Abstract
Protein kinase B (PKB)/Akt is considered to be a key target downstream of insulin receptor substrate 2 (IRS2) in the regulation of β-cell mass. However, while deficiency of IRS2 in mice results in diabetes with insulin resistance and severe failure of β-cell mass and function, only loss of the PKBβ isoform leads to a mild metabolic phenotype with insulin resistance. Other isoforms were reported not to be required for metabolic regulation. To clarify the roles of the three PKB isoforms in the regulation of islet mass and glucose homeostasis, we assessed the metabolic and pancreatic phenotypes of Pkbα, Pkbβ, and Pkbγ-deficient mice. Our study uncovered a novel role for PKBα in the regulation of glucose homeostasis, whereas it confirmed that Pkbβ−/− mice are insulin resistant with compensatory increase of islet mass. Pkbα−/− mice displayed an opposite phenotype with improved insulin sensitivity, lower blood glucose, and higher serum glucagon concentrations. Pkbγ−/− mice did not show metabolic abnormalities. Additionally, our signaling analyses revealed that PKBα, but not PKBβ or PKBγ, is specifically activated by overexpression of IRS2 in β-cells and is required for IRS2 action in the islets.
Adaptation of pancreatic islet mass and function relative to metabolic demand maintains glucose homeostasis and may prevent the development of type 2 diabetes. β-Cell proliferation, apoptosis, growth, and function are tightly regulated by various extracellular factors and intracellular signaling pathways (23, 24, 34). In β-cells, insulin receptor substrate 2 (IRS2) controls maintenance and expansion of islet mass (29, 31, 42). In fact, IRS2-deficient mice are insulin resistant, show β-cell failure and hyperglycemia, and finally develop diabetes (26, 42). In contrast, deficiency of IRS1 only causes insulin resistance without the development of diabetes due to a compensatory increase in functional β-cell mass (1, 38). These observations indicated that IRS2, but not IRS1, is necessary for maintenance and compensatory increase of β-cell mass. Furthermore, experiments with isolated islets revealed that overexpression of IRS2, but not of IRS1, can increase β-cell proliferation and protect cells against high-glucose-induced apoptosis (29). Downstream of IRS2, phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB) signaling is considered to be the critical pathway for the regulation of β-cell mass and function (12, 15, 16, 27). The serine-threonine kinase PKB, also known as Akt, is required for various cellular processes, from the regulation of cell cycle, survival, and growth to glucose and protein metabolism. In mammals, three PKB/Akt isoforms have been characterized and named PKBα/Akt1, PKBβ/Akt2, and PKBγ/Akt3. Although encoded by different genes on different chromosomes, the three isoforms display high homology at the protein level with 80 to 85% identical residues and the same structural organization (43). However, they differ in terms of tissue-specific expression. PKBα is expressed in most tissues and PKBβ is highly expressed in insulin-responsive tissues, whereas PKBγ expression is prominent in the brain and testes (17). All three isoforms are expressed in β-cells (30, 37). The roles of PKB in different tissues have been studied in transgenic-mouse models. While Pkbα−/− and Pkbγ−/− mice show impaired fetal growth and brain development, respectively, glucose homeostasis is unaffected in both models (9, 11, 14, 39, 46). In contrast, Pkbβ−/− mice are insulin resistant and mildly glucose intolerant and have less adipose tissue. Depending on the strain and gender, these mice show either late loss of β-cells followed by the development of diabetes and mild growth deficiency or compensatory increase of β-cell mass without age-dependent progression into overt hyperglycemia (10, 17). These studies suggested that PKBβ is the only isoform playing a role in the regulation of energy homeostasis. On the other hand, constitutive activation of PKBα in β-cells is sufficient to increase growth and proliferation (5, 40), and in INS1 cells it prevents free fatty acid (FFA)-induced apoptosis (44). Furthermore, antagonizing total PKB activity in β-cells by ectopic expression of a kinase-dead mutant causes defects in insulin secretion (4), suggesting that in islets PKB is required mainly for normal function of the β-cells. Although these data support the notion that PKB must play a role in pancreatic β-cells, they are not in line with the stronger metabolic phenotype displayed by IRS2-deficient mice. In fact, PKBα and PKBγ appear not to be required to regulate glucose homeostasis (9, 11, 39), and in the case of Pkbβ−/− mice, even though glucose homeostasis is impaired due to strong peripheral insulin resistance, the overall metabolic phenotype is far less severe than in Irs2−/− mice (10), indicating that the capacity for β-cell compensation is retained in the absence of PKBβ.
The aim of this study was to clarify the role of PKB in the regulation of islet mass and to define the relevance of PKB isoforms for IRS2 action in β-cells. Although it had been shown that PKBα is dispensable for the regulation of glucose homeostasis (9, 11), we found lower blood glucose concentrations in Pkbα−/− mice. Based on this observation, we assessed in more detail the metabolic and the endocrine pancreatic phenotypes of Pkbα-, Pkbβ-, or Pkbγ-deficient mice. In addition, glucose uptake into fat cells, insulin secretion, and islet cell proliferation were investigated. Contrary to previous assumptions implying that PKBβ is the only (or at least the main) isoform playing a role in the regulation of glucose metabolism, we present evidence that both PKBα and PKBβ isoforms are required in the periphery for regulation of glucose homeostasis. While we confirmed that Pkbβ−/− mice are insulin resistant and glucose intolerant with compensatory increase of β-cell mass, Pkbα−/− mice showed lower blood glucose levels, were more insulin sensitive, and revealed higher serum glucagon concentrations accompanied by a mild increase in α-cell mass and proliferation. Moreover, our in vitro experiments showed that PKBα is specifically activated by IRS2 in β-cells and that its activation is required for IRS2-induced proliferation in islets.
MATERIALS AND METHODS
Animals.
Mice deficient in the PKB isoforms (Pkbα−/−, Pkbβ−/−, and Pkbγ−/− mice) were previously generated and described (13, 39, 46). Pkbα−/− and Pkbγ−/− mice were on a mixed 129/Ola and C57BL/6 genetic background, and Pkbβ−/− mice were on a mixed 129/SvJae and C57BL/6 background. Male and female mice at two different age points (2 to 3 and 5 to 6 months old) were investigated, comparing PKB-deficient mice with wild-type littermates. The data presented are from 5- to 6-month-old male mice unless otherwise noted, since the younger age group and the females displayed similar phenotypes. The mice were housed according to the Swiss Animal Protection Laws in groups with 12-h dark-light cycles and with free access to food and water. All procedures were conducted with the relevant approval of the appropriate authorities.
Determination of glucose, insulin, glucagon, and corticosterone.
Blood samples were collected from the tail veins of mice, and d-glucose was measured using a glucose meter (FreeStyle; Disetronic Medical System AG, Burgdorf, Switzerland). After the animals were sacrificed, blood was collected by cardiac puncture using an insulin syringe (BD Micro Fine; BD Consumer Healthcare, Le Pont de Claix, France) and supplemented with aprotinin (Sigma-Aldrich, Saint Louis, MO). After centrifugation, the serum was immediately frozen. Insulin and glucagon were measured with the mouse endocrine multiplex kit (Linco Research, St. Charles, MO). Alternatively, an enzyme-linked immunosorbent assay (ELISA) kit (Ultra Sensitive rat insulin ELISA kit; Crystal Chem, Downers Grove, IL) was used to measure insulin. Corticosterone was measured with the corticosterone double antibody RIA kit (MP Biomedicals, Eschwege, Germany). For all experiments, blood samples for metabolic parameters were drawn at the same time of day, in the morning (9 to 11 a.m.).
Oral GTT, intraperitoneal ITT, and intraperitoneal GC.
For glucose tolerance tests (GTT) and insulin tolerance tests (ITT), mice were fasted overnight. d-Glucose was orally administered at 2 g/kg of bodyweight [d-(+)-glucose anhydrous; Fluka, Buchs, Switzerland]. Insulin was administered by injection (intraperitoneal [i.p.]) at 0.75 U/kg of bodyweight (human recombinant insulin; Sigma-Aldrich, Saint Louis, MO). For glucagon challenge (GC), mice were fasted for 8 h, and glucagon was administered by injection (i.p.) at 30 μg/kg of bodyweight (GlucaGen; Novo Nordisk Pharma, Bagsværd, Denmark). Tail blood was collected at the times indicated, and d-glucose levels were determined as described above.
Morphometric analysis of the pancreatic islets.
Pancreata were dissected, fixed in zinc formalin, embedded in paraffin, and cut into 3-μm sections. To assess the size and number of islets and of α-cells and β-cells, three sections per pancreas were stained for insulin (mouse monoclonal antibody; Sigma-Aldrich, Saint Louis, MO) and glucagon (rabbit polyclonal antibody; Dako, Glostrup, Denmark). Fluorescent secondary antibodies against mouse (fluorescein isothiocyanate [FITC]) and rabbit (Cy3) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Stained sections were analyzed using Axioplan 2 and Axio Vision software (Zeiss, Göttingen, Germany). The areas of the whole sections were assessed with Image J (National Institutes of Health, Bethesda, MD). To visualize proliferating β- and α-cells, Ki-67 immunostaining (goat polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA) was used (19) in combination with DAPI (4′,6′-diamidino-2-phenylindole) (Sigma-Aldrich, Saint Louis, MO) and insulin or glucagon staining, respectively. Apoptosis was assessed with ApopTag peroxidase in situ detection kits (Q-Biogene, Montreal, Quebec, Canada).
Islet isolation and culture.
Islets were isolated from 5-month-old wild-type and PKB-deficient mice by collagenase (Worthington Biochemical Corporation) digestion of the pancreas, as previously described (36). After a density gradient (Histopaque-1119; Sigma-Aldrich, Saint Louis, MO) and hand picking for further purification, the islets were cultured in RPMI 1640 medium containing 11.1 mmol/liter d-glucose (Invitrogen, Carlsbad, CA), 10% FCS (HyClone Laboratories Inc., Logan, UT), 100 units/ml penicillin, 100 μg/ml streptomycin, and 40 g/ml gentamicin (Invitrogen, Carlsbad, CA). Islets were plated on plates coated with extracellular matrix (ECM) derived from bovine corneal cells (Novamed, Jerusalem, Israel) and allowed to attach and flatten for 3 days before the start of the experiments.
Insulin secretion and proliferation assays in isolated islets.
To assess glucose-stimulated insulin secretion, 24 islets of similar sizes per dish were incubated for 1 h in the presence of 2.8 mM glucose and subsequently stimulated for 1 h with 16.7 mM glucose. Overnight acid-alcohol extraction was used to collect total insulin and protein contents. Secreted insulin and total insulin content were measured using the mouse insulin ELISA enzyme immunoassay (Mercodia, Uppsala, Sweden) and normalized by protein content, as measured using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). The BrdU Labeling and Detection Kit II (Roche, Basel, Switzerland) combined with insulin (guinea pig antibody; Dako, Glostrup, Denmark) and DAPI staining was used to assess proliferation in β-cells. Islets were incubated for 2 days in the presence of bromodeoxyuridine (BrdU).
RNA extraction from isolated islets and real-time PCR.
Total RNA was extracted from 80 mouse islets using the Nucleospin RNAII Kit (Macherey-Nagel GmBH, Dueren, Germany) and reverse transcribed using SuperScript II reverse transcriptase with random hexamers as primers (Invitrogen, Carlsbad, CA). Real-Time PCR primers for actin, Pkbα, Pkbβ, Pkbγ, and Glut2 were supplied by Applied Biosystems (Foster City, CA), and changes in mRNA expression were calculated using the difference in cycle threshold values, as previously described (36).
Fat cell isolation and glucose uptake.
Isolation of white adipocytes from 5-month-old wild-type and Pkbα−/− mice and glucose incorporation experiments were performed as described previously (35, 45). Adipocytes were incubated in Kreb's Ringer buffer containing 1% bovine serum albumin (BSA) in the presence of d-[U-14C]glucose with or without 100 nM insulin for 1 h. The radioactivity in lysates was measured by liquid scintillation counting (Kontron Betamatic V, Kontron Instruments, Montigny-Le-Bretonneux, France). Glucose uptake was normalized to the number of adipocytes.
Cell culture.
Rat insulinoma INS1 cells (2), clone 832/13 (21), were cultured in RPMI 1640 medium containing 11 mmol/liter d-glucose supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamax, 1 mM sodium pyruvate, 10 mM HEPES, and 50 μM β-mercaptoethanol (Invitrogen, Carlsbad, CA).
Adenoviral gene transfer into islets and INS1 cells.
Adenoviral vectors (Adv) encoding myc-tagged IRS1, IRS2, or green fluorescent protein (GFP) have been described previously (29). Adenoviral vectors encoding hemagglutinin (HA)-tagged PKBα, PKBβ, or GFP were supplied by Vector BioLabs (Philadelphia, PA). After 3 days of culture on ECM plates, the islets were exposed to viral particles for 2 days at a multiplicity of infection (MOI) of 1,500 to 3,000. INS1 cells were transfected at about 80% of confluence and an MOI of about 10. Viral particles were removed after 6 h by changing the medium. After 2 days, the cells were pelleted in phosphate-buffered saline (PBS) by centrifugation and immediately frozen in liquid nitrogen.
Immunoprecipitation and Western blotting.
INS1 cell pellets were lysed in a buffer containing 50 mM HEPES, 140 mM NaCl, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 3 μg/ml leupeptin, 3 μg/ml aprotenin, 10 mM sodium fluoride, 1 mM disodium pyrophosphate, and 1 mM sodium orthovanadate. The BCA assay was used to measure protein concentrations in the lysates; 500 μg of protein was incubated overnight with the respective antibody. Sera obtained from rabbits immunized with PKB isoform-specific peptides (13, 39, 46) were used for immunoprecipitation. After 3 h of incubation with protein A beads (rpm Protein A Sepharose Fast Flow; GE Healthcare, Buckinghamshire, England), bound beads were washed several times with lysis buffer. Proteins bound to beads were finally dissociated by heating them for 3 min at 70°C in NuPAGE LDS sample buffer with sample reducing agent (Invitrogen, Carlsbad, CA). The eluates were frozen for later analysis. Equal amounts of lysates and eluates were loaded on NuPAGE Novex 4 to 12% Bis-Tris Gels (Invitrogen, Carlsbad, CA) and transferred onto Hybond-P polyvinylidene difluoride (PVDF) membranes (Amersham, GE Healthcare, Buckinghamshire, England). The membranes were incubated overnight at 4°C with primary antibody and for 1 h at room temperature with secondary antibody. Signals were visualized with Lumi-Light Western blotting substrate (Roche, Basel, Switzerland) and quantified using LAS-3000 AIDA software (Fujifilm, Tokyo, Japan). Antibodies against total PKB, phospho-PKB (Ser 473 mouse monoclonal/rabbit polyclonal), PKBβ, and PKBγ were purchased from Cell Signaling Technology (Beverly, MA). Additionally, we used antibodies against PKBα (BD Bioscience, Franklin Lakes, NJ), actin (Millipore-Chemicon International, Billerica, MA), IRS1 (Santa Cruz Biotechnology, Santa Cruz, CA), IRS2 (Millipore-Upstate, Billerica, MA), and myc (self-produced). Secondary antibodies against mouse (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit (Bio-Rad, Hercules, CA) were used.
Statistical analysis.
Data are provided as means ± standard errors of the mean (SEM). Unpaired Student t tests (two tailed) were performed for comparison between data from wild-type and PKB-deficient mice with Welch's correction in case of significantly different variances. Analysis of variance (ANOVA) with Bonferroni's post hoc test was used for multiple-comparison analysis. Results with P values under 0.05 were considered statistically significant.
RESULTS
PKBα is required for regulation of glucose homeostasis.
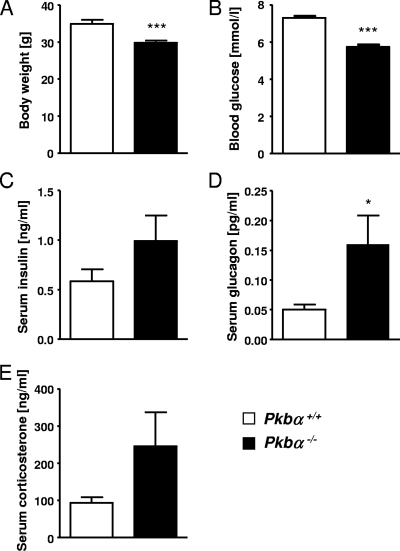
To investigate whether PKBα plays a role in the regulation of glucose homeostasis, we examined the metabolic parameters of Pkbα-deficient mice. Pkbα−/− animals weighed less than control littermates (−14.5% ± 1.5%) (Fig. 1A). In contrast to previously published data (9, 11), we found significantly lower blood glucose (−21.2% ± 4.5%) (Fig. 1B) and higher serum glucagon concentrations (+219% ± 97.3%) (Fig. 1D) in Pkbα−/− mice (random fed) compared to wild-type littermates. Serum insulin (random fed) (Fig. 1C) and serum corticosterone (Fig. 1E) concentrations were not significantly increased in Pkbα−/− mice. The data presented refer to male mice; females showed similar metabolic values (data not shown).
FIG. 1.
PKBα is required for metabolic regulation. Body weight (A), blood glucose (B), serum insulin (C), serum glucagon (D), and serum corticosterone (E) concentrations were examined in random-fed Pkbα−/− mice (filled bars) and control littermates (open bars). The data are presented as means plus SEM (n = 9 to 15). The results are from 5- to 6-month-old male mice. Similar results were obtained with younger mice (2 to 3 months old) and females (data not shown). *, P < 0.05; ***, P < 0.001.
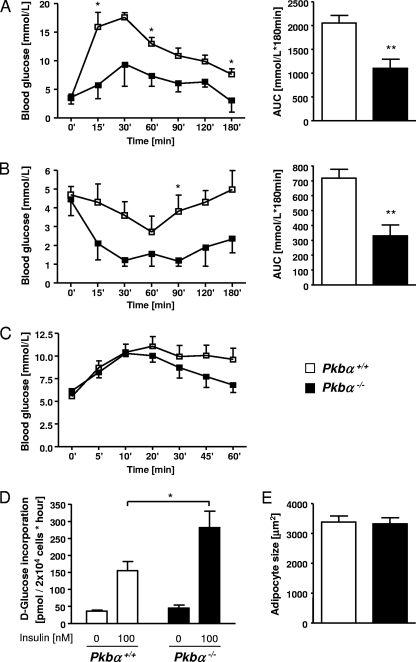
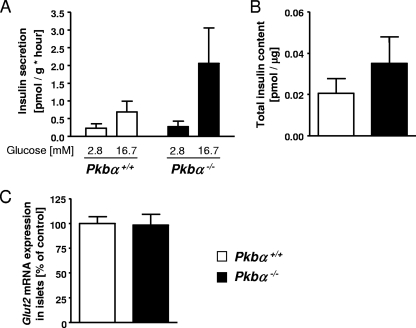
Next, we assessed metabolic responses in Pkbα−/− and wild-type littermates. During oral GTT, blood glucose levels remained consistently lower in Pkbα−/− mice, with a significant reduction in the area under the curve (AUC) (−46.5% ± 12.3%) (Fig. 2A). Intraperitoneal ITT revealed lower levels of blood glucose in Pkbα−/− mice after insulin administration, with a significant reduction of the area under the curve (−54.2% ± 14.3%) (Fig. 2B). During intraperitoneal GC, all animals showed similar increases in blood glucose concentrations after glucagon injection, but a more rapid decrease was observed in Pkbα−/− than in control mice (Fig. 2C). We also assessed glucose incorporation into adipocytes isolated from perigonadal epididymal fat. As shown in Fig. 2D, insulin-induced glucose incorporation was significantly higher (+81.9% ± 31.0%) in adipocytes from Pkbα−/− mice than in those from control littermates. No difference was found in adipocyte size (Fig. 2E). Additionally, we measured glucose-stimulated insulin secretion from isolated islets. Compared to islets from control littermates, insulin secretion from Pkbα−/− islets was higher after stimulation with glucose (+202.0% ± 146.4%) (Fig. 3A), whereas basal values were similar. Glut2 expression in islets was not changed and therefore could not explain this improvement (Fig. 3C). No significant increase in total insulin content was found between controls and Pkbα−/− islets (Fig. 3B).
FIG. 2.
Pkbα−/− mice are more insulin sensitive. (A and B) Curves representing blood glucose concentrations during 180-min oral GTT (A) and intraperitoneal ITT (B) in Pkbα−/− mice (closed squares) and control animals (open squares). The results are means ± SEM (n = 3 to 7) and are from 5- to 6-month-old male mice. The same trends were found in younger animals (2 to 3 months old) and females. The bar graphs on the right show the respective AUCs of GTT and ITT. (C) Blood glucose concentrations during 60-min intraperitoneal GC in Pkbα−/− and control male mice. The data are means ± SEM (n = 4 to 5). Female mice showed similar results (data not shown). (D) Glucose uptake measured after insulin stimulation (100 nM) of perigonadal fat cells isolated from Pkbα−/− female mice and control littermates. (E) Adipocyte size. The data are presented as means plus SEM (n = 5 to 6). *, P < 0.05; **, P < 0.01.
FIG. 3.
Insulin secretion in islets isolated from Pkbα−/− mice. Glucose-stimulated insulin secretion (A) and total insulin content (B) in islets isolated from Pkbα−/− female mice (filled bars) and control littermates (open bars). The data are presented as means plus SEM (n = 3 to 8). (C) Glut2 mRNA expression in islets isolated from Pkbα−/− female mice and control littermates. The data are means plus SEM (n = 5).
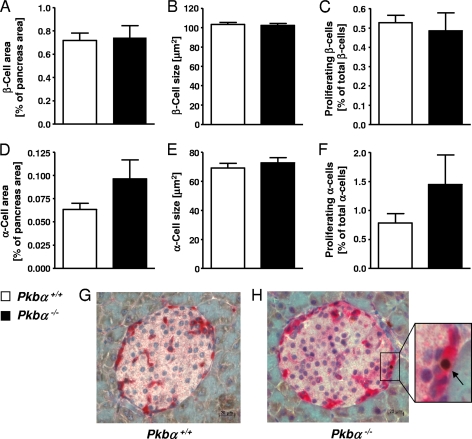
Islet morphology was analyzed by staining pancreatic sections for insulin, glucagon, and the proliferation marker Ki-67. Pancreatic sections from Pkbα−/− mice consistently revealed a trend toward increased α-cell area (1.52- ± 0.3-fold) (Fig. 4D), as well as proliferation (1.85- ± 0.6-fold) (Fig. 4F to H). In contrast, the islet area (Fig. 4A) and proliferation of β-cells (Fig. 4C) were unchanged compared to control animals (1.04- ± 0.14-fold and 0.92- ± 0.18-fold, respectively). The sizes of α-cells (Fig. 4E) and β-cells (Fig. 4B) were unchanged. In females, similar results were found, but no increase in α-cell area (data not shown).
FIG. 4.
Pancreatic phenotype of Pkbα−/− mice. Pancreatic sections from Pkbα−/− mice were immunostained for insulin and glucagon, and islet morphology was analyzed. β-Cell area (A) and size (B), as well as α-cell area (D) and size (E), in sections of Pkbα−/− mice (filled bars) are shown compared to the respective controls (open bars). Expression of Ki-67 was assessed to visualize proliferating cells. Ki-67-positive β-cells (C) and α-cells (F) (insulin and glucagon positive, respectively) were normalized by β- and α-cell area, respectively. (G and H) Representative pancreatic sections from Pkbα+/+ and Pkbα−/− mice costained for glucagon (dark red) and Ki-67 (brown nuclei; arrow). The data are means plus SEM (n = 6), and the results are from 5- to 6-month-old male mice. Females showed similar results but no increase in α-cell area (data not shown).
Expression of PKBβ and PKBγ was assessed in liver, skeletal muscle, fat, and islets isolated from Pkbα-deficient mice and controls. No difference between controls and Pkbα−/− mice was observed (see Fig. S1 in the supplemental material).
A different role for PKBβ in regulation of glucose homeostasis and insulin sensitivity.
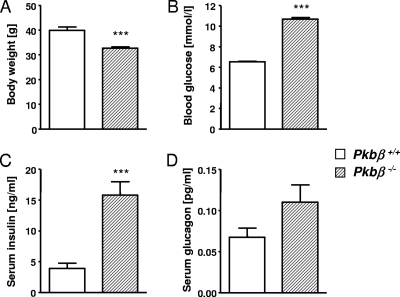
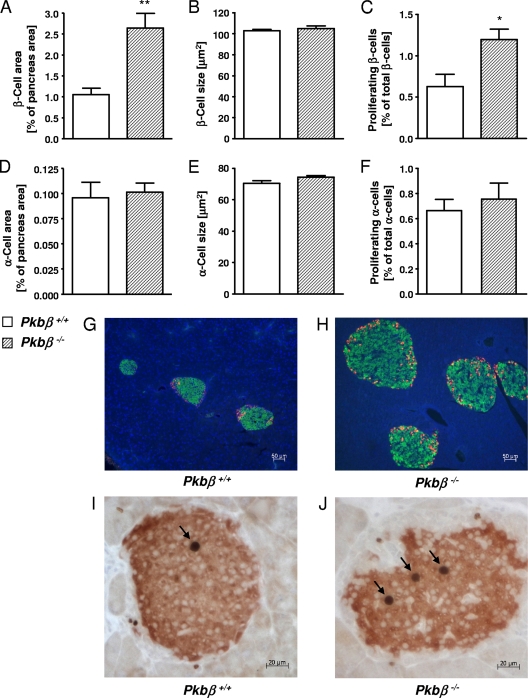
Pkbβ−/− mice weighed less than control littermates (−18.2% ± 1.6%) (Fig. 5A). Analysis of metabolic parameters showed significantly higher blood glucose and serum insulin concentrations for Pkbβ−/− mice (random fed) compared to control littermates (+63.8% ± 4.2% [Fig. 5B] and +306.0% ± 55.9% [Fig. 5C], respectively). Serum glucagon levels were not significantly changed (Fig. 5D). GTT and ITT have previously been performed with Pkbβ−/− mice from this strain and revealed glucose and insulin intolerance (13). Analysis of pancreas morphology in Pkbβ−/− mice revealed significantly increased islet areas (2.4- ± 0.3-fold) (Fig. 6A and G to H), as well as proliferation of β-cells (1.9- ± 0.2-fold) (Fig. 6C and I to J), while the α-cell area (Fig. 6D) and proliferation (Fig. 6F) were unchanged. No changes were found in α-cell (Fig. 6B) and β-cell (Fig. 6E) sizes. A similar phenotype was found for female mice, although increases in blood glucose, serum insulin, and glucagon were milder than in the males and not significant (data not shown). PKBα and PKBγ expression was analyzed in islets of Pkbβ−/− and control mice and showed no significant differences (see Fig. S2 in the supplemental material).
FIG. 5.
Metabolic parameters in Pkbβ−/− mice. Shown is analysis of body weight (A), blood glucose (B), serum insulin (C), and serum glucagon (D) concentrations in random-fed Pkbβ−/− mice (hatched bars) and control littermates (open bars). The data are means plus SEM (n = 11 to 22). The results are from 5- to 6-month-old male mice. Similar results were obtained with younger mice (2 to 3 months old), whereas females showed milder and nonsignificant increases in blood glucose and serum insulin (data not shown). ***, P < 0.001.
FIG. 6.
Pancreatic phenotype of Pkbβ−/− mice. β-Cell area (A) and size (B), α-cell area (D) and size (E), and Ki-67 positive β-cells (C) and α-cells (F) in pancreatic sections of Pkbβ−/− mice (hatched bars) compared to controls (open bars). (G and H) Representative sections of Pkbβ+/+ and Pkbβ−/− mice stained for insulin (green), glucagon (red), and DNA (DAPI; blue). (I and J) Representative sections costained for insulin (red) and Ki-67 (brown nuclei; arrows). The data are presented as mean plus SEM (n = 6 to 7). The results are from 5- to 6-month-old male mice. The same results were obtained with younger mice (2 to 3 months old) and females (data not shown). *, P < 0.05; **, P < 0.01.
PKBγ is dispensable for regulation of glucose homeostasis.
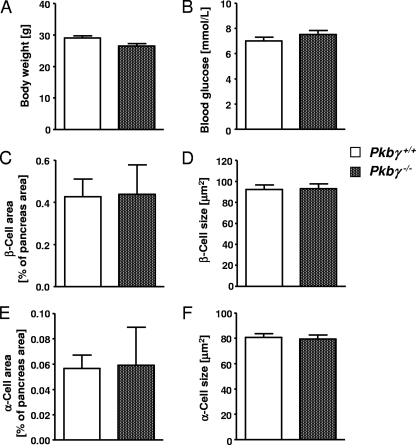
Pkbγ−/− male and female mice showed no differences in body weight (Fig. 7A) and blood glucose concentrations (random fed) (Fig. 7B) compared to their wild-type littermates, confirming previously published results (14, 39). GTT and ITT for Pkbγ−/− mice were previously performed and revealed no changes (13). Analysis of the pancreas morphology of Pkbγ−/− males and females showed normal β-cell (Fig. 7C) and α-cell (Fig. 7E) areas, as well as no changes in β-cell (Fig. 7D) and α-cell (Fig. 7F) size.
FIG. 7.
PKBγ is dispensable for the regulation of glucose homeostasis. (A and B) Body weight (A) and blood glucose concentration (B) in random-fed Pkbγ−/− mice (checked bars) compared to littermate controls (open bars). (C to F) β-Cell area (C) and size (D) and α-cell area (E) and size (F) in pancreatic sections of Pkbγ−/− mice (checked bars) compared to controls (open bars). The data are presented as means plus SEM (n = 24 for metabolic parameters and n = 6 for morphological analysis). The results are from 2- to 3-month-old male mice. The same results were obtained with females (data not shown).
PKBα acts downstream of IRS2 in β-cells.
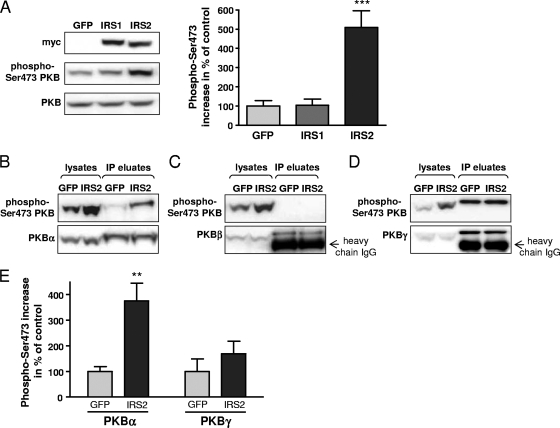
We wanted to analyze which isoforms of PKB are specifically activated by IRS2 in β-cells. In line with published data (28, 32), we found that PKB was strongly activated after overexpression of IRS2 (5.1- ± 0.9-fold) (Fig. 8A), while induction of PKB phosphorylation by IRS1 was only mild and was restricted to certain conditions (i.e., starvation of the cells prior to virus transfection) (data not shown). To analyze activation of the individual PKB isoforms, we performed immunoprecipitations with isoform-specific antibodies and subsequently detected phosphorylation of PKB in immunoprecipitates by Western blotting. This procedure was necessary, as antibodies against specific phosphorylated PKB isoforms are not available. As shown in Fig. 8B and E, PKBα was specifically activated by overexpression of IRS2 (3.7- ± 0.7-fold increase relative to GFP). No phosphorylation was detected for PKBβ (Fig. 8C and E). However, we were able to detect specific activation of PKBβ, but not PKBα, after stimulation of adipocytes with insulin (data not shown), indicating that the antibody against PKBβ used in our study was functional. Finally, PKBγ was activated by overexpression of IRS2 (1.7- ± 0.5-fold increase), but far less than PKBα and with higher variability between the experiments (Fig. 8D and E).
FIG. 8.
PKBα acts downstream of IRS2 in β-cells. (A) Western blot analysis showing phosphorylation of PKB after adenoviral overexpression of myc-tagged IRS1 and IRS2 in INS1 cells. Endogenous expression of PKB was assessed for normalization. As a control, cells were transfected with AdV-GFP. The signals from 7 independent experiments were quantified, and the mean increase in phosphorylation as a percentage of the control is presented as a bar graph on the right. As the antibody against phosphorylated PKB detects all three isoforms, signals in the lysates were not isoform specific. (B to E) To analyze activation of PKB isoforms, lysates of INS1 cells overexpressing IRS2 were immunoprecipitated with isoform-specific antibodies. Representative Western blots for PKBα (B), PKBβ (C), and PKBγ (D) are shown. The signals for phosphorylated PKB were normalized to the signals of the nonphosphorylated PKBα, PKBβ, or PKBγ isoform. For detection of PKBβ and PKBγ, all available antibodies were raised in rabbit; thus, it was not possible to avoid signal of the antibody on the blot. (E) Bar graph showing means plus SEM of 6 to 7 experiments for PKBα (left) and PKBγ (right) after overexpression of GFP (light gray) or IRS2 (dark gray). The data are represented as percentages of the control. **, P < 0.01; ***, P < 0.001.
PKBα is required and sufficient for induction of proliferation in β-cells.
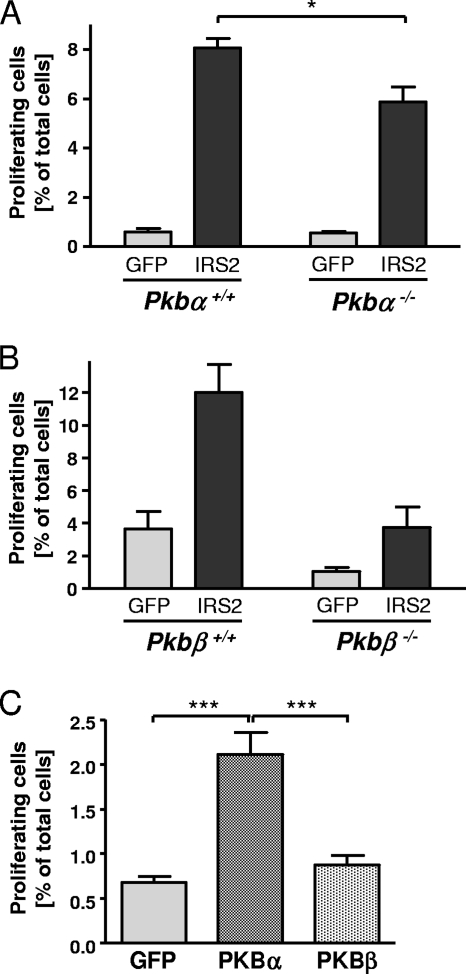
To investigate if PKB isoforms are required for IRS2 action in β-cells, we overexpressed IRS2 in islets isolated from Pkb−/− mice and determined the proliferation rate. In control islets (derived from Pkbα+/+ mice) overexpressing IRS2, we found a strong increase in cell proliferation (13.2- ± 0.6-fold) (Fig. 9A, left). In islets isolated from Pkbα−/− mice, basal values were similar to those of the control, but the increase in proliferation induced by IRS2 (10.4- ± 1.1-fold) was reduced by 27.1% ± 2.82% (Fig. 9A, right). In islets of Pkbβ+/+ mice, IRS2 induced an increase in proliferation of 3.3- ± 0.5-fold (Fig. 9B, left), very similar to islets isolated from Pkbβ−/− mice (3.6- ± 1.2-fold) (Fig. 9B, right). To examine whether PKB isoforms are also sufficient to induce proliferation in islets, PKBα or PKBβ was overexpressed in islets isolated from wild-type C57BL/6 mice. Overexpression of PKBα, but not of PKBβ, resulted in a significant increase in cell proliferation (3.1- ± 0.36-fold) (Fig. 9C).
FIG. 9.
PKBα is required for induction of proliferation by IRS2 in β-cells. Cell proliferation was assessed in isolated islets after adenoviral transfection with IRS2-, PKBα-, PKBβ-, or GFP-encoding vectors. As the islets were counterstained for insulin, the positive cells represent β-cells (although occasionally other islet cells may have been counted). (A and B) Graphs showing the percentages of BrdU-positive cells out of the total number of cells in islets isolated from Pkbα−/− (A) and Pkbβ−/− (B) female mice and the respective controls after overexpression of GFP (light gray) and IRS2 (dark gray). The values are means plus SEM (n = 3 to 5). (C) Graph showing the percentage of BrdU-positive cells in islets isolated from wild-type C57BL/6 male mice after overexpression of GFP (light gray), PKBα (dark stippled), and PKBβ (light stippled). The values are means plus SEM (n = 6). Equal adenoviral expression was tested in parallel experiments in INS1 cells (data not shown). *, P < 0.05; ***, P < 0.001.
DISCUSSION
In β-cells, PKB/Akt is considered to be an important downstream mediator of IRS2 in the regulation of islet mass; however, the available data are contradictory. Our study was designed to reevaluate the relevance of PKB in the control of blood glucose homeostasis and to clarify its role in the regulation of islet mass downstream of IRS2 with a focus on the single isoforms. This included an investigation of metabolic parameters and pancreas morphology in mice deficient for PKBα, -β, or -γ isoforms, as well as in vitro signaling analyses. We discovered a novel role for PKBα in the regulation of insulin sensitivity. In addition, PKBα was found to be the major isoform in β-cell signaling downstream of IRS2. While PKB isoforms are individually dispensable for regulation of the maintenance of islet mass, PKBα may mediate IRS2-dependent compensation for functional β-cell mass.
Metabolic phenotypes differed in the three isoform-specific PKB-deficient mouse strains. Interestingly, we discovered a previously undescribed phenotype for Pkbα−/− mice in addition to growth retardation, which was (at least in part) ascribed to defects in placental development (46, 48). We found that Pkbα−/− mice displayed significantly lower blood glucose and increased serum glucagon concentrations and responded better to insulin, indicating that they are more glucose tolerant and more insulin sensitive than their wild-type littermates (Fig. 2). Improved insulin action was also reflected by increased insulin-stimulated glucose uptake into isolated adipocytes. Since the level of PKBβ in insulin-sensitive tissues, as well as in islets, did not differ between Pkbα−/− and control mice (see Fig. S1 in the supplemental material), we could exclude the possibility that the observed metabolic phenotype was due to a compensatory increase of the PKBβ isoform. Our results contrast with previous work suggesting normal glucose homeostasis in Pkbα−/− mice (9, 11), although a trend toward improved glucose tolerance and insulin sensitivity in Pkbα−/− mice was also apparent in the study by Cho et al. These differences might indicate that the expressivity and penetrance of this phenotype are sensitive to genetic background and might differ among individuals, as was previously described for Pkbβ−/− mice, where high variability was found, not only between different strains, but also among mice of the same strain (17). The mice investigated in our study, as well as by Cho et al., were on a mixed 129 Ola and C57BL/6 background (although they belonged to separate strains), while data published by Chen et al. refer to mice on a 50% 129 R1 and 50% C57BL/6 genetic background. Whereas serum insulin, islet area, and β-cell proliferation were similar in Pkbα−/− mice relative to control littermates, serum glucagon, as well as α-cell area and α-cell proliferation, were increased in the majority of the animals investigated. To the best of our knowledge, this is the first study describing glucagon sensitivity and levels in serum, as well as the islet phenotype of Pkbα−/− mice. Improved insulin sensitivity could lead to the observed decrease in blood glucose levels and a compensatory rise in serum glucagon and corticosterone. In that case, however, insulin secretion would be expected to be downregulated, while we observed normal or even increased serum insulin concentrations in Pkbα−/− mice. This rather suggests a primary β-cell defect and a deregulation of insulin secretion relative to metabolic needs. However, lower blood glucose, increased serum glucagon, and α-cell hyperplasia, together with improved glucose tolerance, were also observed in glucagon receptor knockout mice (18, 33). Therefore, it could be that the phenotype of Pkbα−/− mice is caused by defective glucagon signaling. Nevertheless, after intraperitoneal administration of glucagon, we observed a similar increase in the blood glucose concentration, suggesting that Pkbα−/− and control mice are similarly sensitive to glucagon. The following, more rapid decrease in blood glucose in Pkbα−/− mice is probably attributable to improved insulin sensitivity. Since our mouse model is a whole-body knockout, we cannot exclude the possibility that the observed phenotype, both metabolically and in terms of pancreas morphology, is due to secondary effects. However, the fact that glucose uptake was also improved in isolated fat cells and insulin secretion from isolated islets was enhanced rather suggests a cell-autonomous effect of PKBα absence. To conclusively address this question, tissue-specific knockout for PKBα in β-cells or in specific insulin target tissues (i.e., fat tissue, skeletal muscle, and liver) would be required.
Pkbβ−/− mice showed a phenotype distinct from that of Pkbα−/− mice. Despite the mice being smaller than their control littermates, blood glucose and serum insulin concentrations were increased, consistent with previous studies showing that deficiency of PKBβ leads to insulin resistance (13). We also investigated the pancreas morphology in more detail, as neither the α-cell phenotype nor β-cell proliferation had been described yet. We found a compensatory increase in islet mass with a concomitant increase in β-cell proliferation, while the α-cell area and proliferation were unchanged relative to wild-type littermates. In contrast to β-cell proliferation, the β-cell size was unchanged, indicating that the increase in β-cell mass was due to hyperplasia rather than hypertrophy. Expression of the remaining PKB isoforms was not changed in islets of Pkbβ−/− mice, indicating that the increase in islet mass was not due to a compensatory increase of PKBα expression (see Fig. S2 in the supplemental material). Our results are in line with the findings by Cho et al. (10), though we also found a decrease in body mass, as described by Garofalo et al. (17), thus confirming that the β isoform of PKB is required for both glucose metabolism and growth. Although both PKBα and PKBβ are required to control growth, they seem to have opposite roles in the regulation of glucose homeostasis. While PKBβ improves insulin sensitivity in the periphery, PKBα appears to decrease it. Finally, we found no difference regarding metabolic parameters and pancreas morphology in Pkbγ−/− mice compared to control littermates, confirming published reports showing a dispensable role for PKBγ in glucose metabolism (14, 39).
As PKB function is thought to be required for IRS2-dependent regulation of islet mass (12, 15, 16, 27), we assessed activation/phosphorylation of the PKB isoforms downstream of IRS2. As shown by Lingohr et al. (28), we confirmed a strong (5-fold) induction of PKB phosphorylation in β-cells overexpressing IRS2, but not IRS1. Analysis of activation of the single PKB isoforms revealed that PKBα was specifically activated by IRS2, whereas activation of PKBγ was weak and inconsistent. Interestingly, PKBβ was not activated in β-cells after overexpression of IRS2 (Fig. 8). This implies that in β-cells IRS2 signaling occurs mainly via PKBα, whereas PKBβ does not play a major role downstream of IRS2. Such specific activation suggests distinct nonredundant roles for PKB isoforms in β-cell signaling, in line with previous findings showing isoform-specific activation and functions for PKB downstream of IRS in other tissues (3, 7, 22, 47).
As described previously, IRS2, but not IRS1, can induce a strong increase in cell proliferation in isolated islets (29). To address the question of whether the observed specific activation of PKBα is required for IRS2 action in β-cells, we analyzed the enhancement of cell proliferation in isolated islets overexpressing IRS2 and deficient for PKBα or PKBβ. Our results indicate that PKBα is required downstream of IRS2 in islets, as the IRS2-induced increase in cell proliferation was significantly reduced in islets deficient for PKBα (Fig. 9). Induction of proliferation, however, was only 27% abrogated, indicating that other signaling targets in addition to PKBα must be required for full induction of proliferation by IRS2. Proliferation was similar in control islets and Pkbα−/− islets after transfection with AdV-GFP, implying that the proliferation rate under basal conditions does not depend on PKBα. The requirement for PKBα agrees well with our observation that PKBα is specifically activated by overexpression of IRS2, but not of IRS1. In contrast, we observed a similar IRS2-induced increase of proliferation in control and Pkbβ−/− islets, suggesting that PKBβ is not required downstream of IRS2, which is in line with the finding that overexpression of IRS2 does not activate PKBβ. Although IRS2-induced proliferation did not reach the same extent as in Pkbβ+/+ islets, implying a potential role of PKBβ in this action, basal proliferation was also reduced compared to the controls and could thus explain this difference. The very low proliferation rates found in isolated Pkbβ−/− islets cultured on ECM plates suggests that Pkbβ−/− islets might suffer from a growth disadvantage on this matrix. In fact, even higher β-cell proliferation was found in pancreatic sections of Pkbβ−/− mice. PKB was shown to be required for growth of pancreatic islets on ECM (6, 20, 25, 41), and this observation suggests that PKBβ could be the responsible isoform. This hypothesis is supported by the finding that proliferation in Pkbβ−/− islets can be significantly increased after adenoviral reexpression of the PKBβ isoform (data not shown). We observed differences in basal proliferation rates between Pkbα+/+ and Pkbβ+/+ islets. Because the levels of basal proliferation also varied within strains between experiments, these differences were probably due to experimental variations, such as islet isolation. Nevertheless, the relative differences and induction rates were consistently maintained within the strains. Finally, the prominent role played by PKBα in the regulation of β-cell proliferation is supported by our finding that overexpression of this isoform, but not of PKBβ, in isolated wild-type islets is sufficient to significantly increase cell proliferation (Fig. 9C).
We recently proposed that maintenance and compensatory expansion of islet mass may be regulated by separate pathways downstream of IRS2 (31). It was predicted that PKB is dispensable for maintenance of β-cell mass, whereas PKBα might mediate the expansion of islet mass. The results of the present study are fully compatible with this model and support the idea that none of the three PKB isoforms are required to maintain islet mass, as islet mass was not decreased in any of the isoform-specific PKB-deficient strains and, in the case of Pkbβ−/− mice, was even increased. As others have shown previously, islet mass was not affected even after 80% of PKB activity was removed in β-cells (4), indicating that redundancy between isoforms is an unlikely explanation for the mild phenotype found in isoform-specific PKB-deficient mice. Consistently, absence of one isoform did not lead to compensatory upregulation of the remaining two isoforms in any of the tissues analyzed by us (see Fig. S1 and S2 in the supplemental material), as well as in previous studies (11, 17, 39, 46). In combination with previous data showing that constitutive activation of the α isoform is sufficient to increase β-cell growth and proliferation (5, 40), our results support the notion that PKBα contributes to compensatory increase of functional islet mass. The recent discovery that heterozygosity for PKBα converts the mild metabolic phenotype of Pkbβ−/− mice into overt type 2 diabetes with prominent β-cell dysfunction supports our findings implying an important role played by PKBα in glucose homeostasis and in particular in β-cell function (8).
Supplementary Material
Acknowledgments
We thank Heidi Seiler and Dora Schmid for excellent technical assistance and Stephan Wueest and Reto Rapold for their help in the isolation and handling of primary adipocytes. We are thankful to Michal Neeman and Katrien Vandoorne for kind support during the revision of the manuscript and to Helga Ellingsgaard and Jan Ehses for discussions and various other contributions.
This study was supported by the Takeda Foundation and the Julius Klaus Stiftung. This project was carried out in the framework of COST action BM0602. The FMI is part of the Novartis Research Foundation. O.T. was supported by the Gebert Rüf Stiftung (GRS-027/06). Work performed in the Yang laboratory was supported by the National Basic Research Program of China (2006CB503900 and 2006CB943503).
Footnotes
Published ahead of print on 23 November 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Araki, E., M. A. Lipes, M. E. Patti, J. C. Bruning, B. Haag III, R. S. Johnson, and C. R. Kahn. 1994. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186-190. [DOI] [PubMed] [Google Scholar]
- 2.Asfari, M., D. Janjic, P. Meda, G. Li, P. A. Halban, and C. B. Wollheim. 1992. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167-178. [DOI] [PubMed] [Google Scholar]
- 3.Bae, S. S., H. Cho, J. Mu, and M. J. Birnbaum. 2003. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 278:49530-49536. [DOI] [PubMed] [Google Scholar]
- 4.Bernal-Mizrachi, E., S. Fatrai, J. D. Johnson, M. Ohsugi, K. Otani, Z. Han, K. S. Polonsky, and M. A. Permutt. 2004. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J. Clin. Invest. 114:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal-Mizrachi, E., W. Wen, S. Stahlhut, C. M. Welling, and M. A. Permutt. 2001. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 108:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosco, D., P. Meda, P. A. Halban, and D. G. Rouiller. 2000. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes 49:233-243. [DOI] [PubMed] [Google Scholar]
- 7.Bouzakri, K., A. Zachrisson, L. Al-Khalili, B. B. Zhang, H. A. Koistinen, A. Krook, and J. R. Zierath. 2006. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 4:89-96. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W. S., X. D. Peng, Y. Wang, P. Z. Xu, M. L. Chen, Y. Luo, S. M. Jeon, K. Coleman, W. M. Haschek, J. Bass, L. H. Philipson, and N. Hay. 2009. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol. Cell. Biol. 29:3151-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 11.Cho, H., J. L. Thorvaldsen, Q. Chu, F. Feng, and M. J. Birnbaum. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 12.Dickson, L. M., and C. J. Rhodes. 2004. Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 287:E192-E198. [DOI] [PubMed] [Google Scholar]
- 13.Dummler, B., O. Tschopp, D. Hynx, Z. Z. Yang, S. Dirnhofer, and B. A. Hemmings. 2006. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26:8042-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easton, R. M., H. Cho, K. Roovers, D. W. Shineman, M. Mizrahi, M. S. Forman, V. M. Lee, M. Szabolcs, R. de Jong, T. Oltersdorf, T. Ludwig, A. Efstratiadis, and M. J. Birnbaum. 2005. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol. Cell. Biol. 25:1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elghazi, L., N. Balcazar, and E. Bernal-Mizrachi. 2006. Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int. J. Biochem. Cell Biol. 38:157-163. [DOI] [PubMed] [Google Scholar]
- 16.Elghazi, L., L. Rachdi, A. J. Weiss, C. Cras-Meneur, and E. Bernal-Mizrachi. 2007. Regulation of beta-cell mass and function by the Akt/protein kinase B signalling pathway. Diabetes Obes. Metab. 9(Suppl. 2):147-157. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo, R. S., S. J. Orena, K. Rafidi, A. J. Torchia, J. L. Stock, A. L. Hildebrandt, T. Coskran, S. C. Black, D. J. Brees, J. R. Wicks, J. D. McNeish, and K. G. Coleman. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelling, R. W., X. Q. Du, D. S. Dichmann, J. Romer, H. Huang, L. Cui, S. Obici, B. Tang, J. J. Holst, C. Fledelius, P. B. Johansen, L. Rossetti, L. A. Jelicks, P. Serup, E. Nishimura, and M. J. Charron. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. U. S. A. 100:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdes, J., U. Schwab, H. Lemke, and H. Stein. 1983. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31:13-20. [DOI] [PubMed] [Google Scholar]
- 20.Hammar, E., G. Parnaud, D. Bosco, N. Perriraz, K. Maedler, M. Donath, D. G. Rouiller, and P. A. Halban. 2004. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes 53:2034-2041. [DOI] [PubMed] [Google Scholar]
- 21.Hohmeier, H. E., H. Mulder, G. Chen, R. Henkel-Rieger, M. Prentki, and C. B. Newgard. 2000. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424-430. [DOI] [PubMed] [Google Scholar]
- 22.Huang, C., A. C. Thirone, X. Huang, and A. Klip. 2005. Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J. Biol. Chem. 280:19426-19435. [DOI] [PubMed] [Google Scholar]
- 23.Imai, Y., H. R. Patel, N. M. Doliba, F. M. Matschinsky, J. W. Tobias, and R. S. Ahima. 2008. Analysis of gene expression in pancreatic islets from diet-induced obese mice. Physiol. Genomics 36:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn, S. E., R. L. Hull, and K. M. Utzschneider. 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840-846. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser, N., A. P. Corcos, A. Tur-Sinai, Y. Ariav, and E. Cerasi. 1988. Monolayer culture of adult rat pancreatic islets on extracellular matrix: long term maintenance of differentiated B-cell function. Endocrinology 123:834-840. [DOI] [PubMed] [Google Scholar]
- 26.Kubota, N., K. Tobe, Y. Terauchi, K. Eto, T. Yamauchi, R. Suzuki, Y. Tsubamoto, K. Komeda, R. Nakano, H. Miki, S. Satoh, H. Sekihara, S. Sciacchitano, M. Lesniak, S. Aizawa, R. Nagai, S. Kimura, Y. Akanuma, S. I. Taylor, and T. Kadowaki. 2000. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49:1880-1889. [DOI] [PubMed] [Google Scholar]
- 27.Lingohr, M. K., L. M. Dickson, C. E. Wrede, I. Briaud, J. F. McCuaig, M. G. Myers, Jr., and C. J. Rhodes. 2003. Decreasing IRS-2 expression in pancreatic beta-cells (INS-1) promotes apoptosis, which can be compensated for by introduction of IRS-4 expression. Mol. Cell. Endocrinol. 209:17-31. [DOI] [PubMed] [Google Scholar]
- 28.Lingohr, M. K., L. M. Dickson, C. E. Wrede, J. F. McCuaig, M. G. Myers, Jr., and C. J. Rhodes. 2003. IRS-3 inhibits IRS-2-mediated signaling in pancreatic beta-cells. Mol. Cell. Endocrinol. 204:85-99. [DOI] [PubMed] [Google Scholar]
- 29.Mohanty, S., G. A. Spinas, K. Maedler, R. A. Zuellig, R. Lehmann, M. Y. Donath, T. Trub, and M. Niessen. 2005. Overexpression of IRS2 in isolated pancreatic islets causes proliferation and protects human beta-cells from hyperglycemia-induced apoptosis. Exp. Cell Res. 303:68-78. [DOI] [PubMed] [Google Scholar]
- 30.Muller, D., G. C. Huang, S. Amiel, P. M. Jones, and S. J. Persaud. 2006. Identification of insulin signaling elements in human beta-cells: autocrine regulation of insulin gene expression. Diabetes 55:2835-2842. [DOI] [PubMed] [Google Scholar]
- 31.Niessen, M. 2006. On the role of IRS2 in the regulation of functional beta-cell mass. Arch. Physiol. Biochem. 112:65-73. [DOI] [PubMed] [Google Scholar]
- 32.Niessen, M., F. Jaschinski, F. Item, M. P. McNamara, G. A. Spinas, and T. Trub. 2007. Insulin receptor substrates 1 and 2 but not Shc can activate the insulin receptor independent of insulin and induce proliferation in CHO-IR cells. Exp. Cell Res. 313:805-815. [DOI] [PubMed] [Google Scholar]
- 33.Parker, J. C., K. M. Andrews, M. R. Allen, J. L. Stock, and J. D. McNeish. 2002. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem. Biophys. Res. Commun. 290:839-843. [DOI] [PubMed] [Google Scholar]
- 34.Prentki, M., and C. J. Nolan. 2006. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 116:1802-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudich, A., D. Konrad, D. Torok, R. Ben-Romano, C. Huang, W. Niu, R. R. Garg, N. Wijesekara, R. J. Germinario, P. J. Bilan, and A. Klip. 2003. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46:649-658. [DOI] [PubMed] [Google Scholar]
- 36.Rutti, S., J. A. Ehses, R. A. Sibler, R. Prazak, L. Rohrer, S. Georgopoulos, D. T. Meier, N. Niclauss, T. Berney, M. Y. Donath, and A. von Eckardstein. 2009. Low and high-density lipoproteins modulate function, apoptosis and proliferation of primary human and murine pancreatic beta cells. Endocrinology 150:4521-4530. [DOI] [PubMed] [Google Scholar]
- 37.Stenson Holst, L., H. Mulder, V. Manganiello, F. Sundler, B. Ahrén, C. Holm, and E. Degerman. 1998. Protein kinase B is expressed in pancreatic beta cells and activated upon stimulation with insulin-like growth factor I. Biochem. Biophys. Res. Commun. 250:181-186. [DOI] [PubMed] [Google Scholar]
- 38.Tamemoto, H., T. Kadowaki, K. Tobe, T. Yagi, H. Sakura, T. Hayakawa, Y. Terauchi, K. Ueki, Y. Kaburagi, S. Satoh, et al. 1994. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182-186. [DOI] [PubMed] [Google Scholar]
- 39.Tschopp, O., Z. Z. Yang, D. Brodbeck, B. A. Dummler, M. Hemmings-Mieszczak, T. Watanabe, T. Michaelis, J. Frahm, and B. A. Hemmings. 2005. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943-2954. [DOI] [PubMed] [Google Scholar]
- 40.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 7:1133-1137. [DOI] [PubMed] [Google Scholar]
- 41.Wang, R. N., and L. Rosenberg. 1999. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J. Endocrinol. 163:181-190. [DOI] [PubMed] [Google Scholar]
- 42.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. Bonner-Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900-904. [DOI] [PubMed] [Google Scholar]
- 43.Woodgett, J. R. 2005. Recent advances in the protein kinase B signaling pathway. Curr. Opin. Cell Biol. 17:150-157. [DOI] [PubMed] [Google Scholar]
- 44.Wrede, C. E., L. M. Dickson, M. K. Lingohr, I. Briaud, and C. J. Rhodes. 2002. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J. Biol. Chem. 277:49676-49684. [DOI] [PubMed] [Google Scholar]
- 45.Wueest, S., R. A. Rapold, J. M. Rytka, E. J. Schoenle, and D. Konrad. 2009. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 52:541-546. [DOI] [PubMed] [Google Scholar]
- 46.Yang, Z. Z., O. Tschopp, M. Hemmings-Mieszczak, J. Feng, D. Brodbeck, E. Perentes, and B. A. Hemmings. 2003. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278:32124-32131. [DOI] [PubMed] [Google Scholar]
- 47.Yun, S. J., D. F. Tucker, E. K. Kim, M. S. Kim, K. H. Do, J. M. Ha, S. Y. Lee, J. Yun, C. D. Kim, M. J. Birnbaum, and S. S. Bae. 2009. Differential regulation of Akt/protein kinase B isoforms during cell cycle progression. FEBS Lett. 583:685-690. [DOI] [PubMed] [Google Scholar]
- 48.Yung, H. W., S. Calabrese, D. Hynx, B. A. Hemmings, I. Cetin, D. S. Charnock-Jones, and G. J. Burton. 2008. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 173:451-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.