-inhibitory activity. Malaria episodes were recorded by passive surveillance over the subsequent 6-month malaria season. Logistic regression showed that greater age (odds ratio [OR], 0.78; 95% confidence interval [95% CI], 0.63 to 0.95; P = 0.02) and growth-inhibitory activity (OR, 0.50; 95% CI, 0.30 to 0.85; P = 0.01) were significantly associated with decreased malaria risk in children. A growth-inhibitory activity level of 40% was determined to be the optimal cutoff for discriminating malaria-immune and susceptible individuals in this cohort, with a sensitivity of 97.0%, but a low specificity of 24.3%, which limited the assay's ability to accurately predict protective immunity and to serve as an in vitro correlate of antibody-mediated immunity. These data suggest that antibodies which block merozoite invasion of RBC and/or inhibit the intra-RBC growth of the parasite contribute to but are not sufficient for the acquisition of malaria immunity.
Plasmodium falciparum malaria persists as a global public health threat (23). The development of an effective malaria vaccine is widely viewed as a critical step toward combating this disease, but replicating naturally acquired malaria immunity, which develops only after repeated P. falciparum infections, is proving to be a formidable challenge (54). At the heart of this challenge is the apparent complexity of the immune response that underlies naturally acquired malaria immunity, a poorly understood process that likely involves several immune effector mechanisms (28). Antibodies clearly play a critical role in acquired immunity against the blood stages of P. falciparum, as evidenced by passive transfer studies in humans (10), but the antigen specificity of protective antibodies and their effector functions remain unclear.
An in vitro assay which correlates with in vivo protective immunity has long been sought to address these critical knowledge gaps and to facilitate the development and evaluation of malaria vaccine candidates. The growth inhibition assay (GIA) measures the capacity of antibodies to limit red blood cell (RBC) invasion and/or intra-RBC growth of P. falciparum in vitro. Several versions of this assay have been used in the preclinical phases of malaria vaccine development, particularly for apical membrane antigen 1 (AMA1) and merozoite surface protein 1 (MSP1), proteins expressed in apical organelles and on the surfaces of merozoites, the invasive form of the blood-stage parasite, respectively (1, 9, 14, 18, 25, 27, 36, 47, 49, 52, 53). For example, vaccination of Aotus monkeys with MSP1, the most widely studied blood-stage vaccine candidate, elicits antibodies with growth-inhibitory activity (including invasion-inhibitory activity) that correlate with protection against a virulent P. falciparum challenge in vivo (53).
In humans, however, the relationship between growth-inhibitory activity and protection against malaria is less clear, due in part to the fact that blood-stage malaria vaccine candidates have not yet demonstrated protective efficacy in clinical trials. In separate phase 1 studies, vaccination with MSP142 formulated on Alhydrogel/CPG 7909 (34) or AS02 (42) induced growth-inhibitory activity in malaria-naïve individuals, but in a subsequent phase 2b trial, vaccination with MSP142/AS02 failed to protect Kenyan children from malaria (GIA results not reported) (44). Similarly, vaccination with AMA1 formulated on Alhydrogel induced growth-inhibitory activity in malaria-naïve adults (30) but had no impact on the frequency of parasitemic episodes in a phase 2 study of Malian children (GIA results not reported) (50). Taken together, these clinical trials suggest that vaccine-induced antibodies which block RBC invasion and/or intra-RBC growth of the parasite are not sufficient to confer protective immunity, at least not at the levels achieved in these trials; however, the results of these trials do not rule out the possibility that such antibodies are a necessary component of a multifaceted protective immune response.
Given that there are currently no effective blood-stage vaccines, the relative contribution of antibodies with growth-inhibitory activity to protective immune responses in humans can be addressed only with studies of individuals who have acquired malaria immunity through natural P. falciparum exposure. Many studies in areas where malaria is endemic have demonstrated that P. falciparum exposure is associated with growth-inhibitory activity in the total (6, 33, 37, 45), MSP1-specific (17, 37, 43), and AMA1-specific (24, 35, 39) IgG fractions, but relatively few studies have investigated the relationship between growth-inhibitory activity and malaria risk, and of these studies, most have used the appearance of asexual parasites in the blood as a surrogate for clinical disease. The results of these studies have varied, with growth-inhibitory activity being associated with protection from blood-stage infection in some (15, 26), but not others (11, 37). The few published studies to use clinical malaria as an end point did not find an association between growth-inhibitory activity and malaria risk (32, 33, 45). Variations in the study design (particularly the clinical end point used to measure malaria risk), the intensity and timing of P. falciparum transmission at each site, and the GIA procedure employed make the collective interpretation of these studies difficult, and thus the relationship between growth-inhibitory activity and malaria risk remains unclear.
The aim of the study described here was to more clearly define the relationship between in vitro growth-inhibitory activity and the risk of clinical malaria. To this end, we used a standardized method to measure the growth-inhibitory activity of purified IgGs (30) from the plasmas of 220 children and adults collected before the malaria season. Unlike previous studies, we correlated this activity with three common measures of malaria risk: (i) whether or not malaria was experienced (odds of malaria), (ii) the time to the first malaria episode, and (iii) the number of malaria episodes per subject (incidence of malaria). The study was carried out at a site with an intense, sharply demarcated malaria season (13), ideal for prospectively examining the correlation between growth-inhibitory activity and the subsequent risk of clinical malaria. We found that growth-inhibitory activity was significantly associated with a reduction in the odds of experiencing malaria but that it lacked the specificity to reliably discriminate malaria-immune and susceptible individuals. This is consistent with a model in which invasion and/or growth-inhibitory antibodies contribute to but do not fully explain the full range of naturally acquired malaria immunity.
MATERIALS AND METHODS
Study cohort and clinical end points.
This study was carried out in Kambila, a small (<1 km2), rural village with a population of 1,500 situated 20 kilometers north of Bamako, the capital of Mali. P. falciparum transmission is seasonal and intense at this site from July through December. A detailed description of the study site and cohort has been published elsewhere (13). In May 2006, during a 2-week period just prior to the malaria season, 225 individuals aged 2 to 10 years and 18 to 25 years were enrolled after random selection from an age-stratified census of the entire village population. Enrollment exclusion criteria were a hemoglobin level of <7 g/dl, fever of ≥37.5°C, acute systemic illness, use of antimalarial or immunosuppressive medications in the past 30 days, or pregnancy. During the 2-week enrollment period, blood smears and venous blood samples were collected, and plasma was stored at −80°C. Stool and urine were examined at enrollment for the presence of helminth infections. From May 2006 through January 2007, participants were instructed to report symptoms of malaria at the village health center, staffed 24 h a day by a study physician. For individuals with signs or symptoms of malaria, blood smears were examined for the presence of P. falciparum. Patients with positive smear results were treated according to international standards. The case definition of malaria for research purposes was an axillary temperature of ≥37.5°C, P. falciparum asexual parasitemia of ≥5,000 parasites/μl, and a nonfocal physical examination by the study physician. Severe malaria, as defined by the WHO (2), was included in this definition. The following three clinical end points were used to evaluate the relationship between growth-inhibitory activity measured before the malaria season and malaria risk during the 9-month study period: (i) whether or not malaria was experienced (odds of malaria), (ii) the time to the first malaria episode, and (iii) the number of malaria episodes per subject (incidence of malaria).
The ethics committee of the Faculty of Medicine, Pharmacy, and Odonto-Stomatology and the institutional review board at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, approved this study. Written, informed consent was obtained from adult participants and from the parents or guardians of participating children.
GIA.
A detailed description of IgG purification and the GIA has been reported elsewhere (30). In brief, total IgG fractions were purified from individual plasma samples by use of protein G columns, and the eluted fractions were dialyzed against RPMI 1640 and concentrated to 25 mg/ml. Purified IgGs were incubated with O+ human RBC to remove nonspecific antibodies. Test IgGs (6.3 mg/ml in the final test well) were incubated with P. falciparum-parasitized RBC for ∼40 h, and increases in parasitemia were quantified by biochemical measurement of P. falciparum lactate dehydrogenase.
Malaria slides.
Thick blood smears were stained with Giemsa stain and counted against 300 leukocytes. P. falciparum densities were recorded as the number of asexual parasites/μl of whole blood, based on an average leukocyte count of 7,500/μl. Each smear was evaluated separately by two expert microscopists blinded to the clinical status of study participants. Any discrepancies were resolved by a third expert microscopist.
Hemoglobin typing.
Hemoglobin was typed by high-performance liquid chromatography (HPLC) (D-10 instrument; Bio-Rad, Hercules, CA).
Stool and urine exams for helminth infection.
At enrollment, duplicate stool samples were examined for Schistosoma mansoni eggs and other intestinal helminths, using the semiquantitative Kato-Katz method. To detect Schistosoma haematobium eggs, 10 ml of urine was poured over Whatman filter paper. One or two drops of ninhydrin was placed on the filter and left to air dry. After drying, the filter was dampened with tap water and helminth eggs detected by microscopy.
Statistical analysis.
Data were analyzed using STATA (release 10.0; StataCorp LP) and JMP software (SAS, version 7.0). The Kruskal-Wallis test was used to compare continuous variables between groups, and Fisher's exact test was used to compare categorical variables. The malaria-free probability over the 9-month study period was estimated by the Kaplan-Meier curve, and times to the first malaria episode were compared by applying the log rank test. A logistic regression model was used to assess the relationship between growth-inhibitory activity before the malaria season and the subsequent odds of malaria during the study period, controlling for the effects of age, gender, ethnicity, distance lived from study clinic, self-reported bed-net use, and hemoglobin type. The same list of variables was included in Cox's proportional hazard and Poisson regression models to determine the relationships between growth-inhibitory activity and the hazard ratio and the repeated infection rate of malaria, respectively. For this analysis, growth-inhibitory activity was divided into deciles so that the regression coefficient for this variable represents the change in malaria risk with each 10% increase in growth-inhibitory activity, holding all other independent variables constant. To account for overdispersion, bootstrap test and bootstrap confidence intervals were adopted for Poisson regression. We performed the Hosmer and Lemeshow goodness-of-fit test for the logistic regression model and the deviance goodness-of-fit test for the Poisson regression model. The proportional hazard assumption was examined by using tests based on the scaled Schoenfeld residuals (20). Partition analysis was used to determine the level of growth-inhibitory activity that best discriminated malaria-immune and susceptible individuals. This method finds a set of cuts or groupings of x values that best predict a y value by exhaustively searching all possible cuts or groupings, recursively forming a tree of decision rules until the best fit is reached (19). Sensitivity and specificity were calculated using the cutoff value for growth-inhibitory activity. For all tests, two-tailed P values were considered significant if they were <0.05.
RESULTS
Baseline characteristics and clinical outcomes.
In May 2006, we initiated an observational cohort study in Mali to investigate the mechanisms underlying the acquisition and maintenance of malaria immunity. A detailed description of this study site and cohort has been reported elsewhere (13). During a 2-week period 1 month prior to the 6-month malaria season, we enrolled 225 individuals (of 237 screened) in four predefined age groups: 2 to 4 years (n = 73), 5 to 7 years (n = 52), 8 to 10 years (n = 51), and 18 to 25 years (n = 49). The proportion of individuals who attended scheduled follow-up visits within the cohort design was >99% for children (2 to 10 years) and 82% for adults (18 to 25 years) during the 9-month study period. The baseline demographic and clinical characteristics of the cohort are shown in Table 1, according to age group. As previously reported, only three of the characteristics shown in Table 1 were associated with a decreased malaria risk—increasing age, the sickle cell trait (HbAS), and asymptomatic P. falciparum parasitemia at study enrollment (13). Notably, individuals with asymptomatic P. falciparum infection at enrollment (the end of the dry season) were not treated with antimalarials. During 495 unscheduled clinic visits, 298 cases of malaria were diagnosed, 5 of which met WHO criteria for severe malaria (2). As expected, the incidence of malaria and the proportion of individuals experiencing at least one malaria episode decreased with age, whereas the time to the first malaria episode increased with age (Table 2).
TABLE 1.
Baseline characteristics of the study cohort
| Characteristic | Value for age group |
Value for all participants (n = 225) | |||
|---|---|---|---|---|---|
| 2-4 yr (n = 73) | 5-7 yr (n = 52) | 8-10 yr (n = 51) | 18-25 yr (n = 49) | ||
| Gender (no. [%] of females) | 42 (57.5) | 25 (48.1) | 19 (37.3) | 30 (61.2) | 116 (51.6) |
| Ethnicity (no. [%]) | |||||
| Bambara | 48 (65.8) | 26 (50.0) | 27 (53.0) | 33 (67.4) | 134 (59.6) |
| Sarakole | 21 (28.8) | 23 (44.2) | 20 (39.2) | 13 (26.5) | 77 (34.2) |
| Fulani | 3 (4.1) | 3 (5.8) | 3 (5.9) | 2 (4.1) | 11 (4.9) |
| Malinke | 1 (1.4) | 0 (0.0) | 1 (2.0) | 1 (2.0) | 3 (1.3) |
| No. (%) of individuals with P. falciparum-positive smear at enrollment | 4 (5.5) | 4 (7.7) | 5 (9.8) | 3 (6.1) | 16 (7.1) |
| Level of parasitemia if smear was positive at enrollment (no. of parasites/μl) (geometric mean [95% CI]) | 1,438 (159-12,973) | 3,616 (1,500-8,715) | 415 (134-1,287) | 953 (39-23,382) | 1,137 (579-2,232) |
| No. (%) of individuals positive for gastrointestinal helminths at enrollmenta | 9 (13.0) | 4 (8.3) | 4 (9.3) | 0 (0.0) | 17 (9.0) |
| No. (%) of individuals positive for urine schistosomiasis at enrollmentb | 0 (0.0) | 0 (0.0) | 2 (4.3) | 9 (28.1) | 11 (6.0) |
| Hemoglobin level at enrollment (g/l) (mean ± SD) | 11.2 ± 1.2 | 11.8 ± 1.0 | 12.3 ± 1.1 | 13.6 ± 1.4 | 12.1 ± 1.5 |
| Distance lived from clinic (km) (mean ± SD) | 0.39 ± 0.11 | 0.40 ± 0.14 | 0.37 ± 0.10 | 0.36 ± 0.09 | 0.38 ± 0.11 |
| No. (%) of individuals who used bed netsc | 21 (28.8) | 16 (30.8) | 9 (17.6) | 15 (30.6) | 61 (27.1) |
| No. (%) of individuals with hemoglobin typed | |||||
| AA | 50 (72.5) | 43 (86.0) | 35 (68.6) | 32 (76.2) | 160 (75.5) |
| AS | 8 (11.6) | 4 (8.0) | 5 (9.8) | 5 (11.9) | 22 (10.4) |
| AC | 10 (14.5) | 3 (6.0) | 11 (21.6) | 5 (11.9) | 29 (13.7) |
| CC | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
Data available for 190 subjects.
Data available for 184 subjects.
Nightly bed-net use was self-reported at the end of the malaria season.
Data available for 212 subjects.
TABLE 2.
Malaria-related clinical outcomes during the 9-month study period, by age group
| Outcome | Value for age group |
Value for all individuals (n = 225) | P valuea | |||
|---|---|---|---|---|---|---|
| 2-4 yr (n = 73) | 5-7 yr (n = 52) | 8-10 yr (n = 51) | 18-25 yr (n = 49) | |||
| Malaria incidence (mean ± SD)b | 1.99 ± 1.25 | 1.94 ± 1.21 | 0.98 ± 1.05 | 0.08 ± 0.28 | 1.33 ± 1.30 | <0.001 |
| No. of cases of severe malariac | 4 | 1 | 0 | 0 | 5 | |
| Time to first malaria episode for those who experienced malaria (days [median])d | 101 | 114 | 130 | 153 | 115 | 0.02 |
| No. (%) of individuals with at least one malaria episode | 63 (86.3) | 45 (86.5) | 31 (60.8) | 4 (8.2) | 143 (63.6) | <0.001 |
| Level of parasitemia at first malaria episode (no. of parasites/μl) (geometric mean [95% CI]) | 34,374 (24,955-47,348) | 15,687 (9,623-25,574) | 10,433 (5,079-21,427) | 8,816 (4,082-19,037) | 19,625 (15,004-25,668) | 0.04 |
P values were derived by using Fisher's exact test for categorical variables and the Kruskal-Wallis test for continuous variables.
A malaria episode was defined as a temperature of >37.5°C, asexual parasitemia of >5,000 parasites/microliter, and nonfocal physical examination.
According to WHO definition of severe malaria.
Days since study enrollment.
Growth-inhibitory activity increases with age and asymptomatic P. falciparum infection.
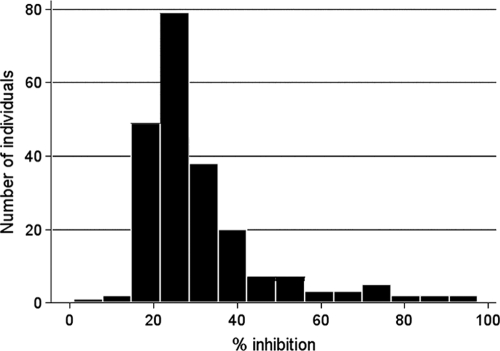
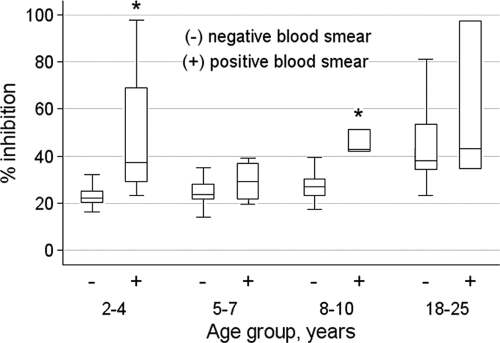
Of the 225 study participants, 220 had plasma samples available for analysis. The growth-inhibitory activity distribution for all 220 subjects is shown in Fig. 1. Inhibitory activity ranged from −7% to 97.6%, with a median of 26.0%. Growth-inhibitory activity increased with age among those with negative blood smears at enrollment (Fig. 2) (median GIA activities were 22.3% [2- to 4-year-olds], 23.8% [5- to 7-year-olds], 26.9% [8- to 10-year olds], and 38.1% [18- to 25-year-olds]; P < 0.001). In all age groups, growth-inhibitory activity was higher among those with concurrent asymptomatic P. falciparum infection (Fig. 2), although the increase in inhibitory activity reached statistical significance only for the 2- to 4-year-old (P < 0.01) and 8- to 10-year-old (P = 0.02) age groups. Interestingly, growth-inhibitory activity in parasitemic children approached adult levels (Fig. 2). It is notable that growth-inhibitory activity did not differ significantly by hemoglobin type (i.e., HbAA, HbAS, or HbAC).
FIG. 1.
Distribution of growth-inhibitory activities in the study cohort. The histogram shows the growth-inhibitory activities for the 220 study participants measured just prior to the malaria season. Inhibitory activities ranged from −7% to 97.6%, with a median of 26.0%. Only one individual had a negative value for % growth inhibition (−7% [data not shown]).
FIG. 2.
Growth-inhibitory activity by age group and whether individuals were parasitemic with P. falciparum just prior to the malaria season. Box-and-whisker plots represent the smallest and largest (whiskers), the lower and upper quartiles of (top and bottom of box), and the median (horizontal line across box) growth-inhibitory activity. Growth-inhibitory activity in nonparasitemic individuals increased with age (P < 0.001). Within each age group, the median growth-inhibitory activity was higher in parasitemic than in nonparasitemic individuals, but this difference reached statistical significance only in the 2- to 4-year-old (P < 0.01) and 8- to 10-year-old (P = 0.02) age groups, as indicated by an asterisk above the bar. Sample sizes for nonparasitemic individuals were as follows: 2- to 4-year-olds, n = 68; 5- to 7-year-olds, n = 45; 8- to 10-year-olds, n = 45; and 18- to 25-year-olds, n = 46. Those for parasitemic individuals were as follows: 2- to 4-year-olds, n = 4; 5- to 7-year-olds, n = 4; 8- to 10-year-olds, n = 5; and 18- to 25-year-olds, n = 3. The nonparametric Kruskal-Wallis test was used for statistical analysis.
Multivariate analysis of growth-inhibitory activity and malaria risk.
We determined prospectively whether growth-inhibitory activity measured just prior to the 6-month malaria season was associated with subsequent malaria risk. For this analysis, a malaria episode was defined as an axillary temperature of ≥37.5°C, P. falciparum asexual parasitemia of ≥5,000 parasites/μl, and a nonfocal examination by the study physician. Three measures of malaria risk were analyzed: (i) whether or not malaria was experienced (odds of malaria), (ii) the time to the first malaria episode, and (iii) the number of malaria episodes per subject (incidence of malaria). Because the incidence rate of malaria was very low in adults during the study period (Table 2), they were excluded from further analysis.
To adjust for potential confounding factors of the relationship between growth-inhibitory activity and malaria risk in children aged 2 to 10 years, the following variables were included in multivariate regression models: age, gender, ethnicity, distance lived from study clinic, self-reported bed-net use, and hemoglobin type (Table 1). Because individuals with asymptomatic P. falciparum infection before the malaria season (7.1% of the cohort) had decreased malaria risk (13) and increased growth-inhibitory activity (Fig. 1), they were excluded from this analysis. The results of logistic regression analysis (Table 3) showed that age (odds ratio [OR], 0.78; 95% confidence interval [95% CI], 0.63 to 0.95; P = 0.02), HbAS (OR, 0.23; 95% CI, 0.06 to 0.87; P = 0.03), and growth-inhibitory activity (OR, 0.50; 95% CI, 0.30 to 0.85; P = 0.01) were associated with decreased malaria risk. Cox regression showed that age (hazard ratio [HR], 0.86; 95% CI, 0.80 to 0.94; P < 0.001) and HbAS (HR, 0.41; 95% CI, 0.21 to 0.79; P = 0.01) were associated with decreased malaria risk, as measured by the time to first malaria episode, whereas growth-inhibitory activity was not (HR, 0.80; 95% CI, 0.63 to 1.02; P = 0.07). Finally, Poisson regression revealed that age (incidence rate ratio [IRR], 0.91; 95% CI, 0.86 to 0.96; P = 0.001), the Sarakole ethnic group (IRR, 1.53; 95% CI, 1.07 to 2.18; P = 0.02), and HbAS (IRR, 0.43; 95% CI, 0.27 to 0.68; P < 0.001) were significant predictors of decreased malaria incidence, whereas growth-inhibitory activity was not. Diagnostic tests indicated that the multivariate regression analyses did not violate the assumptions of each model type.
TABLE 3.
Results of multivariate regression analysis for children aged 2 to 10 years
| Explanatory variable | Result of regression analysis for malaria outcome |
|||||
|---|---|---|---|---|---|---|
| Whether malaria was experienced |
Time to first malaria episode |
Malaria incidence |
||||
| OR (95% CI) | P | HR (95% CI) | P | IRR (95% CI) | P | |
| Age (yr) | 0.78 (0.63-0.95) | 0.02 | 0.86 (0.80-0.94) | <0.001 | 0.91 (0.86-0.96) | 0.001 |
| Gender | 2.04 (0.76-5.46) | 0.16 | 1.38 (0.94-2.03) | 0.10 | 1.03 (0.82-1.30) | 0.80 |
| Ethnicity, Bambara | 0.23 (0.03-2.08) | 0.19 | 0.68 (0.34-1.34) | 0.26 | 1.12 (0.79-1.58) | 0.52 |
| Ethnicity, Sarakole | 0.84 (0.08-8.96) | 0.89 | 1.00 (0.49-2.06) | 0.99 | 1.53 (1.07-2.18) | 0.02 |
| Distance lived from clinic (km) | 0.30 (0.004-24.02) | 0.59 | 0.48 (0.07-3.23) | 0.45 | 0.77 (0.23-2.59) | 0.67 |
| Bed-net usea | 0.70 (0.25-1.97) | 0.50 | 0.89 (0.59-1.35) | 0.59 | 0.96 (0.75-1.23) | 0.77 |
| HbAS type | 0.23 (0.06-0.87) | 0.03 | 0.41 (0.21-0.79) | 0.01 | 0.43 (0.27-0.68) | <0.001 |
| HbAC type | 0.56 (0.16-1.95) | 0.36 | 0.81 (0.47-1.40) | 0.44 | 1.14 (0.80-1.63) | 0.47 |
| Growth-inhibitory activityb | 0.50 (0.30-0.85) | 0.01 | 0.80 (0.63-1.02) | 0.07 | 0.93 (0.75-1.16) | 0.53 |
Nightly bed-net use was self-reported at the end of the malaria season.
For this analysis, growth-inhibitory activity was divided into deciles, and thus the regression coefficient for this variable represents the change in malaria risk with each 10% increase in growth-inhibitory activity, holding all other independent variables constant.
Sensitivity and specificity analysis of growth-inhibitory activity and malaria risk.
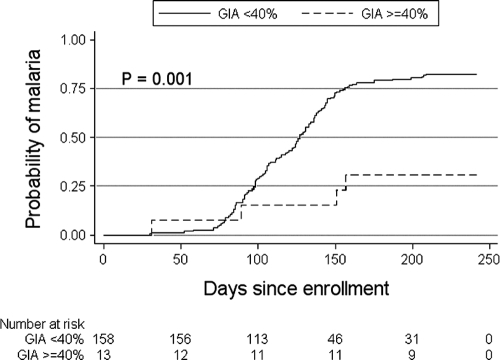
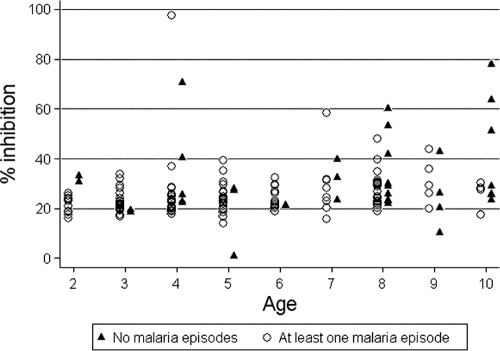
An ideal in vitro surrogate of malaria immunity would be highly sensitive and specific in discriminating between malaria-immune and susceptible individuals. We analyzed growth-inhibitory activity as a dichotomous variable—defined by the level of growth-inhibitory activity that best discriminated children aged 2 to 10 years who did not experience malaria during the study period from those who experienced at least one episode. Children who were parasitemic at enrollment were included. By partition analysis, the optimal cutoff was determined to be 40%. The odds of experiencing a malaria episode among those with growth-inhibitory activity of <40% was 10.4 times higher than that among those with growth-inhibitory activity of ≥40%. Time-to-event analysis (Fig. 3) showed that a growth-inhibitory activity of ≥40% was associated with a significant delay in the time to the first malaria episode (P < 0.001 by log rank test). The growth-inhibitory activity cutoff of 40% had a sensitivity and specificity of 97.0% and 24.3%, respectively, where the high sensitivity and low specificity reflect the overlapping distributions of inhibitory activity among those who did and did not experience at least one malaria episode (Fig. 4).
FIG. 3.
Kaplan-Meier estimates of the cumulative probability of malaria in children aged 2 to 10 years during the 6-month malaria season, by whether growth-inhibitory activity was greater or less than 40%. Malaria was defined as an axillary temperature of ≥37.5°C, P. falciparum asexual parasitemia of ≥5,000 parasites/μl, and a nonfocal physical examination by the study physician. The number of individuals at risk for the first malaria episode in the two groups over the course of the study period is shown below the plot. The P value was obtained using the log rank test.
FIG. 4.
Distribution of growth-inhibitory activities in malaria-immune (no malaria episodes) and susceptible (≥1 malaria episode) children aged 2 to 10 years. Malaria was defined as an axillary temperature of ≥37.5°C, P. falciparum asexual parasitemia of ≥5,000 parasites/μl, and a nonfocal physical examination by the study physician.
DISCUSSION
In this prospective study, we found that growth-inhibitory activity measured before the malaria season was associated with a decreased probability of experiencing clinical malaria during the subsequent 6-month malaria season, an association that remained statistically significant after adjusting for age, sickle cell trait, and other potential confounders of malaria risk. Growth-inhibitory activity was also associated with a delayed time to the first malaria episode, although this association remained only marginally significant in multivariate analysis. We found no significant association between growth-inhibitory activity and the number of malaria episodes per individual during the study period (i.e., malaria incidence). Although a growth-inhibitory activity of ≥40% best predicted protection from malaria, this cutoff lacked the specificity to discriminate those who did and did not experience malaria. This is best illustrated by the data in Fig. 4 that show overlapping distributions of growth-inhibitory activity for malaria-immune and susceptible children; in other words, there were individuals with a relatively high level of growth-inhibitory activity who got malaria and individuals with a relatively low level who did not get malaria. Taken together, these findings suggest that antibodies which block merozoite invasion and/or inhibit parasite growth contribute to naturally acquired malaria immunity but that these antibodies do not appear to be necessary or sufficient for protection.
This finding is not unexpected, since growth-inhibitory activity is a putative measure of antibodies that interfere with merozoite invasion and/or parasite growth and not with other possible components of a protective antibody response, such as (i) blocking the binding of infected RBC (iRBC) to endothelial cells (3, 4), (ii) opsonization and destruction of merozoites and iRBC by phagocytic cells (8, 21, 40), (iii) neutralization of toxic molecules such as glycosylphosphatidylinositol (38, 51), and (iv) antibody-dependent cellular inhibition of parasite growth (7).
Several features of this cohort study made it well suited for assessing the relationship between growth-inhibitory activity and malaria risk. The study population was an age-stratified, random sample representing 15% of all individuals living in a rural, well-circumscribed, nonmigratory community where antimalarial drugs were provided exclusively by the study investigators. Enrollment of all study participants was completed during a 2-week period approximately 1 month prior to the abrupt onset of intense P. falciparum transmission. At enrollment, 93% of participants had blood smears that were negative for P. falciparum, obviating the need for a treatment-reinfection study design, an approach that may alter the subsequent risk of malaria (12). Moreover, several aspects of the study favored an unbiased detection of malaria episodes: (i) the proportion of individuals who attended scheduled follow-up visits was >99% among children aged 2 to 10 years, indicating a high degree of study awareness and participation; (ii) the average distance from individuals' homes to the study clinic was 382 m, minimizing geographic and logistic barriers to the diagnosis of malaria episodes; and (iii) a study physician was available at all times at the only easily accessible health care facility in the area.
Relatively few studies have examined the relationship between growth-inhibitory activity and protection from clinical malaria. In a longitudinal study in Gambia, Marsh et al. (32) found no correlation between growth-inhibitory activity measured in dialyzed serum and malaria incidence or the odds of experiencing malaria. Perraut et al. (45) conducted a prospective study in Senegal in an area of seasonal P. falciparum transmission and found no correlation between growth-inhibitory activity measured in undialyzed serum and the incidence of malaria. More recently, McCallum et al. (33) performed a prospective study in Kenya in an area of seasonal P. falciparum transmission and found no correlation between growth-inhibitory activity measured with dialyzed serum and purified IgG and the time to the first malaria episode. In the present study, we also failed to find a correlation between growth-inhibitory activity and malaria incidence, and after adjusting for age, the association between growth-inhibitory activity and time to the first malaria episode was only marginally significant. However, unlike the study by Marsh et al. (32), we found that inhibitory activity correlated with a reduction in the odds of experiencing malaria, based on the dichotomous variable of whether or not an individual experienced clinical malaria during the study period. Whether the various measures of malaria risk—incidence, the time to first malaria episode, and the odds of experiencing malaria—reflect the same underlying immune response is not clear, but we believe that categorizing individuals by whether or not they experience malaria is the least ambiguous one clinically and likely gives the cleanest separation of relevant immune phenotypes. The present study highlights the importance of including all three clinical end points in observational studies of naturally acquired malaria immunity whenever possible.
Other field studies have either demonstrated that growth-inhibitory activity can be induced by P. falciparum exposure (6, 17, 33, 42) or focused on the relationship between total (15) or MSP119-specific (11, 26, 37) growth-inhibitory activity and the risk of P. falciparum parasitemia as a surrogate for clinical malaria. Using a treatment-time-to-infection design in an area of Kenya where malaria is holoendemic, Dent et al. (15) found that higher total growth-inhibitory activities in both dialyzed and undialyzed plasmas were associated with a delay in time to infection by blood smear. The three studies which focused on MSP119-specific growth-inhibitory activity used a transgenic P. falciparum line that differs only in the MSP119 region so that the growth-inhibitory activity attributable to MSP119-specific antibodies could be quantified (43). Two of the studies, one a cross-sectional study in Gambia (11) and the other employing a treatment-time-to-infection design in an area of Vietnam where malaria is mesoendemic (37), found no relationship between MSP119-specific growth-inhibitory activity and the prevalence/density of P. falciparum parasitemia and the time to asymptomatic infection by blood smear, respectively. The third study, a treatment-time-to-infection study conducted during a malaria epidemic in Western Kenya, found that a higher MSP119-specific growth-inhibitory activity delayed the time to asymptomatic P. falciparum infection (26). It is important that these studies relied on the presence of asexual parasites in the blood as a surrogate for clinical malaria, but the interpretation of this end point is not clear, since asymptomatic parasitemia has been associated with both an increase (41) and decrease (13, 29) in the risk of symptomatic malaria.
The inconsistent results reported in studies of growth-inhibitory activity and malaria risk may be due to differences in P. falciparum transmission dynamics or genetic backgrounds of the study populations or to artifacts of study design (e.g., differences in the age distribution of the study population). Another important factor is the variability in serum/plasma sample preparation methods. Unprocessed plasma may contain drugs, cytokines, complement, and other nonspecific factors that inhibit parasite growth, resulting in inconsistent growth-inhibitory activity (46). Even for studies that use purified IgG, significant qualitative and quantitative differences have been reported between IgG purification methods (5). In going forward, efforts to standardize the GIA may facilitate the comparison of results from malaria vaccine trials and observational studies of acquired immunity.
Intriguingly, many field studies showed an inverse correlation between growth-inhibitory activity and age (15, 33) or no correlation (11, 37, 45), which is a paradox, since the gradual development of malaria immunity is thought to depend in part on the acquisition of antibodies directed against asexual blood-stage parasites (31), an assertion strongly supported by passive transfer studies (10). Therefore, growth-inhibitory activity, a purported in vitro correlate of the in vivo functional activity of these antibodies, would be expected to increase with age in a P. falciparum-exposed population, as we observed in the present study. The lack of correlation in other studies may be explained partly by saturation of inhibitory activity in older study populations, with mean ages reaching 23.9 years (45) and 27.5 years (37) in some studies, versus 8.9 years in our study. This possibility is consistent with results from a phase 1 trial of the AMA1 vaccine in semi-immune Malian adults, which showed no increase in growth-inhibitory activity after vaccination despite a significant increase in antibody titers (16). Regarding the inverse correlation between age and growth-inhibitory activity, it is possible that polyclonal antibody responses to repeated infections over time generate antibodies to merozoite proteins that block invasion of RBC but also interfere with invasion-blocking antibodies (22, 35). However, it remains difficult to reconcile this observation with the fact that malaria risk decreases with repeated P. falciparum infections and that antibodies play a key role in this response, unless protective antibodies target stages of the parasite life cycle that are not represented in the growth-inhibitory assay. Notably, we found that growth-inhibitory activity in parasitemic children approached adult levels, suggesting that children are capable of generating functional antibodies against blood-stage antigens, but without concurrent P. falciparum infection this response appeared to be short-lived compared to that in adults, who maintained relatively high growth-inhibitory activity in the absence of concurrent parasitemia. However, this does not appear to be an explanation for the inverse correlation between growth-inhibitory activity and age observed in other studies, since McCallum et al. (33) found that growth-inhibitory activities were not significantly different between parasitemic and aparasitemic individuals and Dent et al. (15) showed a trend toward higher growth-inhibitory activity in aparasitemic children and adults.
The study reported here suggests that antibodies which block merozoite invasion of RBC and/or inhibit the intra-RBC growth of the parasite contribute to but are not sufficient for the acquisition of malaria immunity. Given the complexity of P. falciparum infection and the corresponding immune response, it is likely that protective immunity against this pathogen requires multiple effector mechanisms. It therefore seems unlikely that any single assay which measures a limited subset of effector mechanisms will correlate unequivocally with protection from malaria. Rather, a panel of assays which captures innate B- and T-cell responses will probably be necessary to accurately predict protection. A comprehensive systems biology approach (48) applied to rigorously conducted prospective studies in P. falciparum-exposed populations may ultimately be needed to fully understand the signature of naturally acquired malaria immunity. Such information could facilitate malaria vaccine development by identifying correlates of protection and expediting the early screening of multiple vaccine candidates.
Acknowledgments
We sincerely thank the residents of Kambila, Mali, for participating in this study. We also thank Seydou Dia and Tonkoro Diarra for assisting at the study clinic, Bakary Coulibaly and Daouda Kane for helping to prepare the study site, and Richard Sakai and Julie Kim for logistical support. We also thank Zhou Hong for performing the GIA.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The GIA Reference Center is supported by the PATH/Malaria Vaccine Initiative.
Editor: J. H. Adams
Footnotes
Published ahead of print on 16 November 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Angov, E., B. M. Aufiero, A. M. Turgeon, M. Van Handenhove, C. F. Ockenhouse, K. E. Kester, D. S. Walsh, J. S. McBride, M. C. Dubois, J. Cohen, J. D. Haynes, K. H. Eckels, D. G. Heppner, W. R. Ballou, C. L. Diggs, and J. A. Lyon. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 128:195-204. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 3.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann-Leitner, E. S., R. M. Mease, E. H. Duncan, F. Khan, J. Waitumbi, and E. Angov. 2008. Evaluation of immunoglobulin purification methods and their impact on quality and yield of antigen-specific antibodies. Malar. J. 7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolad, A., I. Nebie, N. Cuzin-Ouattara, A. Traore, F. Esposito, and K. Berzins. 2003. Antibody-mediated in vitro growth inhibition of field isolates of Plasmodium falciparum from asymptomatic children in Burkina Faso. Am. J. Trop. Med. Hyg. 68:728-733. [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celada, A., A. Cruchaud, and L. H. Perrin. 1982. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin. Exp. Immunol. 47:635-644. [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, S. P., H. L. Gibson, C. T. Lee-Ng, P. J. Barr, and G. S. Hui. 1992. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 149:548-555. [PubMed] [Google Scholar]
- 10.Cohen, S., G. I. Mc, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 11.Corran, P. H., R. A. O'Donnell, J. Todd, C. Uthaipibull, A. A. Holder, B. S. Crabb, and E. M. Riley. 2004. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect. Immun. 72:6185-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulibaly, D., D. A. Diallo, M. A. Thera, A. Dicko, A. B. Guindo, A. K. Kone, Y. Cissoko, S. Coulibaly, A. Djimde, K. Lyke, O. K. Doumbo, and C. V. Plowe. 2002. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am. J. Trop. Med. Hyg. 67:604-610. [DOI] [PubMed] [Google Scholar]
- 13.Crompton, P. D., B. Traore, K. Kayentao, S. Doumbo, A. Ongoiba, S. A. Diakite, M. A. Krause, D. Doumtabe, Y. Kone, G. Weiss, C. Y. Huang, S. Doumbia, A. Guindo, R. M. Fairhurst, L. H. Miller, S. K. Pierce, and O. K. Doumbo. 2008. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J. Infect. Dis. 198:1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darko, C. A., E. Angov, W. E. Collins, E. S. Bergmann-Leitner, A. S. Girouard, S. L. Hitt, J. S. McBride, C. L. Diggs, A. A. Holder, C. A. Long, J. W. Barnwell, and J. A. Lyon. 2005. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 73:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent., A. E., E. S. Bergmann-Leitner, D. W. Wilson, D. J. Tisch, R. Kimmel, J. Vulule, P. O. Sumba, J. G. Beeson, E. Angov, A. M. Moormann, and J. W. Kazura. 2008. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One 3:e3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dicko, A., D. J. Diemert, I. Sagara, M. Sogoba, M. B. Niambele, M. H. Assadou, O. Guindo, B. Kamate, M. Baby, M. Sissoko, E. M. Malkin, M. P. Fay, M. A. Thera, K. Miura, A. Dolo, D. A. Diallo, G. E. Mullen, C. A. Long, A. Saul, O. Doumbo, and L. H. Miller. 2007. Impact of a Plasmodium falciparum AMA1 vaccine on antibody responses in adult Malians. PLoS One 2:e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan, A. F., P. Burghaus, P. Druilhe, A. A. Holder, and E. M. Riley. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. [DOI] [PubMed] [Google Scholar]
- 18.Epstein, N., L. H. Miller, D. C. Kaushel, I. J. Udeinya, J. Rener, R. J. Howard, R. Asofsky, M. Aikawa, and R. L. Hess. 1981. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J. Immunol. 127:212-217. [PubMed] [Google Scholar]
- 19.Gaudard, M., P. Ramsey, and M. Stephens. 2009. Interactive data mining informs designed experiments. Q. Reliab. Eng. Int. 25:299-315. [Google Scholar]
- 20.Grambsch, P. M., and T. M. Therneau. 1994. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515-526. [Google Scholar]
- 21.Groux, H., and J. Gysin. 1990. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res. Immunol. 141:529-542. [DOI] [PubMed] [Google Scholar]
- 22.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay, S. I., C. A. Guerra, P. W. Gething, A. P. Patil, A. J. Tatem, A. M. Noor, C. W. Kabaria, B. H. Manh, I. R. Elyazar, S. Brooker, D. L. Smith, R. A. Moyeed, and R. W. Snow. 2009. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 6:e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui, G. S., and W. A. Siddiqui. 1987. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp. Parasitol. 64:519-522. [DOI] [PubMed] [Google Scholar]
- 26.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173:666-672. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langhorne, J., F. M. Ndungu, A. M. Sponaas, and K. Marsh. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9:725-732. [DOI] [PubMed] [Google Scholar]
- 29.Males, S., O. Gaye, and A. Garcia. 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. 46:516-522. [DOI] [PubMed] [Google Scholar]
- 30.Malkin, E. M., D. J. Diemert, J. H. McArthur, J. R. Perreault, A. P. Miles, B. K. Giersing, G. E. Mullen, A. Orcutt, O. Muratova, M. Awkal, H. Zhou, J. Wang, A. Stowers, C. A. Long, S. Mahanty, L. H. Miller, A. Saul, and A. P. Durbin. 2005. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect. Immun. 73:3677-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh, K., and S. Kinyanjui. 2006. Immune effector mechanisms in malaria. Parasite Immunol. 28:51-60. [DOI] [PubMed] [Google Scholar]
- 32.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 33.McCallum, F. J., K. E. Persson, C. K. Mugyenyi, F. J. Fowkes, J. A. Simpson, J. S. Richards, T. N. Williams, K. Marsh, and J. G. Beeson. 2008. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 3:e3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura, K., H. Zhou, A. Diouf, S. E. Moretz, M. P. Fay, L. H. Miller, L. B. Martin, M. A. Pierce, R. D. Ellis, G. E. Mullen, and C. A. Long. 2009. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin. Vaccine Immunol. 16:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura, K., H. Zhou, S. E. Moretz, A. Diouf, M. A. Thera, A. Dolo, O. Doumbo, E. Malkin, D. Diemert, L. H. Miller, G. E. Mullen, and C. A. Long. 2008. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J. Immunol. 181:8776-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura, K., H. Zhou, O. V. Muratova, A. C. Orcutt, B. Giersing, L. H. Miller, and C. A. Long. 2007. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect. Immun. 75:5827-5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murhandarwati, E. E., L. Wang, C. G. Black, D. H. Nhan, T. L. Richie, and R. L. Coppel. 2009. Inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein 1 do not correlate with delayed appearance of infection with Plasmodium falciparum in semi-immune individuals in Vietnam. Infect. Immun. 77:4510-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik, R. S., G. Krishnegowda, C. F. Ockenhouse, and D. C. Gowda. 2006. Naturally elicited antibodies to glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum require intact GPI structures for binding and are directed primarily against the conserved glycan moiety. Infect. Immun. 74:1412-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair, M., M. G. Hinds, A. M. Coley, A. N. Hodder, M. Foley, R. F. Anders, and R. S. Norton. 2002. Structure of domain III of the blood-stage malaria vaccine candidate, Plasmodium falciparum apical membrane antigen 1 (AMA1). J. Mol. Biol. 322:741-753. [DOI] [PubMed] [Google Scholar]
- 40.Newbold, C. I., A. G. Craig, S. Kyes, A. R. Berendt, R. W. Snow, N. Peshu, and K. Marsh. 1997. PfEMP1, polymorphism and pathogenesis. Ann. Trop. Med. Parasitol. 91:551-557. [DOI] [PubMed] [Google Scholar]
- 41.Njama-Meya, D., M. R. Kamya, and G. Dorsey. 2004. Asymptomatic parasitaemia as a risk factor for symptomatic malaria in a cohort of Ugandan children. Trop. Med. Int. Health 9:862-868. [DOI] [PubMed] [Google Scholar]
- 42.Ockenhouse, C. F., E. Angov, K. E. Kester, C. Diggs, L. Soisson, J. F. Cummings, A. V. Stewart, D. R. Palmer, B. Mahajan, U. Krzych, N. Tornieporth, M. Delchambre, M. Vanhandenhove, O. Ofori-Anyinam, J. Cohen, J. A. Lyon, and D. G. Heppner. 2006. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 24:3009-3017. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogutu, B. R., O. J. Apollo, D. McKinney, W. Okoth, J. Siangla, F. Dubovsky, K. Tucker, J. N. Waitumbi, C. Diggs, J. Wittes, E. Malkin, A. Leach, L. A. Soisson, J. B. Milman, L. Otieno, C. A. Holland, M. Polhemus, S. A. Remich, C. F. Ockenhouse, J. Cohen, W. R. Ballou, S. K. Martin, E. Angov, V. A. Stewart, J. A. Lyon, D. G. Heppner, and M. R. Withers. 2009. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One 4:e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perraut, R., L. Marrama, B. Diouf, C. Sokhna, A. Tall, P. Nabeth, J. F. Trape, S. Longacre, and O. Mercereau-Puijalon. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191:264-271. [DOI] [PubMed] [Google Scholar]
- 46.Persson, K. E., C. T. Lee, K. Marsh, and J. G. Beeson. 2006. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J. Clin. Microbiol. 44:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirson, P., and M. Perkins. 1985. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J. Immunol. 134:1946-1951. [PubMed] [Google Scholar]
- 48.Querec, T. D., R. S. Akondy, E. K. Lee, W. Cao, H. I. Nakaya, D. Teuwen, A. Pirani, K. Gernert, J. Deng, B. Marzolf, K. Kennedy, H. Wu, S. Bennouna, H. Oluoch, J. Miller, R. Z. Vencio, M. Mulligan, A. Aderem, R. Ahmed, and B. Pulendran. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed, Z. H., M. P. Kieny, H. Engers, M. Friede, S. Chang, S. Longacre, P. Malhotra, W. Pan, and C. Long. 2009. Comparison of immunogenicity of five MSP1-based malaria vaccine candidate antigens in rabbits. Vaccine 27:1651-1660. [DOI] [PubMed] [Google Scholar]
- 50.Sagara, I., A. Dicko, R. D. Ellis, M. P. Fay, S. I. Diawara, M. H. Assadou, M. S. Sissoko, M. Kone, A. I. Diallo, R. Saye, M. A. Guindo, O. Kante, M. B. Niambele, K. Miura, G. E. Mullen, M. Pierce, L. B. Martin, A. Dolo, D. A. Diallo, O. K. Doumbo, L. H. Miller, and A. Saul. 2009. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 27:3090-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schofield, L., M. C. Hewitt, K. Evans, M. A. Siomos, and P. H. Seeberger. 2002. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418:785-789. [DOI] [PubMed] [Google Scholar]
- 52.Singh, S., M. C. Kennedy, C. A. Long, A. J. Saul, L. H. Miller, and A. W. Stowers. 2003. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 71:6766-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh, S., K. Miura, H. Zhou, O. Muratova, B. Keegan, A. Miles, L. B. Martin, A. J. Saul, L. H. Miller, and C. A. Long. 2006. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 74:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vekemans, J., and W. R. Ballou. 2008. Plasmodium falciparum malaria vaccines in development. Expert Rev. Vaccines 7:223-240. [DOI] [PubMed] [Google Scholar]