Abstract
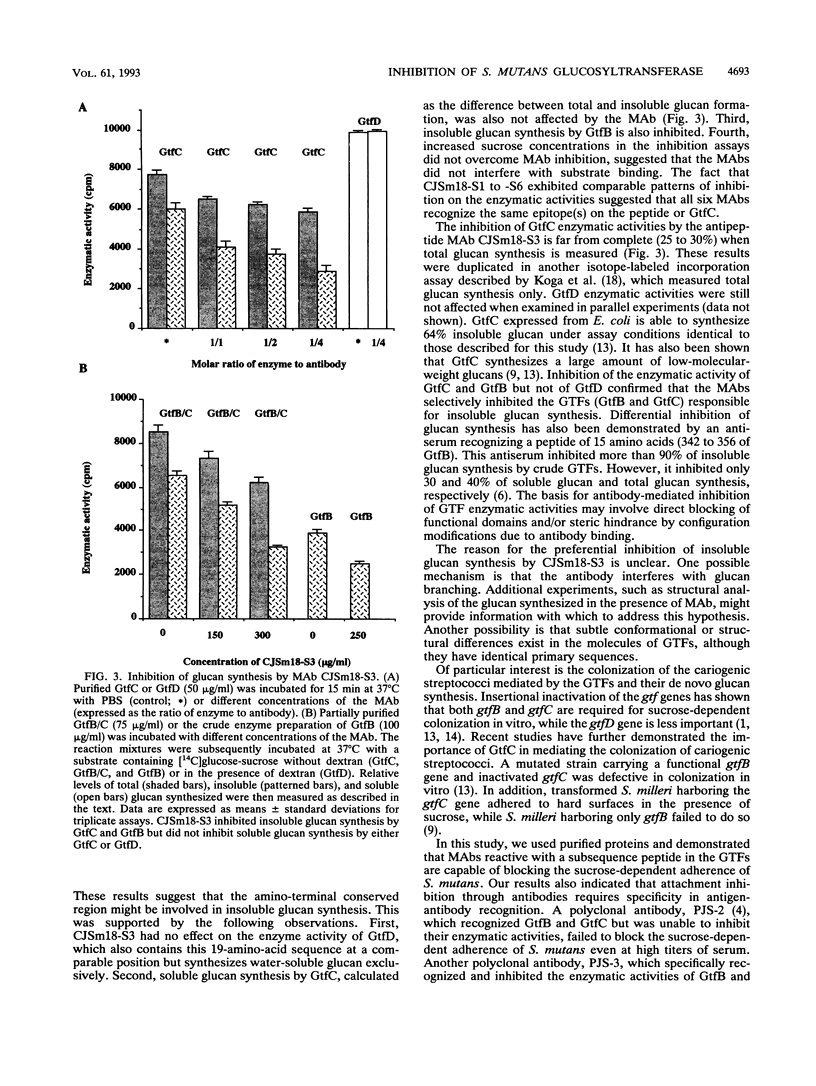
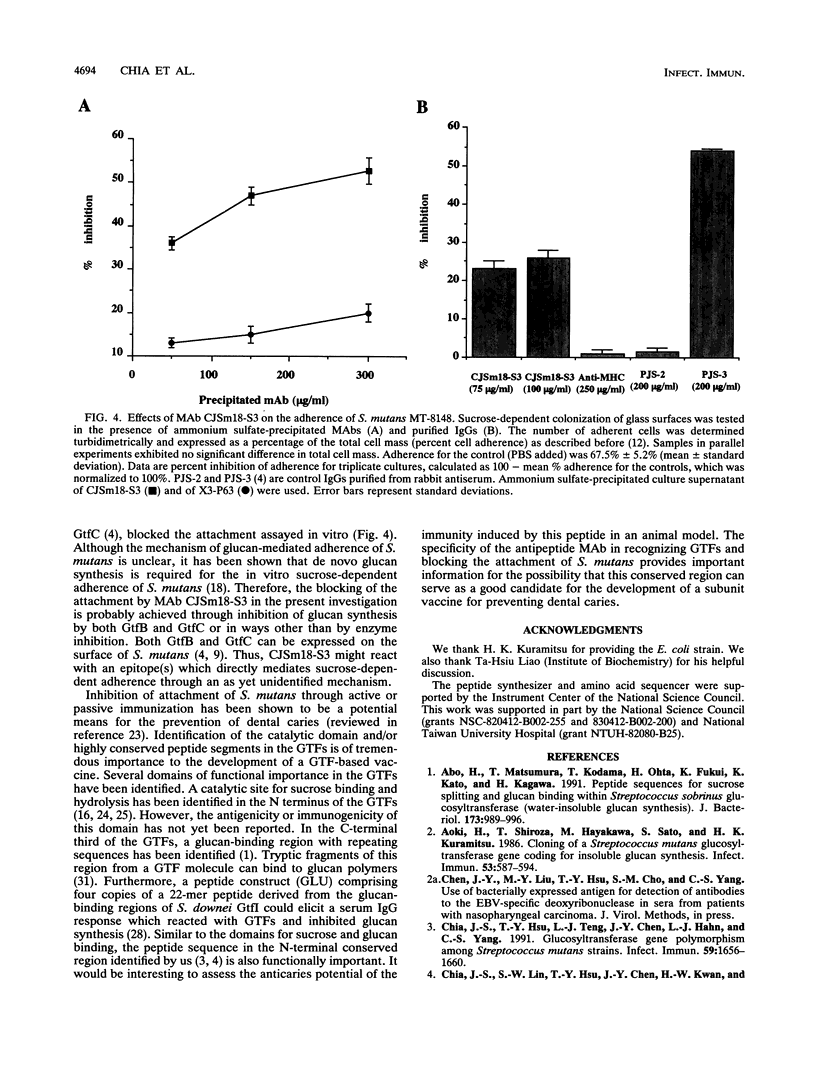
Preliminary analysis indicated that a 19-amino-acid peptide sequence (435 to 453 of GtfC) within a highly conserved region of the glucosyltransferases of the cariogenic streptococci might be functionally important (J.-S. Chia, S.-W. Lin, T.-Y. Hsu, J.-Y. Chen, H.-W. Kwan, and C.-S. Yang, Infect. Immun. 61:1563-1566, 1993). To obtain antipeptide monoclonal antibodies (MAbs), the 19-amino-acid peptide was conjugated to bovine serum albumin and used as an antigen in BALB/c mice. Six immunoglobulin G-secreting hybridoma clones, CJSm18-S1 to -S6, specifically reacted with this peptide and with purified GtfC and GtfD but not with bovine serum albumin in an enzyme-linked immunosorbent assay. The concentrated hybridoma supernatant of all six MAbs inhibited GtfC enzymatic activity but failed to inhibit GtfD, although GtfD contains the same peptide sequence. Further analysis of a purified immunoglobulin G2b MAb from one of the clones, CJSm18-S3, confirmed that this MAb specifically inhibited GtfC enzymatic activity for insoluble-glucan synthesis in a dose-dependent manner. CJSm18-S3, even at high concentrations, had no effect on GtfD, which synthesizes water-soluble glucan exclusively. Furthermore, the in vitro sucrose-dependent adherence of Streptococcus mutans was also inhibited by CJSm18-S3 in a dose-dependent manner. Our results indicate that the peptide containing the N-terminal conserved region of glucosyltransferases is functionally important for both enzymatic activity and bacterial adherence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo H., Matsumura T., Kodama T., Ohta H., Fukui K., Kato K., Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase). J Bacteriol. 1991 Feb;173(3):989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Hsu T. Y., Teng L. J., Chen J. Y., Hahn L. J., Yang C. S. Glucosyltransferase gene polymorphism among Streptococcus mutans strains. Infect Immun. 1991 May;59(5):1656–1660. doi: 10.1128/iai.59.5.1656-1660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Lin S. W., Hsu T. Y., Chen J. Y., Kwan H. W., Yang C. S. Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans. Infect Immun. 1993 Apr;61(4):1563–1566. doi: 10.1128/iai.61.4.1563-1566.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Teng L. J., Wong M. Y., Hsieh C. C. Association between dental caries prevalence and Streptococcus mutans among 13-year-old children. Taiwan Yi Xue Hui Za Zhi. 1989 Jun;88(6):589–594. [PubMed] [Google Scholar]
- Dertzbaugh M. T., Macrina F. L. Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect Immun. 1990 Jun;58(6):1509–1513. doi: 10.1128/iai.58.6.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Ikeda T., Kuramitsu H. K. Expression of Streptococcus mutans gtf genes in Streptococcus milleri. Infect Immun. 1992 Jul;60(7):2815–2822. doi: 10.1128/iai.60.7.2815-2822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore K. S., Russell R. R., Ferretti J. J. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun. 1990 Aug;58(8):2452–2458. doi: 10.1128/iai.58.8.2452-2458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Tsuchitani Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect Immun. 1981 Apr;32(1):364–372. doi: 10.1128/iai.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda O., Kato C., Kuramitsu H. K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990 Oct;136(10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- Kato C., Nakano Y., Lis M., Kuramitsu H. K. Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1184–1188. doi: 10.1016/0006-291x(92)92329-v. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Liao T. H. Isoelectric focusing of multiple forms of DNase in thin layers of polyacrylamide gel and detection of enzymatic activity with a zymogram method following separation. Anal Biochem. 1982 Jan 1;119(1):96–101. doi: 10.1016/0003-2697(82)90671-6. [DOI] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Hamada S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J Gen Microbiol. 1986 Oct;132(10):2873–2883. doi: 10.1099/00221287-132-10-2873. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Childers N. K. Development and outlook for a caries vaccine. Crit Rev Oral Biol Med. 1990;1(1):37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- Mooser G., Hefta S. A., Paxton R. J., Shively J. E., Lee T. D. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J Biol Chem. 1991 May 15;266(14):8916–8922. [PubMed] [Google Scholar]
- Mooser G., Iwaoka K. R. Sucrose 6-alpha-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989 Jan 24;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Holmberg C. F., Eastcott J., King W. F., Ali-Salaam P. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect Immun. 1993 Jul;61(7):2899–2905. doi: 10.1128/iai.61.7.2899-2905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Wong C., Hefta S. A., Paxton R. J., Shively J. E., Mooser G. Size and subdomain architecture of the glucan-binding domain of sucrose:3-alpha-D-glucosyltransferase from Streptococcus sobrinus. Infect Immun. 1990 Jul;58(7):2165–2170. doi: 10.1128/iai.58.7.2165-2170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


