Abstract
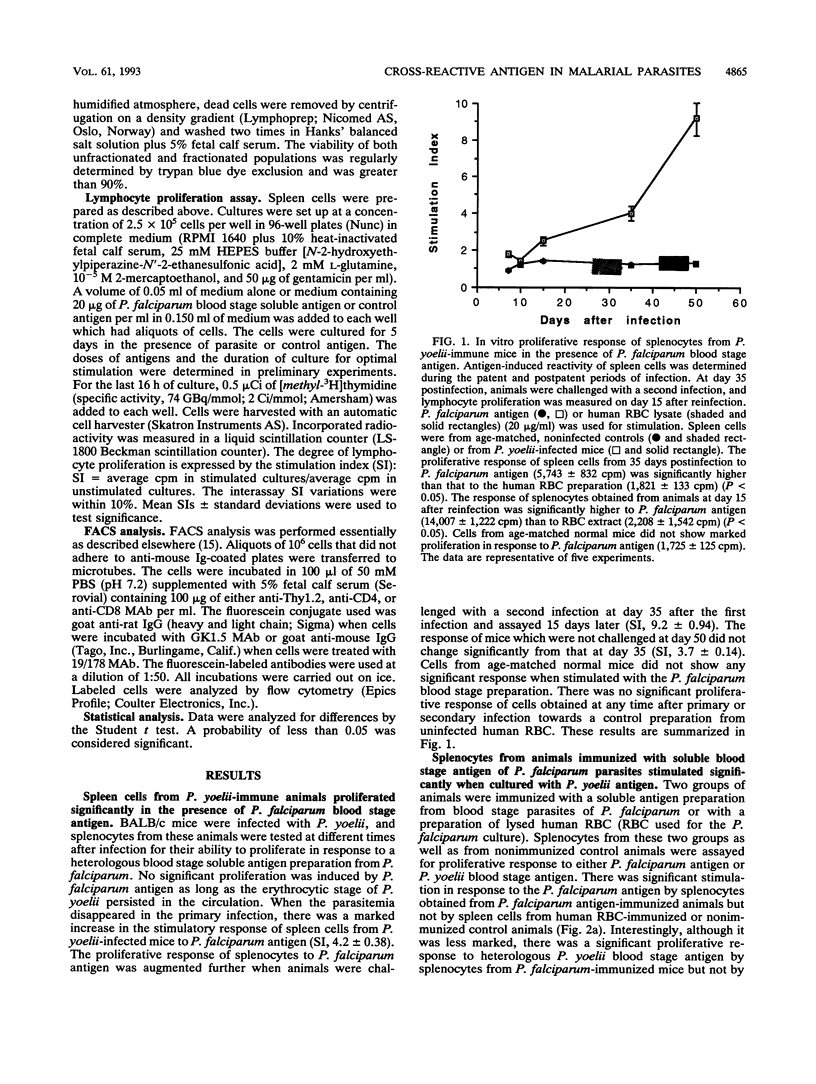
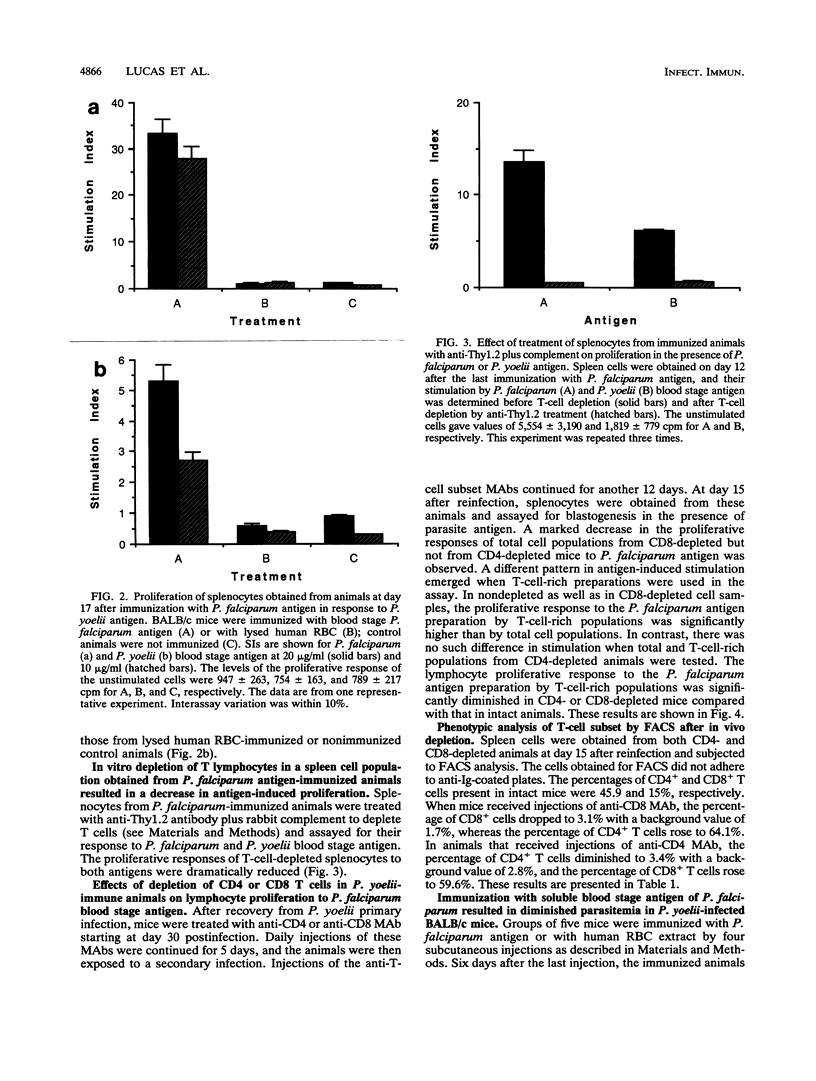
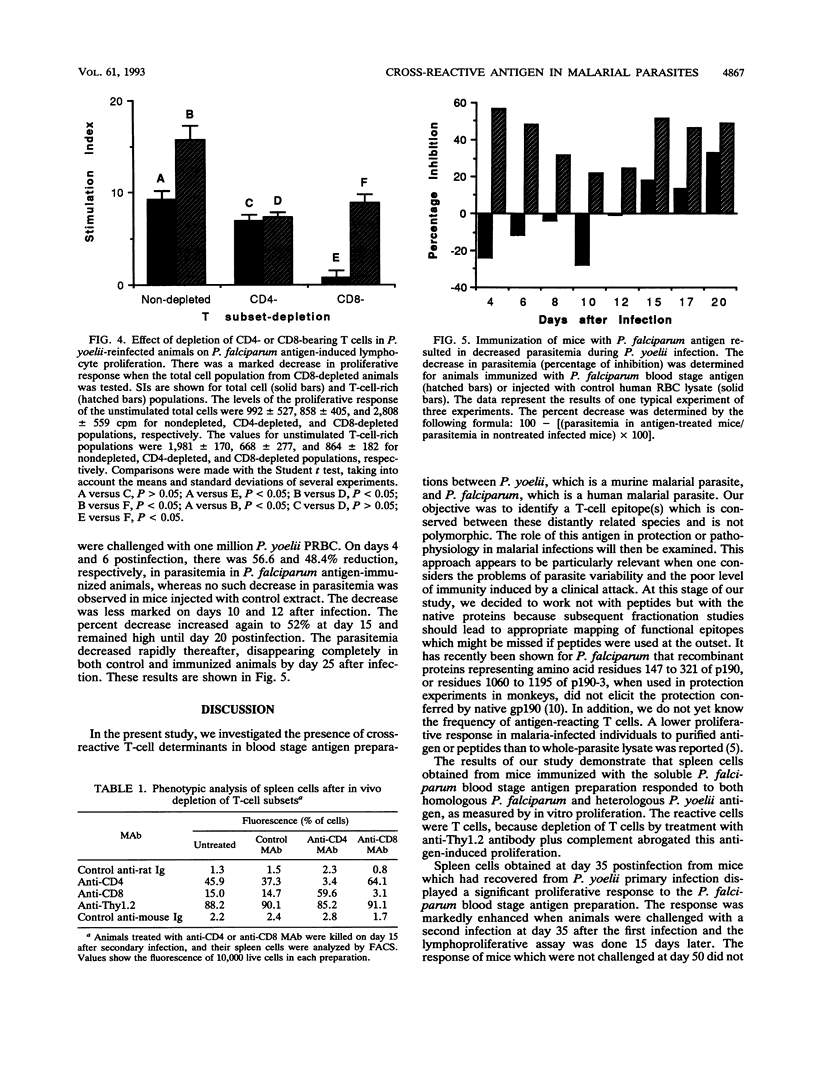
In the current study, we investigated the presence of a cross-reactive antigen(s) in the erythrocyte stage from Plasmodium yoelii (265 BY strain) and Plasmodium falciparum through recognition by T cells primed in vivo with antigens from each of these parasites. BALB/c mice are naturally resistant to P. falciparum but are susceptible to P. yoelii infection. Mice that had recovered from P. yoelii primary infection became resistant to a second infection. A higher in vitro proliferative response to a soluble blood stage preparation of P. falciparum was observed in splenic cells from immune animals than in those from mice with a patent P. yoelii infection. The antigen-induced proliferative response was enhanced when animals were exposed to a secondary infection. Animals exposed to a challenge infection were treated with anti-CD4 or anti-CD8 monoclonal antibodies to deplete the corresponding subset of T cells. There was a marked diminution in P. falciparum antigen-induced proliferative response in the total splenic cell populations from CD8-depleted but not from CD4-depleted mice. In CD8-depleted and nondepleted animals, the antigen-induced proliferation in the total cell populations was markedly lower than in the T-cell-rich populations, indicating inhibitory activities of B cells and/or macrophages. There was no such difference in the stimulation between total and T-enriched cell populations from CD4-depleted animals. Flow cytometry analysis demonstrated the presence of an almost equal percentage of CD8+ (59.6%) and CD4+ (64%) T cells in the spleen preparations following in vivo depletion of CD4- and CD8-bearing T cells, respectively. When cultured with P. yoelii blood stage antigen, splenocytes from animals immunized with P. falciparum antigen displayed a significant proliferative response which was markedly diminished by treatment with anti-Thy-1.2 antibody plus complement. Animals immunized with P. falciparum antigen and then challenged with P. yoelii blood stage parasites displayed about a 50% lower level of parasitemia. These results demonstrated the existence of a cross-reactive antigen(s) between a murine and a human Plasmodium species, as determined from both in vivo and in vitro biological assays, and indicated the reactivity of mainly CD8+ T cells with this antigen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Brown J., Greenwood B. M., Terry R. J. Cellular mechanisms involved in recovery from acute malaria in Gambian children. Parasite Immunol. 1986 Nov;8(6):551–564. doi: 10.1111/j.1365-3024.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Chizzolini C., Nicholson J. K., Geinoz A., Olsen-Rasmussen M. A., Schrijvers D. In vivo decreased expression of CD25 (p55 chain of IL-2 receptor) on CD3+ T cells correlates with low in vitro responsiveness to Plasmodium falciparum antigen in subjects living in a malaria endemic area. Clin Immunol Immunopathol. 1991 Aug;60(2):209–219. doi: 10.1016/0090-1229(91)90064-h. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Arnot D. E., Tam J. P., Nussenzweig V., Enea V. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol Cell Biol. 1986 Nov;6(11):3965–3972. doi: 10.1128/mcb.6.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K., Ehrfeld A., Falk I., Goebel H., Kupsch J., Reimann A., Zgaga-Griesz A., Saizawa K. M., Yachelini P., Tomonari K. Affinity enhancement and transmembrane signaling are associated with distinct epitopes on the CD8 alpha beta heterodimer. J Immunol. 1991 Oct 1;147(7):2075–2081. [PubMed] [Google Scholar]
- Etlinger H. M., Caspers P., Matile H., Schoenfeld H. J., Stueber D., Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991 Oct;59(10):3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Miller L. H. T-cell antigens and epitopes in malaria vaccine design. Curr Top Microbiol Immunol. 1990;155:65–78. doi: 10.1007/978-3-642-74983-4_5. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Bradley A. K., Greenwood A. M., Byass P., Jammeh K., Marsh K., Tulloch S., Oldfield F. S., Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81(3):478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Vick R. M. Evidence for a malaria mitogen in human malaria. Nature. 1975 Oct 16;257(5527):592–594. doi: 10.1038/257592a0. [DOI] [PubMed] [Google Scholar]
- Gross A., Geva S., Frankenburg S. Plasmodium berghei: lymphocyte and macrophage dynamics in the spleen of Balb/c mice in the course of infection and after rechallenge of cured mice. Exp Parasitol. 1988 Feb;65(1):50–60. doi: 10.1016/0014-4894(88)90106-3. [DOI] [PubMed] [Google Scholar]
- Haque A., Chamekh M., Cornelis J., Capron A., Haque S. A monoclonal antibody to Ly-6 gene product inhibits generation of functionally active T cells and recognizes single antigenic specificity whose expression is up-regulated in virus-transformed rat fibroblast. Immunology. 1990 Apr;69(4):558–563. [PMC free article] [PubMed] [Google Scholar]
- Haque A., Cuna W., Pestel J., Capron A., Bonnel B. Tolerance in rats by transplacental transfer of Dipetalonema viteae microfilariae: recognition of putative tolerogen(s) by antibodies that inhibit antigen-specific lymphocyte proliferation. Eur J Immunol. 1988 Aug;18(8):1167–1172. doi: 10.1002/eji.1830180804. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Rosero F., Herrera S., Caspers P., Rotmann D., Sinigaglia F., Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992 Jan;60(1):154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Webster H. K. Immunology of human malaria. A cellular perspective. Parasite Immunol. 1989 Mar;11(2):105–116. doi: 10.1111/j.1365-3024.1989.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Newbold C. I. Serological cross-reaction between high molecular weight proteins synthesized in blood schizonts of Plasmodium yoelii, Plasmodium chabaudi and Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):191–196. doi: 10.1016/0166-6851(83)90096-8. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose L. D. Characterization of macrophage dysfunction in rodent malaria. J Leukoc Biol. 1984 Dec;36(6):703–718. doi: 10.1002/jlb.36.6.703. [DOI] [PubMed] [Google Scholar]
- Lucas B., Engel A., Camus D., Haque A. Plasmodium yoelii in mice: antigen reactivity of CD4- and CD8-bearing T cells. Cell Immunol. 1993 Aug;150(1):59–71. doi: 10.1006/cimm.1993.1178. [DOI] [PubMed] [Google Scholar]
- Malik A., Egan J. E., Houghten R. A., Sadoff J. C., Hoffman S. L. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Oyeyinka G. O. Malaria--mitogens or super antigens? Nature. 1986 Feb 13;319(6054):543–544. doi: 10.1038/319543b0. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Rijnbeek A. M., Reber-Liske R., Sinigaglia F. Plasmodium falciparum-specific human T cell clones: recognition of different parasite antigens. Eur J Immunol. 1987 Feb;17(2):193–196. doi: 10.1002/eji.1830170207. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., Dockrell H., Taverne J. Macrophages as effector cells in immunity to malaria. Immunol Lett. 1985;11(3-4):233–237. doi: 10.1016/0165-2478(85)90173-7. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Belosevic M., van der Meide P. H., Podoba J. E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990 Oct;58(10):3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Wanidworanun C., Barnwell J. W., Nagasawa H., Aikawa M., Shear H. Cross-reacting antigens to Pc96, a protective antigen of Plasmodium chabaudi, in P. falciparum, P. vivax, and P. cynomolgi. Am J Trop Med Hyg. 1989 Jun;40(6):579–584. doi: 10.4269/ajtmh.1989.40.579. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Berzofsky J. A., Houghten R. A., Sedegah M., Hollindale M., Hoffman S. L. A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei. J Immunol. 1992 Sep 15;149(6):2103–2109. [PubMed] [Google Scholar]
- Wyler D. J., Herrod H. G., Weinbaum F. I. Response of sensitized and unsensitized human lymphocyte subpopulations to Plasmodium falciparum antigens. Infect Immun. 1979 Apr;24(1):106–110. doi: 10.1128/iai.24.1.106-110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


